Abstract
Purpose
Autonomic dysfunction has been reported in autism spectrum disorder (ASD). Less is known about autonomic function during sleep in ASD. The objective of this study is to provide insight into the autonomic cardiovascular control during different sleep stages in ASD. We hypothesized that patients with ASD have lower vagal and higher sympathetic modulation with elevated heart rate (HR), as compared to typical developing children (TD).
Methods
We studied 21 children with ASD and 23 TD children during overnight polysomnography. Heart rate and spectral parameters were calculated for each vigilance stage during sleep. Data from the first four sleep cycles were used to avoid possible effects of different individual sleep lengths and sleep cycle structures. Linear regression models were applied to study the effects of age and diagnosis (ASD and TD).
Results
In both groups, HR decreased during non-REM sleep and increased during REM sleep. However, HR was significantly higher in stages N2, N3 and REM sleep in the ASD group. Children with ASD showed less high frequency (HF) modulation during N3 and REM sleep. LF/HF ratio was higher during REM. Heart rate decreases with age at the same level in ASD and in TD. We found an age effect in LF in REM different in ASD and TD.
Conclusion
Our findings suggest possible deficits in vagal influence to the heart during sleep, especially during REM sleep. Children with ASD may have higher sympathetic dominance during sleep but rather due to decreased vagal influence.
Keywords: Autism, Sleep, Autonomic system, Heart rate variability
Background
The autonomic nervous system (ANS) controls the heart rate (HR), blood pressure (BP), and other physiological functions. The ANS may also contribute to cognitive, affective, and behavioral responses in children [1]. Several models have linked to possible changes in the development of ANS function to autism. The polyvagal theory postulates that the myelinated vagal branch of the autonomic nervous system affects social behavior [2] and may be compromised in individuals with autism spectrum disorder (ASD) given their social deficits [3].
Autonomic function is also related to other stress systems such as the hypothalamic–pituitary–adrenal axis. Dysregulation of the cortisol rhythm, with diminished reduction of the expected fall in evening cortisol, has been observed in ASD [4]. Individuals with ASD may be in a state of hyperarousal, exacerbated by daytime stressors, which in turn may contribute to sleep, anxiety, and gastrointestinal symptoms [5–7]. Understanding how sympathetic and vagal systems function in children with ASD may lead to targeted treatments for these symptoms.
Heart rate variability (HRV) is a well-established tool to characterize autonomic vagal and sympathetic modulation (Electrophysiology 1996). Spectral analysis of HRV is a non-invasive tool and advantageous method to characterize autonomic modulation in children. High frequency (HF) oscillations of HRV reflect vagal modulations while low frequency (LF) oscillations result from sympathetic and parasympathetic cardiac modulation [8]. Increase in LF/HF and normalized LF power may reflect sympathetic activation under certain conditions such as orthostatic stress [9]. It is unknown if this relation between spectral parameters and sympathetic activation is maintained during sleep. Microneurography, a direct continuous assessment of sympathetic activity, supports that sleep-related sympathetic activity parallels HR and BP, with lower values during non-REM sleep as compared to wakefulness. During REM sleep, sympathetic nerve activity increases above the levels recorded during wakefulness, and the values for BP and HR return to those recorded during wakefulness [10–12].
Spectral analysis of HRV during sleep has not been studied to date in the ASD population, and has high potential to contribute to our knowledge of the neuro-physiological processes associated with ASD. Spectral analysis of HRV has been applied to quantify vagal modulation in ASD during resting and during challenging social tasks [13–15]. According to the polyvagal theory, children with ASD have lower vagal influence on body functions, as reflected by respiratory sinus arrhythmia (RSA). Some authors found increased HR and decreased RSA [16, 17]. However, approximately 50 % of studies did not find group differences in resting vagal activity in children with ASD compared with typically developing children (TD). There are several reasons for these discrepancies. First, a critical obstacle in performing analysis of HRV is the requirement of stationarity in HR data. This is difficult to achieve, especially in awake children. The stereotypic behavior and excessive movements found in ASD can furthermore skew HR and HRV [18]. Second, baseline conditions or tasks present a stressful situation for a child with ASD [15]. Third, medications used in ASD can affect HR and HRV. Finally, children with ASD show reduced responsiveness of HRV during challenging tasks [1].
Sleep provides an opportunity to study the child during a time of decreased stressful psychological inputs and fewer body movements. While data exist regarding fluctuations of autonomic nervous system activity during sleep in healthy children [19], little is known about HRV and overall sleep in children with autism. For example, to our knowledge, changes in autonomic activity changes during different sleep stages in children with ASD have not been studied.
The objective of this study is to provide insight into the autonomic cardiovascular control during different sleep stages in patients with ASD. We hypothesized that during sleep, patients with ASD have lower vagal modulation (HF of heart rate variability) and elevated HR, as compared to TD children.
Methods
Subjects and clinical conditions
The Vanderbilt Institutional Review Board and Colorado Multiple Institutional Review Board approved this study. Children and parents were informed about the protocol. After children agreed to participate verbally (assuming child was developmentally able to provide verbal assent), a written informed consent was obtained from the parents. We included data from children with ASD, and those who were TD, who had participated in two studies examining the relationship between polysomnography and sleep questionnaires [20, 21] and a study examining the relationship between polysomnography and home-based measures. None of the participants had sleep disorders (e.g., REM sleep without atonia) or abnormalities in breathing (e.g., sleep apnea) on polysomnography. A more detailed clinical representation has been published previously [20, 21]. Our dataset consisted of 21 children with ASD (all boys) between 4 and 10 years (median age: 8.0, lower quartile-upper quartile Q1–Q3: 6.2–9.4) years and 23 typically developing children (TD, 18 boys and 5 girls) between 4 and 10 years (median age 8.3, Q1–Q2: 6.6–9.3 years). All children underwent physical examination and did not have signs of cardiovascular disease or history of other illness. They did not use caffeine (for at least 72 h prior to testing) or psychotropic medications, including melatonin, or other medications. The median body mass index (BMI) of ASD was 16.2 (Q1–Q3: 15.6–18.3) kg/m2. The median BMI of TD was 18.1 (Q1–Q3: 15.6–19.8) kg/m2. ASD and TD participants did not differ in growth, age adjusted BMI, or blood pressure (Table 1).
Table 1.
Demographics, anthropometrics, and vital signs of children with autism spectrum disorder (ASD) and typically developing children (TD).
| ASD | TD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | p |
| Age (years) | 7.8 | 1.8 | 7.6 | 6.2 | 10 | 8.0 | 1.9 | 8.3 | 6.6 | 9.3 | p>0.05 |
| Height (cm) | 127.4 | 12.9 | 123.1 | 117.5 | 140.6 | 127.7 | 11.8 | 127.0 | 120.6 | 137.5 | p>0.05 |
| Weight (kg) | 30.4 | 11.4 | 26.9 | 23.8 | 31.9 | 29.4 | 7.1 | 30.0 | 23.5 | 35.5 | p>0.05 |
| Percentile Growth | 61.2 | 21.2 | 75 | 50.0 | 75.0 | 60.9 | 31.7 | 75.0 | 43.8 | 90.0 | p>0.05 |
| Percentile BMI | 63.8 | 29.2 | 50.0 | 50.0 | 90.0 | 54.6 | 39.7 | 62.5 | 19.5 | 91.3 | p>0.05 |
| Systolic BP (mmHg) | 104.3 | 15.9 | 102.0 | 92.8 | 113.5 | 102.1 | 11.6 | 99.5 | 94.8 | 112.0 | p>0.05 |
| Diastolic BP (mmHg) | 60.3 | 8.0 | 59.5 | 53.8 | 66.0 | 58.2 | 7.6 | 59.5 | 54.3 | 64.8 | p>0.05 |
| Pulse Rate (beats per min) | 90.0 | 19.8 | 90.0 | 83.0 | 97.0 | 87.3 | 18.0 | 86.0 | 78.3 | 92.0 | P>0.05 |
Percentile Growth and BMI determined from Growth Charts of Center for Disease Control and Prevention (CDC 2001)
BP office blood pressure (n=10 of each group);
Mean, standard deviation (SD), median (Median) values, and 1st quartile (Q1) and 3rd quartile (Q3) are reported.
Polysomnography
Overnight polysomnography (PSG) with video was performed at the Vanderbilt University Sleep Research Core and the University of Colorado affiliated Children's Hospital Colorado Sleep Center. Digital PSG diagnostic systems (Neurofax EEG-1100, Polysmith 6.0, Nihon-Kohden, Irvine, CA) and Sandman Digital 32+ Amplifier (Sandman, Elite Sleep Diagnostic Software, Natus Medical Embla, San Carlos, CA) were used to analyze sleep.
The American Academy of Sleep Medicine (AASM) guidelines were followed for the collection of PSG data [22]. Neurophysiological channels included two frontal (F3 and F4), two central (C3 and C4), and two occipital (O1 and O2) EEGs referenced to common electrodes (M2 and M1, respectively), chin electromyography (EMG), and electrooculography (EOG) E1 and E2. Limb movements were monitored using EMG placement on the anterior tibialis of each leg. Cardiorespiratory data were collected using inductive plethysmography with non-calibrated sum signal to monitor respiratory effort of both the chest and abdomen. Airflow was measured by both nasal pressure cannula and by oral/nasal thermocouple. Pulse oximetry signal (SpO2) was recorded to monitor oxygen saturations. A lead II electrocardiogram (ECG) was used to monitor heart rhythm.
PSG data were classified for stages wake (W), non-rapid eye movement sleep N1, N2, N3, and rapid eye movement sleep (REM) following standard AASM guidelines [23]. Sleep efficiency (SE) was defined as the percentage of total sleep time to total time in bed. A single registered polysomnography technologist performed the staging to avoid inter-rater reliability concerns. A board-certified sleep specialist reviewed each study to ensure accuracy.
Heart rate and heart rate variability
ECG signal was digitized at a sampling rate of 200 or 500 Hz limited by the PSG system. Data were processed with software written in MATLAB (Mathworks, Houston, TX). QRS detection was performed using a modified Pan-Tompkins algorithm and verified manually [24]. For spectral analysis, beat-to-beat values were linear interpolated, low-pass filtered (cutoff 0.5 Hz) and re-sampled at 5 Hz. Data segments of 180 s without any artefacts were identified for each sleep stage. Linear trends were removed and power spectral density was estimated with the Fast Fourier Transformation (FFT) based Welch algorithm using segments of 256 data points with 50 % overlapping and Hanning window. The power in the frequency range of low frequencies (LF: 0.04–0.15 Hz) and high frequencies (HF: 0.15–0.40 Hz) was calculated following the HRV Task Force recommendation [9].
Statistical analysis
HR and spectral parameters were calculated for each vigilance stage during sleep. Data from the first four sleep cycles were used to avoid possible effects of different individual sleep lengths and sleep cycle structures. Mean, standard deviation (SD), median values, and 1st quartile (Q1) and 3rd quartile (Q3) are reported. Linear regression models were applied to study the effects of age and diagnosis (ASD and TD) using R (http://www.r-project.org/) and MATLAB software. First, we assessed whether the slope for age was equal for the two diagnosis groups using the model with the sleep parameter as the dependent variable, “diagnosis”, “age” and “age × diagnosis” as independent variables. When the interaction term was not significant, we then used the simpler model with diagnosis and age as independent variables. P-values of less than 0.05 were considered statistically significant.
Results
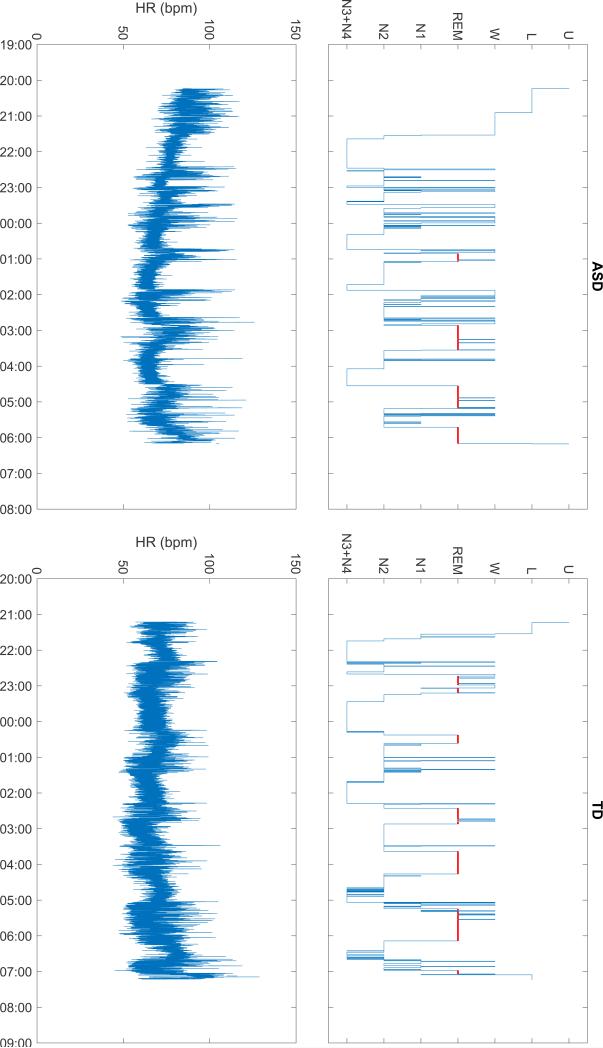
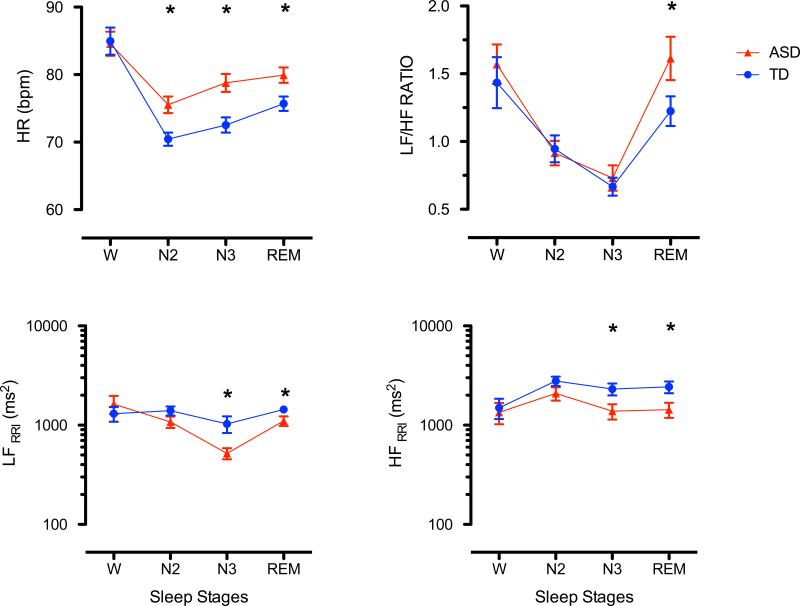
Sleep parameters were obtained from all subjects. An example of sleep structure and heart rate of one child with ASD and one child who was TD are shown in Fig. 1. Spectral analysis was not possible in one ASD child. Figure 2 shows the HR dynamic and its spectral components averaged over the first four sleep cycles and sleep stages (wake, N2, N3 and REM sleep) for children with TD and ASD. LF component decreased and HF component increased during non-REM sleep in all children. Changes were reversed during REM sleep (Fig. 2).
Fig. 1.
Sleep structure (hypnogram, top) and heart rate (HR, bottom) in a child with autism spectrum disorder (ASD, left) and a typically developing child (TD, right). Hypnogram definitions: U undefined; L light, W wake; N1, N2, N3 + N4 non-REM N1, N2 and N3 + N4; REM rapid eye movement sleep
Fig. 2.
Dynamic of heart rate (HR, top left panel), LF/HF ratio (top right panel), and absolute and normalized (LF, middle panels) and high (HF, bottom panels) frequency heart rate variability averaged over the first 4 sleep cycles and stages of wake (W), non-REM (N2 and N3) and REM sleep in children with ASD (red, mean and standard error, n = 20) and TD (blue, mean and standard error, n = 23)
Statistical model and age effect
The slope for age was equal for the two diagnosis groups using the model with the sleep and spectral parameters as the dependent variable, “diagnosis”, “age” and “age × diagnosis” as independent variables (p > 0.05). The interaction term was not significant for all studied sleep and spectral parameters. This satisfied the choice of the simpler statistical model with diagnosis and age as independent variables. The probability of statistical tests for independent age effect (p_age) and effect of group diagnosis (p_diag) are displayed in the last two columns of Tables 2, 3, 4, 5. Analysis of logarithmic transformed data revealed similar results and were not reported.
Table 2.
Parameters of one night polysomnography in children with autism spectrum disorder (ASD) and typically developing children (TD) during Wake (W), Non-REM (N1, N2, N3) and REM-sleep (REM) of the first sleep cycle.
| ASD | TD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | p_age | p_diag |
| Time in Bed (min) | 558.10 | 43.63 | 562.25 | 533.75 | 582.00 | 580.85 | 48.05 | 580.00 | 556.25 | 609.25 | 0.617 | 0.119 |
| Total Sleep Time (min) | 503.55 | 44.23 | 514.50 | 463.63 | 532.00 | 532.59 | 58.61 | 534.00 | 492.25 | 571.00 | 0.580 | 0.081 |
| Sleep Latency (min) | 54.55 | 41.63 | 44.00 | 32.13 | 67.00 | 48.26 | 38.51 | 34.50 | 27.00 | 55.25 | 0.882 | 0.240 |
| Rem_latency | 184.95 | 66.42 | 188.00 | 137.13 | 215.63 | 162.67 | 61.04 | 160.00 | 111.75 | 215.25 | 0.794 | 0.266 |
| Wake %) | 15.75 | 9.57 | 13.67 | 8.22 | 19.86 | 10.04 | 6.22 | 8.62 | 5.52 | 11.18 | 0.804 | 0.026 |
| N1 (%) | 3.75 | 1.72 | 3.18 | 2.57 | 5.29 | 3.33 | 1.26 | 3.09 | 2.70 | 3.82 | 0.908 | 0.372 |
| N2 (%) | 32.42 | 7.74 | 34.48 | 28.20 | 38.56 | 34.00 | 5.71 | 33.99 | 31.58 | 37.70 | 0.282 | 0.456 |
| N3 (%) | 24.99 | 4.83 | 25.41 | 21.57 | 28.82 | 26.94 | 5.53 | 27.19 | 22.83 | 31.15 | 0.019 | 0.192 |
| REM (%) | 17.96 | 5.80 | 19.27 | 15.61 | 21.84 | 20.73 | 3.91 | 21.27 | 18.31 | 23.49 | 0.451 | 0.074 |
| Sleep Efficiency (%) | 0.79 | 0.11 | 0.82 | 0.75 | 0.87 | 0.85 | 0.07 | 0.87 | 0.83 | 0.90 | 0.927 | 0.047 |
| Stage Change Rate (1/min) | 0.20 | 0.05 | 0.19 | 0.17 | 0.23 | 0.23 | 0.05 | 0.22 | 0.20 | 0.26 | 0.817 | 0.070 |
| Awakening Rate (1/min) | 0.04 | 0.02 | 0.05 | 0.03 | 0.05 | 0.05 | 0.01 | 0.05 | 0.04 | 0.06 | 0.445 | 0.269 |
Mean, standard deviation (SD), median (Median) values, and 1st quartile (Q1) and 3rd quartile (Q3) are reported.
Table 3.
Heart Rate (HR) and respiration in children with autism spectrum disorder (ASD) and typically developing children (TD) averaged over the first 4 sleep cycles and stages of Wake (W), non-REM (N1. N2, N3) and REM sleep.
| ASD | TD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stages | Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | p_age | p_diag |
| HR (bpm) | W | 84.6 | 9.8 | 85.3 | 77.6 | 89.6 | 85.0 | 9.9 | 85.8 | 78.0 | 90.2 | 0.000 | 0.449 |
| N1 | 70.9 | 5.9 | 68.9 | 66.2 | 76.7 | 74.9 | 5.6 | 74.6 | 69.5 | 80.6 | 0.280 | 0.345 | |
| N2 | 75.5 | 10.0 | 73.1 | 68.1 | 81.3 | 70.4 | 8.8 | 69.2 | 65.0 | 76.2 | 0.000 | 0.000 | |
| N3 | 78.8 | 9.6 | 77.2 | 72.6 | 84.1 | 72.5 | 9.1 | 70.1 | 66.8 | 77.4 | 0.000 | 0.000 | |
| REM | 79.9 | 9.1 | 77.9 | 74.2 | 85.5 | 75.7 | 9.4 | 74.2 | 68.4 | 83.2 | 0.000 | 0.001 | |
| Respiration (breath/min) | W | 20.2 | 3.6 | 20.7 | 17.8 | 22.8 | 20.6 | 3.1 | 19.9 | 19.0 | 21.7 | 0.494 | 0.762 |
| N1 | 17.0 | 3.4 | 17.3 | 14.1 | 19.6 | 20.6 | 2.0 | 20.3 | 18.8 | 22.9 | 0.837 | 0.247 | |
| N2 | 17.6 | 2.2 | 18.0 | 15.4 | 19.3 | 17.0 | 2.3 | 16.3 | 15.5 | 18.5 | 0.233 | 0.133 | |
| N3 | 18.6 | 2.5 | 18.3 | 16.8 | 20.4 | 18.0 | 2.5 | 17.8 | 16.3 | 19.6 | 0.166 | 0.192 | |
| REM | 18.1 | 2.8 | 18.3 | 15.8 | 20.0 | 17.9 | 2.8 | 17.2 | 15.7 | 20.1 | 0.930 | 0.638 | |
HR heart rate
Mean, standard deviation (SD), median (Median) values, and 1st quartile (Q1) and 3rd quartile (Q3) are reported.
Table 4.
Results of absolute heart rate variability in children with autism spectrum disorder (ASD) and typically developing children (TD) averaged over the first 4 sleep cycles and stages of Wake (W), non-REM (N1, N2, N3) and REM sleep.
| ASD | TD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stages | Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | p_age | p_diag |
| LF (msec2) | W | 1630.6 | 1848.2 | 1003.7 | 576.4 | 1890.1 | 1304.6 | 1089.3 | 851.6 | 404.0 | 2056.9 | 0.198 | 0.577 |
| N1 | 1740.8 | 1375.7 | 1780.2 | 448.8 | 3013.1 | 2240.2 | 2847.2 | 966.0 | 252.6 | 5502.0 | 0.028 | 0.348 | |
| N2 | 1080.9 | 1157.4 | 619.8 | 379.2 | 1575.9 | 1398.7 | 1319.2 | 1000.4 | 531.1 | 1810.9 | 0.660 | 0.126 | |
| N3 | 519.0 | 473.6 | 339.1 | 186.1 | 653.5 | 1030.2 | 1587.4 | 520.9 | 149.4 | 1287.2 | 0.590 | 0.029 | |
| REM | 1104.2 | 972.5 | 808.2 | 512.0 | 1573.4 | 1441.0 | 1105.7 | 1167.6 | 537.4 | 2189.5 | 0.005 | 0.042 | |
| HF (msec2) | W | 1347.9 | 1794.9 | 847.9 | 345.7 | 1239.8 | 1498.2 | 1687.5 | 877.6 | 359.3 | 2348.5 | 0.187 | 0.612 |
| N1 | 3343.0 | 2744.2 | 2974.7 | 1122.0 | 5748.2 | 2407.8 | 3388.8 | 794.8 | 126.9 | 6301.9 | 0.285 | 0.801 | |
| N2 | 2086.5 | 2631.8 | 1124.9 | 566.0 | 2186.6 | 2782.2 | 2730.1 | 1630.6 | 594.0 | 4497.9 | 0.642 | 0.123 | |
| N3 | 1382.2 | 1739.9 | 705.8 | 311.2 | 1527.3 | 2312.7 | 2601.5 | 1303.7 | 376.7 | 3607.4 | 0.362 | 0.031 | |
| REM | 1432.7 | 1985.1 | 703.4 | 304.2 | 1383.9 | 2427.7 | 2921.4 | 1432.7 | 518.5 | 2853.4 | 0.875 | 0.023 | |
| TP (msec2) | W | 4828.2 | 5163.0 | 3479.7 | 1182.8 | 4389.4 | 4476.7 | 4068.1 | 2824.5 | 1197.0 | 6884.4 | 0.144 | 0.959 |
| N1 | 8452.8 | 5418.0 | 9697.0 | 3205.5 | 13077.9 | 6598.9 | 7593.8 | 2380.3 | 2050.9 | 15365.4 | 0.010 | 0.858 | |
| N2 | 4252.1 | 4658.3 | 2504.7 | 1438.9 | 5691.3 | 5337.9 | 3965.4 | 4332.5 | 1861.9 | 8305.1 | 0.896 | 0.130 | |
| N3 | 2335.5 | 2429.4 | 1318.1 | 769.1 | 3041.2 | 3963.7 | 4278.5 | 2749.7 | 818.9 | 5607.6 | 0.432 | 0.017 | |
| REM | 3928.2 | 3352.9 | 2646.3 | 1644.9 | 5382.1 | 5473.0 | 4247.1 | 4207.7 | 2205.3 | 8609.1 | 0.152 | 0.017 | |
LF low frequency power, HF high frequency power, TP total power of heart rate variability
Mean, standard deviation (SD), median (Median) values, and 1st quartile (Q1) and 3rd quartile (Q3) are reported.
Table 5.
Results of normalized heart rate variability in children with autism spectrum disorder (ASD) and typically developing children (TD) averaged over the first 4 sleep cycles and stages of Wake (W), non-REM (N1, N2, N3) and REM sleep.
| ASD | TD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Stages | Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | p_age | p_diag |
| nLF (nu) | W | 57.8 | 11.1 | 57.6 | 47.9 | 66.9 | 53.6 | 15.5 | 55.9 | 45.4 | 64.1 | 0.182 | 0.174 |
| N1 | 36.5 | 21.8 | 37.4 | 17.2 | 55.4 | 56.1 | 10.0 | 55.2 | 46.6 | 66.6 | 0.556 | 0.230 | |
| N2 | 41.7 | 16.7 | 39.5 | 30.4 | 53.5 | 40.4 | 19.8 | 43.3 | 22.0 | 56.7 | 0.155 | 0.682 | |
| N3 | 36.6 | 15.5 | 35.7 | 25.2 | 44.5 | 34.8 | 17.2 | 33.9 | 22.3 | 49.2 | 0.321 | 0.597 | |
| REM | 53.7 | 18.5 | 57.3 | 39.3 | 68.7 | 47.3 | 18.8 | 47.4 | 31.4 | 61.4 | 0.429 | 0.048 | |
| nHF (nu) | W | 42.2 | 11.1 | 42.4 | 33.1 | 52.1 | 46.4 | 15.5 | 44.1 | 35.9 | 54.6 | 0.182 | 0.174 |
| N1 | 63.5 | 21.8 | 62.6 | 44.6 | 82.8 | 43.9 | 10.0 | 44.8 | 33.4 | 53.4 | 0.556 | 0.230 | |
| N2 | 58.3 | 16.7 | 60.5 | 46.5 | 69.6 | 59.6 | 19.8 | 56.7 | 43.3 | 78.0 | 0.155 | 0.682 | |
| N3 | 63.4 | 15.5 | 64.3 | 55.5 | 74.8 | 65.2 | 17.2 | 66.1 | 50.8 | 77.7 | 0.321 | 0.597 | |
| REM | 46.3 | 18.5 | 42.7 | 31.3 | 60.7 | 52.7 | 18.8 | 52.6 | 38.6 | 68.6 | 0.429 | 0.048 | |
| LF/HF | W | 1.6 | 0.8 | 1.4 | 0.9 | 2.0 | 1.4 | 0.9 | 1.3 | 0.8 | 1.8 | 0.193 | 0.437 |
| N1 | 0.8 | 0.8 | 0.6 | 0.2 | 1.5 | 1.4 | 0.6 | 1.2 | 0.9 | 2.0 | 0.597 | 0.355 | |
| N2 | 0.9 | 0.7 | 0.7 | 0.4 | 1.2 | 0.9 | 0.9 | 0.8 | 0.3 | 1.3 | 0.168 | 0.801 | |
| N3 | 0.7 | 0.7 | 0.6 | 0.3 | 0.8 | 0.7 | 0.5 | 0.5 | 0.3 | 1.0 | 0.265 | 0.608 | |
| REM | 1.6 | 1.3 | 1.3 | 0.6 | 2.2 | 1.2 | 1.0 | 0.9 | 0.5 | 1.7 | 0.990 | 0.041 | |
nLF normalized low frequency power, nHF normalized high frequency power, LF/HF ratio
Mean, standard deviation (SD), median (Median) values, and 1st quartile (Q1) and 3rd quartile (Q3) are reported.
In all children, the percentage of N3 decreased with age (p = 0.019). Other sleep parameters did not show an age effect (Table 2). The HR decreased significantly with age for WAKE (p = 0.000), N2 (p = 0.000), N3 (p = 0.000), and REM (p = 0.000) in both groups (Table 3). No significant age effects were found in HF or any normalized spectral parameters (Tables 4, 5). The LF in REM sleep decreased with age (p = 0.005) (Table 4).
Group differences
Primary outcome parameters
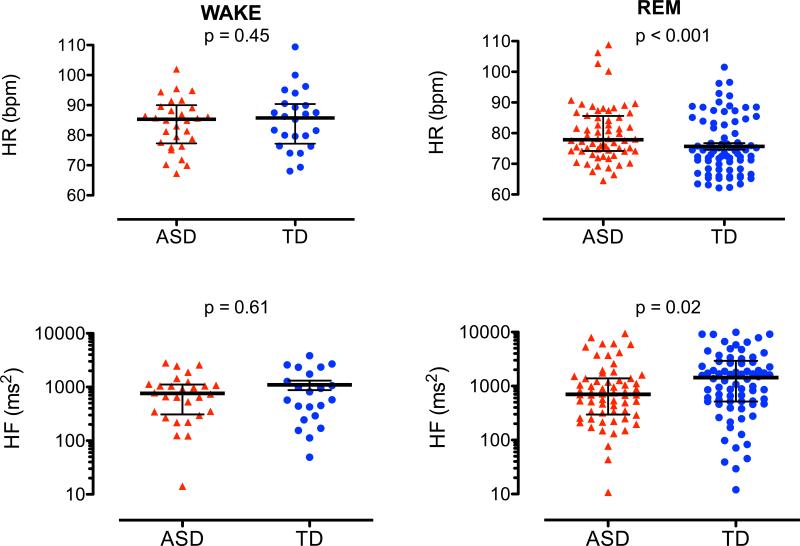
HR decreased with onset of sleep, with minimal levels during N2 sleep, and increased during REM sleep in both groups. Children with ASD had higher HR during N2 (p = 0.000), N3 (p = 0.000), and REM sleep (p = 0.001) as compared to TD children (Table 3; Fig. 2 left top panel, Fig. 3 top panels).
Fig. 3.
Individual heart rates (HR, top panels), and high frequency heart rate variability ((HF, bottom panels) during wake (W) and REM sleep in children with ASD (red) and TD (blue). Median and quartiles (black horizontal lines)
Absolute HF power increases from wake to N2 and decreases during N3 and REM. Children with ASD had significant lower values in N3 (p = 0.031) and REM (p = 0.023) as compared to TD (Table 4; Fig. 1, left bottom panel). ASD had lower total power during N3 and REM sleep (Table 4; Fig. 2 left bottom panel, Fig. 3 top panels).
Secondary parameters
During non-REM sleep, LF power reached minimal values at N3. LF power increased in REM sleep and reaches similar levels of WAKE in TD, but not in AD. No group differences in absolute LF power were found during WAKE and N2. Children with ASD had significant lower LF values in N3 (p = 0.029), and REM (p = 0.042) as compared with TD children. (Table 4; Fig. 2 left middle panel).
Normalized LF decreased during sleep with minimal values in N3, followed by increases during REM sleep. ASD had significant higher values in REM (p = 0.048, Table 2). Normalized HF values showed a reversed dynamic with significant lower values during REM in ASD (p = 0.048, Table 2). LF to HF ratio decreased during sleep with minimal vales during N3 and then increasing values during REM. ASD showed significant higher LF to HF ratios in REM (p = 0.041, Table 4; Fig. 2 right top panel).
Furthermore, we found differences in percentage of WAKE and sleep efficiency based on diagnosis (TD or ASD). Children with ASD had significantly higher percentage of wake (p = 0.026) and lower sleep efficiency (p = 0.047) (Table 2). The respiratory rates for wake, N1, N2, and N3, and REM were not different (for more details, see Table 3).
Discussion
Heart rate modulation during sleep
Our primary hypothesis, that children with ASD would have higher HR during sleep, was supported with higher values during both non-REM sleep and REM sleep. Our secondary hypothesis, that children with ASD would have lower vagal modulation during sleep, was also supported by lower absolute HF values in N3 and REM sleep. Furthermore, we found that children with ASD have lower absolute LF values in N3 and REM sleep with the LF to HF ratio higher in REM sleep. The HR decrease during N2 sleep was associated with increasing dominance of vagal modulation. It is unclear if the higher HR during REM sleep in ASD is a result of decreased vagal activation, sympathetic activation dominance or both. The LF/HF ratio (e.g., sympatho-vagal balance), followed the dynamic of HR with a decrease during non-REM sleep and return to initial wake levels during REM sleep. This dynamic could indicate higher sympathetic dominance during REM which has been described in sleep studies with direct recordings of sympathetic activity during sleep in healthy individuals [10].
In summary, the increase of HF and lower LF to HF ratio during non-REM supports the model of vagal dominance during non-REM sleep. The reduction of HF during REM sleep and increase in LF to HF ratio supports the assumption of sympathetic dominance and but due to vagal withdrawal. Our results are in agreement with several authors who demonstrated that NREM sleep is associated with vagal dominance reflected in reduced sympatho-vagal tone [19, 25–29]. Taken together with our findings of higher HR in ASD, it suggests that these children may have higher sympathethic dominance during sleep (e.g., LF to HF ratio) but due to decreased vagal influence.
Age dependency of autonomic modulation during sleep
Another important finding of our study is that HR decreases with age at the same level in ASD and in TD but was always higher in ASD. This age dependency of HR was found through all sleep stages. Our data are consistent with others who described this relation between the slowing of HR and the increase in body size associated with maturation [17]. Higher HR in ASD children could be explained with a delayed autonomic maturation with lack of vagal cardiac slowing (e.g., lower vagal modulation). This is in agreement with the polyvagal theory about reduced vagal influences on behavior in ASD [2, 3, 30]. Porges documented an age dependency for HR and decreased RSA which was not correlated to age [17]. Others found that HF of HRV increases with age in infants and children [31–33] supporting the assumption that ASD might have a delayed developmental trajectory.
Additionally, there are indications that the vagal component of HRV during sleep is reduced and that there is less vagal modulation to the heart during REM sleep. This has implications for the challenging daytime behaviors seen in ASD as REM sleep has restorative functions, such as information processing of memories between the hippocampus and neo cortex [34]. Conversely, sleep quality affects the performance and autonomic function the next day [35]. We found lower sleep efficiency and higher amount of wakefulness during the sleep period, which could affect the autonomic function during the next day.
HR can be affected by many factors including exercise, stress and anxiety, dehydration, and neurotransmitter abnormalities. The intrinsic HR can become important especially if the vagal modulation is attenuated, such as during REM sleep. It is less known that the intrinsic HR shows age dependency with decreases of intrinsic HR with age [36]. Abnormalities in adrenergic and cholinergic pathways may be responsible for different HR modulation. Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity has been found in ASD [37]. The majority of published studies showed higher concentrations in subjects with ASD as compared with controls [38].
Physical activity in healthy children during the day increases HR and reduces parasympathetic nervous activity the following night [39]. We did not control for physical activity in our population, which could affect our results. The response to anxiety is another possible factor that may have affected our results. Kushki et al. [5] found that children with ASD have elevated HR during both resting baseline and anxiety. They concluded that ASD may be associated with an atypical autonomic response to anxiety that is most consistent with sympathetic over-arousal and parasympathetic under-arousal, similar to our findings during REM sleep [5]. One of the strengths of our work is that by recording during sleep, we were able to minimize the confounding effects of daytime anxiety on autonomic parameters.
In summary, our findings suggest possible deficits in vagal influence to the heart during sleep, especially during REM sleep. Future work will be needed to clarify whether sympathetic activity is elevated or dominant. Our data also emphasize the importance of studying autonomic modulation in children during sleep in addition to wakefulness as differences may be revealed during sleep only. Differences in children in HR and HRV might be masked during wake but become more obvious during sleep. During the day, differences between ASD and TD may be difficult to detect as vagal and sympathetic tone is dominated by other factors such as visceral inputs or stress factors. Our data also point out the importance of accounting for age in physiological studies in ASD, and raise the possibility of a developmental delay seen in ASD may extend to the autonomic nervous system. Deriving simple parameters from sleep like heart rate and heart rate variability may be useful to monitor therapeutic progress in children with autism, particularly in those undergoing treatment with medications that mitigate hyperarousal. Our study is a pilot study and further research is needed to evaluate the clinical relevance in this area.
Supplementary Material
Acknowledgments
This study was funded by an administrative supplement to Grant NIH/NCRR1 UL1 RR024975-01 in part by CTSA award UL1TR000445. Additional support came from the National Alliance for Autism Research, a Vanderbilt University Interdisciplinary Discovery Grant and the Vanderbilt Kennedy Center for Research in Human Development. Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Health under Award Number NIH 2P01HL056693-19. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflict of interest Nothing to disclose.
References
- 1.Benevides TW, Lane SJ. A review of cardiac autonomic measures: considerations for examination of physiological response in children with autism spectrum disorder. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1971-z. doi:10.1007/s10803-013-1971-z. [DOI] [PubMed] [Google Scholar]
- 2.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 3.Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. doi:10.1016/S0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 4.Corbett BA, Mendoza S, Wegelin JA, et al. Variable cortisol circadian rhythms in children with autism and anticipatory stress. J Psychiatry Neurosci JPN. 2008;33:227–234. [PMC free article] [PubMed] [Google Scholar]
- 5.Kushki A, Drumm E, Pla Mobarak M, et al. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE. 2013;8:e59730. doi: 10.1371/journal.pone.0059730. doi:10.1371/journal.pone.0059730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazurek MO, Vasa RA, Kalb LG, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol. 2013;41:165–176. doi: 10.1007/s10802-012-9668-x. doi:10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr Clin North Am. 2011;58:685–698. doi: 10.1016/j.pcl.2011.03.009. doi:10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 9.Malik M. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 10.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 11.Iwase S, Mano T, Okada H, et al. Sleep-related changes in sympathetic outflow to muscle and skin in humans. J Auton Nerv Syst. 1993;43:88. doi: 10.1016/0165-1838(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 12.Okada H, Iwase S, Mano T, et al. Changes in muscle sympathetic nerve activity during sleep in humans. Neurology. 1991;41:1961. doi: 10.1212/wnl.41.12.1961. [DOI] [PubMed] [Google Scholar]
- 13.Althaus M, Mulder LJ, Mulder G, et al. Cardiac adaptivity to attention-demanding tasks in children with a pervasive developmental disorder not otherwise specified (PDD-NOS). Biol Psychiatry. 1999;46:799–809. doi: 10.1016/s0006-3223(98)00374-6. [DOI] [PubMed] [Google Scholar]
- 14.Ming X, Julu POO, Brimacombe M, et al. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. doi:10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Sheinkopf SJ, Neal-Beevers AR, Levine TP, et al. Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Res Treat. 2013;2013:1–7. doi: 10.1155/2013/868396. doi:10.1155/2013/868396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bal E, Harden E, Lamb D, et al. Emotion Recognition in Children with Autism Spectrum Disorders: relations to Eye Gaze and Autonomic State. J Autism Dev Disord. 2010;40:358–370. doi: 10.1007/s10803-009-0884-3. doi:10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- 17.Porges SW, Macellaio M, Stanfill SD, et al. Respiratory sinus arrhythmia and auditory processing in autism: modifiable deficits of an integrated social engagement system? Int J Psychophysiol. 2013;88:261–270. doi: 10.1016/j.ijpsycho.2012.11.009. doi:10.1016/j.ijpsycho.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willemsen-Swinkels SH, Buitelaar JK, Dekker M, van Engeland H. Subtyping stereotypic behavior in children: the association between stereotypic behavior, mood, and heart rate. J Autism Dev Disord. 1998;28:547–557. doi: 10.1023/a:1026008313284. [DOI] [PubMed] [Google Scholar]
- 19.Baharav A, Kotagal S, Gibbons V, et al. Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology. 1995;45:1183–1187. doi: 10.1212/wnl.45.6.1183. [DOI] [PubMed] [Google Scholar]
- 20.Goldman SE, Surdyka K, Cuevas R, et al. Defining the sleep phenotype in children with autism. Dev Neuropsychol. 2009;34:560–573. doi: 10.1080/87565640903133509. doi:10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malow BA, Marzec ML, McGrew SG, et al. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep N Y Then Westchest. 2006;29:1563. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med JCSM. 2012;8:597–619. doi: 10.5664/jcsm.2172. doi:10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C, Chesson ALJ, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st edn. American Academy of Sleep Medicine; Westchester: 2007. [Google Scholar]
- 24.Pan J, Tompkins WJ. A real-time QRS detection algorithm. Biomed Eng IEEE Trans On. 1985:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 25.Berlad I, Shlitner A, Ben-Haim S, Lavie P. Power spectrum analysis and heart rate variability in Stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance. J Sleep Res. 1993;2:88–90. doi: 10.1111/j.1365-2869.1993.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 26.Busek P, Vanková J, Opavskỳ J, et al. Spectral analysis of heart rate variability in sleep. Physiol Res. 2005;54:369–376. [PubMed] [Google Scholar]
- 27.Elsenbruch S, Harnish MJ, Orr WC. Heart rate variability during waking and sleep in healthy males and females. Sleep. 1999;22:1067–1071. doi: 10.1093/sleep/22.8.1067. [DOI] [PubMed] [Google Scholar]
- 28.Trinder J. Cardiac activity and sympathovagal balance during sleep. Sleep Med Clin. 2007;2:199–208. doi:10.1016/j.jsmc. 2007.04.001. [Google Scholar]
- 29.Vaughn BV, Quint SR, Messenheimer JA, Robertson KR. Heart period variability in sleep. Electroencephalogr Clin Neurophysiol. 1995;94:155–162. doi: 10.1016/0013-4694(94)00270-u. doi:10.1016/0013-4694(94)00270-U. [DOI] [PubMed] [Google Scholar]
- 30.Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: a polyvagal perspective. Infant Child Dev. 2011;20:106–118. doi: 10.1002/icd.688. doi:10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patriquin MA, Lorenzi J, Scarpa A, Bell MA. Developmental trajectories of respiratory sinus arrhythmia: associations with social responsiveness: trajectories of RSA. Dev Psychobiol. 2014;56:317–326. doi: 10.1002/dev.21100. doi:10.1002/dev.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. doi:10.1002/1098-2302(200007)37:1<44:AID-DEV6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Goto M, Nagashima M, Baba R, et al. Analysis of heart rate variability demonstrates effects of development on vagal modulation of heart rate in healthy children. J Pediatr. 1997;130:725–729. doi: 10.1016/s0022-3476(97)80013-3. [DOI] [PubMed] [Google Scholar]
- 34.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. doi:10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michels N, Clays E, De Buyzere M, et al. Children's sleep and autonomic function: low sleep quality has an impact on heart rate variability. Sleep. 2013 doi: 10.5665/sleep.3234. doi:10.5665/sleep.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus B, Gillette PC, Garson A., Jr Intrinsic heart rate inchildren and young adults: an index of sinus node function isolated from autonomic control. Am Heart J. 1990;119:911–916. doi: 10.1016/s0002-8703(05)80331-x. [DOI] [PubMed] [Google Scholar]
- 37.Lake CR, Ziegler MG, Murphy DL. Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Arch Gen Psychiatry. 1977;34:553–556. doi: 10.1001/archpsyc.1977.01770170063005. [DOI] [PubMed] [Google Scholar]
- 38.Lam KSL, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. doi:10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Iwasa Y, Nakayasu K, Nomura M, et al. The relationship between autonomic nervous activity and physical activity in children. Pediatr Int. 2005;47:361–371. doi: 10.1111/j.1442-200x.2005.02082.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.