Abstract
When orthodontic patients desire shorter treatment times with aesthetic results and long-term stability, it is important for the orthodontist to understand the potential limitations and problems that may arise during standard and/or technology-assisted accelerated treatment. Bone density plays an important role in facilitating orthodontic tooth movement (OTM), such that reductions in bone density can significantly increase movement velocity. Lifestyle, genetic background, environmental factors and disease status all can influence a patients’ overall health and bone density. In some individuals, these factors may create specific conditions that influence systemic-wide bone metabolism. Both genetic variation and the onset of a bone-related disease can influence systemic bone density and local bone density, such as is observed in the mandible and maxilla. These types of localized density changes can affect the rate of OTM and may also influence the risk of unwanted outcomes, i.e., the occurrence of dental external apical root resorption (EARR).
Keywords: orthodontics, tooth movement, bone density, genetics, root resorption, EARR, P2RX7, IL1B, OPG
Introduction
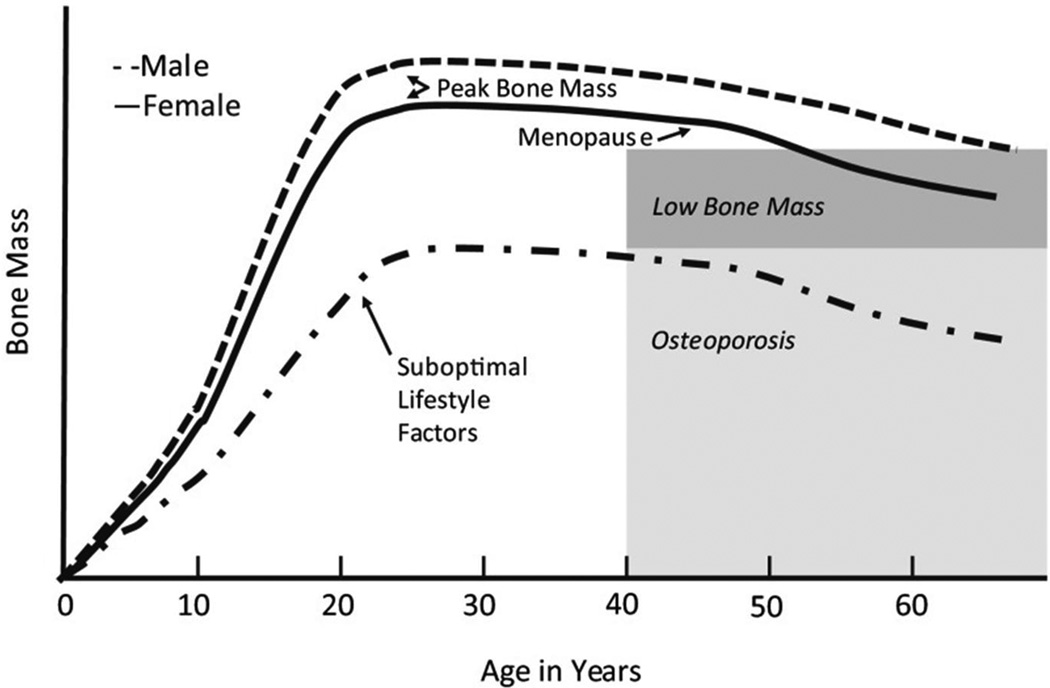
Bone Mass is a measure of the combined amount of bone matrix and mineral content within a segment of bone. Bone Mineral Density (BMD) is a clinical proxy for estimating bone mass that takes into account the concentration of calcium and other minerals, and estimates bone strength. An individuals’ BMD changes over a lifetime such that it peaks in or around the middle twenties to thirties and then declines, particularly in women who exhibit a dramatic decline with the onset of menopause. Calcium intake can aid in increasing and/or maintaining BMD, especially later in life, as can regular, weigh-bearing exercise. Environmental factors, such as diet, smoking, drinking status and types of bacteria found in our bodies (particularly the microbiome of the digestive track),[1, 2] can also alter BMD (Figure 1).
Figure 1.
Changes in bone mineral density (BMD) over a lifetime. (Used with permission from Weaver C, Gordon C, Janz K, Kalkwarf H, Lappe J, Lewis R et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. (Used with permission from Osteoporosis International. 2016;27(4):1281–386).[67]
Genetics, and epigenetics, also contribute to characteristics of our BMD. Various disease states have been shown to have profound effects on overall bone health. Measuring BMD is especially important in the diagnosis, monitoring, treatment and/or management of such bone-centered diseases as osteomalacia/rickets, osteopenia, osteoporosis and osteogenesis imperfecta. Chronic inflammatory diseases including obesity, diabetes, and inflammatory bowel disease (IBD) are known to have systemic effects that have the ability to affect bone quality and mass.
BMD measurements of the jaws and spine show similar rates of age-related deterioration.[3] With this in mind, measurement of jawbone density has been proposed as a good predictor of systemic-wide alteration in bone metabolism; such as with the onset of osteoporosis which could affect other anatomical regions that are distant to the jaws like the lumbar vertebrae. It can be inferred from this evidence that systemic variation or alterations of bone metabolism would directly affect maxillary and mandibular bone density, specifically of alveolar bone.[4]
Orthodontists use force to move teeth in a controlled fashion in order to facilitate the proper positioning of the teeth, and achieve a uniform distribution of forces during occlusion. Tooth movement, through the alveolar bone envelope triggered by orthodontic strain, is a phenomenon that depends directly on the coordinated activity of osteoblasts, osteocytes, and osteoclasts. External apical root resorption (EARR) is root resorption that can be seen on standard diagnostic radiographs caused by undesirable activity of osteoclastic cells on the root surface.[5] Although It can occur in the absence of orthodontic treatment, its incidence increases when concurrent with orthodontic treatment.[6]
Irrespective of whether or not EARR is facilitated by orthodontic mechanical factors, the process leading to EARR implicate specific molecular pathways that orchestrate non-physiological cellular activation for root demineralization and the creation of dental root resorption pits.[6] Differing alveolar bone densities and bone modeling/remodeling processes affect the strain on the dental root, thus influencing the orthodontic tooth movement (OTM) process and the increased occurrence of EARR as a deleterious secondary effect.
Natural Tooth Position and the Modeling and/or Remodeling of Bone Associated With OTM
Under normal circumstances, the positions of individual teeth in the oral cavity are determined by an equilibrium state created between the soft tissue forces being applied to the crowns of the teeth (i.e., from the tongue, lips, and/or buccal mucosal), and the occlusal forces generated on each tooth root encased within the alveolar bone. A familiar saying in orthodontics is that there is nothing more stable than a malocclusion. This adage infers that external forces must be placed on teeth; forces strong enough to alter the natural equilibrium and to promote movement of the teeth into a new position. OTM requires the purposeful application of force on teeth to correct a malocclusion. Modeling of the alveolar bone through the periodontal ligament (PDL) enables a tooth to change its position while maintaining periodontal support.
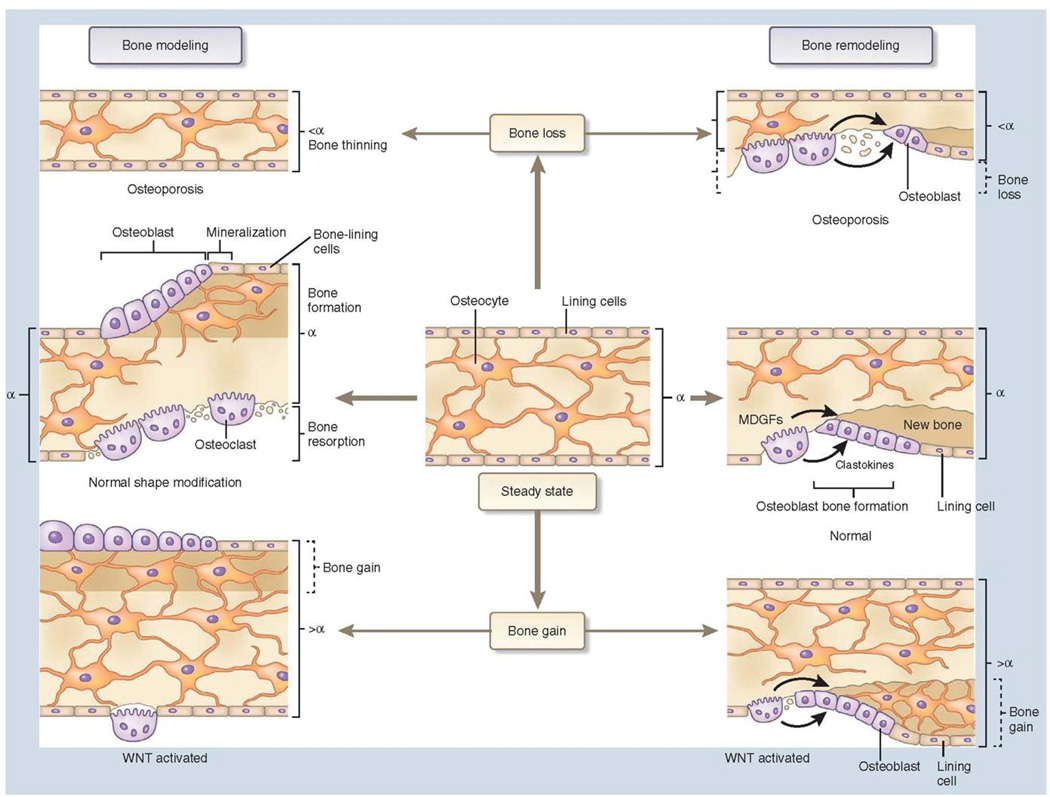
Bone modeling changes the shape of bone resulting in changes in bone morphology. The ability to change bone morphology is due to bone resorption and formation occurring in an uncoupled manner and on separate surfaces. In contrast, bone remodeling is the mechanism based on the coupled and balanced activities of bone resorption and formation along specific sites on the same bone surface that ensures turnover while maintaining bone mass and gross morphology. This allows for adaptation to both mechanical loading and the requirements of calcium and phosphate metabolism (Figure 2).[7, 8] While modeling along the periosteal surface is key for maintaining alveolar bone support during tooth movement,[9] both bone modeling and remodeling are involved in the orthodontic response (Figure 3).[8, 10]
Figure 2.
Comparison of bone modeling and remodeling. (Used with permission from Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. (Used with permission from Nature medicine. 2013;19(2):179–92).[7]
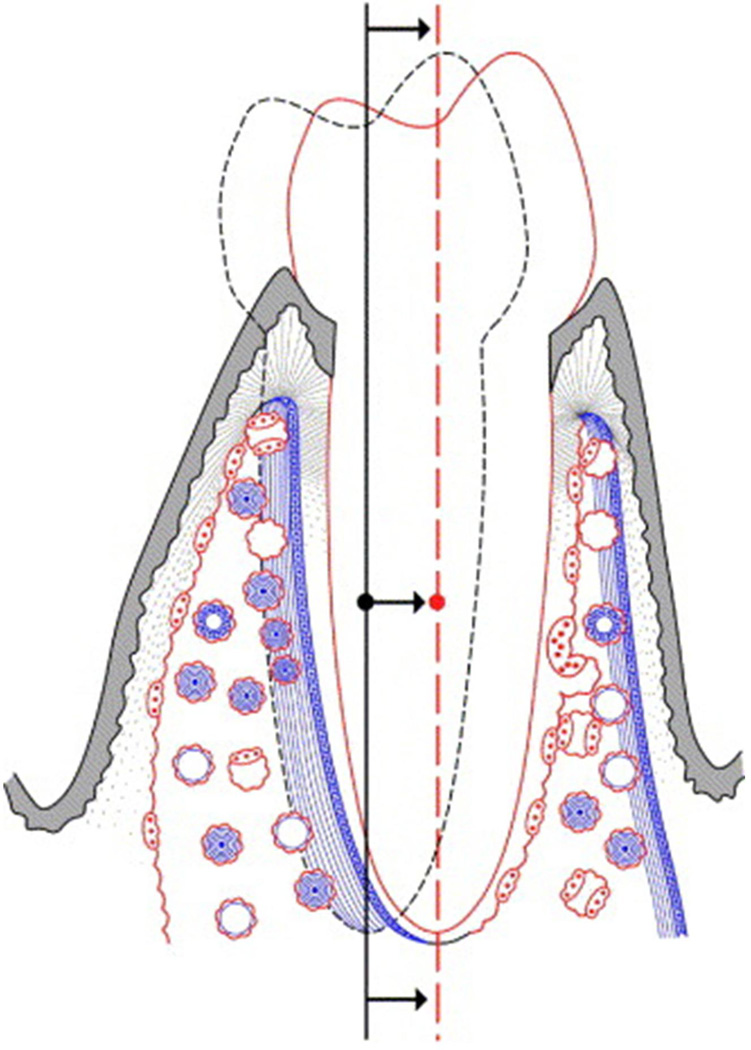
Figure 3.
A schematic diagram shows the bone physiology associated with translation of a tooth. Note that there is a coordinated bone modeling and remodeling response leading and trailing the moving tooth. This mechanism allows a tooth to move relative to basilar bone while maintaining a normal functional relationship with its periodontium. Osteoclastic and osteoblastic activity are in red and blue, respectively. (Used with permission from Roberts WE, Roberts JA, Epker BN, Burr DB, Hartsfield JK, editors. Remodeling of mineralized tissues, part I: the Frost legacy. Seminars in orthodontics; 2006: Elsevier).[8]
When the PDL is maximally compressed by orthodontic force, heavy functional loads are transferred directly to a relatively small area of the lamina dura. Thus, orthodontic force is a static load superimposed on function.[11] It is hypothesized that the dynamic (repetitive) loading of mastication results in accelerated fatigue damage in bone adjacent to the compressed necrotic PDL. This leads to undermining resorption removing the resisting bone, allowing the tooth to move. The unique biomechanical properties of the PDL allow it to generate a bone modeling response to even light loads.[12, 13] However, bone modeling of the alveolar process during sustained tooth movement appears to utilize the same atrophic and hypertrophic mechanisms for functional adaptation throughout the skeleton.
Root Strain as a Testable Mechanism of EARR in Animal Models
Animal studies on root resorption historically used histological sections of root resorption lacunae to measure the extent of root resorption. Human studies comparing control teeth that were to be extracted for orthodontic treatment to teeth that have some orthodontic forced placed on them prior to extraction for treatment reasons have also historically used histological sections. Micro CT or cone-beam CT are now also used to measure the resorption lacunae and changes in volume. These microscopic resorption lacunae are not seen on standard diagnostic radiographs. It has been presumed that an increase in resorption lacunae over a short period of time would indicate the start of a process that if continued would result in an extent of root loss that would be observable on standard radiographs, i.e., external apical root resorption (EARR).
In lactating calcium-deficient female Sprague-Dawley rats, a decrease in alveolar bone density resulted in more rapid orthodontic tooth movement of maxillary molars compared to non-lactating rats on a control diet. In addition to the observed increase in tooth movement velocity, there was decreased cratering of the outer cementum layer of the roots for the teeth being moved. This data suggested that a reduction in bone density effectively reduced the orthodontically-induced strain on the dental root, enabling the tooth to move faster with differential resorption of alveolar bone versus root structure.[14]
Stress analysis of orthodontically-stimulated rat molars has indicated that mechanically-induced bone resorption is due to fatigue failure in the bone itself.[15] This supports the hypothesis that root resorption might be related to a reduced rate of bone resorption at the PDL interface manifested as a prolonged inductive (lag) phase that is associated with compressed necrotic areas in the PDL.[16] Furthermore, root resorption under loading stress in the apical and cervical thirds was found to be more severe than in root regions that were not mechanically stressed. In this respect, the mean hardness and elastic modulus of premolars subjected to orthodontic loading were similar to those of untreated control premolars observed in other studies, decreasing from the cervical to the apical regions.[17] in contrast, the direction as well as the magnitude of stress in the PDL, root and alveolar bone may help define mechanotransduction during tooth movement. In silico models found an approximately 3:1:1 ratio between radial, circumferential and longitudinal stress magnitudes for tipping movement and 2:1:1 for translation movement.[18] These results suggest that direction plays a critical role in triggering a biological response for tooth movement to take place, as well as an iatrogenic response, like root resorption, in particular regions of the root specific to each type of movement.
A hypothetical scheme of the model that focused on the structure of the root judged that when an orthodontic force was applied, tensile and compressive longitudinal stresses would tend to simultaneously separate and crush the dentinal tubules, so affecting the underlying cementum layer and provoking microcracks; furthermore when the cementum layer was also defective, these simultaneous differential tensile and compressive effects might have an increased impact on root resorption, taking into account that osteoclastic cell activity is upregulated due to the stimulus to orthodontic tooth movement.[18] The single-tooth numeric simulation provided other explanations of mechanically induced root resorption, even though single-tooth stress levels were reported as being considerably less than in the multi-tooth system. [19] Numeric studies have helped explain how root resorption might be initiated by hydrostatic compressive stress-induced tissue necrosis.[19, 20]
Genetic Influence in EARR and its Biological Scenario
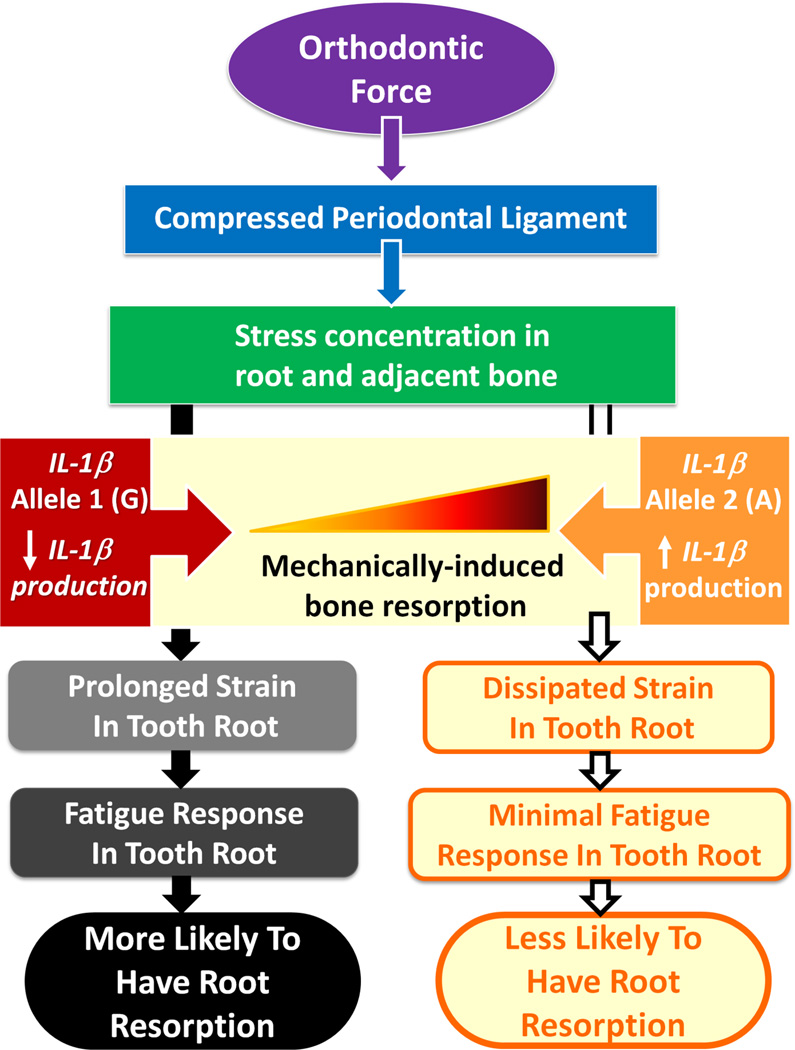
Incorporating the effect of pro-resorptive cytokines on alveolar bone modeling during OTM to the treatment factors, yields a more complex explanatory model. Interleukin-1β (IL-1β) is a potent stimulus for osteoclastic cell recruitment and bone resorption during OTM.[21, 22] It is proposed that a deficiency of IL-1β inhibits the alveolar bone resorptive response to orthodontic loads. Thus less bone resorption might result in prolonged stress concentrated on the root of the tooth, triggering a cascade of fatigue-related events leading to root resorption (Figure 4).[23, 14]
Figure 4.
Proposed model for pathway through which the IL-1B +3954 genotype (rs1143634) modulates the extent of root resorption experienced during orthodontic tooth movement. This model suggests that low IL-1 production in case of allele 1 (G) results in relatively less catabolic bone modeling in cortical bone interface of periodontal ligament (PDL) because of decreased number of osteoclasts associated with lower levels of this cytokine. Inhibition of bone resorption in direction of tooth movement results in maintaining prolonged dynamic loading of tooth root adjacent to compressed PDL, resulting in more root resorption because of fatigue failure of root. In case of high IL-1 production associated with allele 2 (A), compressed PDL space is restored by resorption of bone interface of PDL, resulting in only mild root resorption that is controlled by cementum-healing mechanism. This is one model for how these various factors might be implicated in clinical expression of root resorption. However, as EARR is a complex trait, it is not projected as the only model in its development. (Used with permission from Al-Qawasmi RA, Hartsfield JK, Everett ET, Flury L, Liu L, Foroud TM et al. Genetic predisposition to external apical root resorption. Am J Orthod Dentofacial Orthop. 2003;123(3):242–52).[16]
It is notable that the cluster of IL1 gene polymorphisms associated with EARR concurrent with orthodontic treatment has a positive correlation with IL-1β production rates in vitro. Specifically, allele 1 (G) of the IL1B polymorphism at +3954 (rs1143634) is related with relatively low production of IL-1β.[24, 25] Monocytes from subjects homozygous for the IL1B 3954 allele 2 (A) produce 4-fold more IL-1β, while heterozygous cells produce approximately 2-fold more IL-β, than cells from those homozygous for allele 1.[26, 25] Allele 2 (A) of IL1B +3954 has in some studies been associated with adult periodontitis; consistent with the observation that excessive IL-1β activates the degradation of extracellular matrix and bone in the periodontal tissues.[27, 28] As a potent stimulus of osteoclast recruitment and bone resorption, IL-1β,[21, 22] low IL-1β production in the case of allele 1 (G) might result in relatively less catabolic bone modeling (resorption) at the cortical bone interface with the PDL. Essentially, increased risk of root resorption associated with allele 1 (G) of IL-1B might be facilitated through impairment of alveolar resorption, resulting in prolonged stress and strain of the adjacent tooth root due to dynamic functional loads.
The pro-inflammatory function of IL-1β is antagonized by the interleukin 1 receptor antagonist (IL1Ra) protein encoded by the IL1RN gene. In this regard, two other genes of the IL1 gene cluster, IL1A and IL1RN, have been explored in populations of different origin. The polymorphisms analyzed (rs1800587 and rs419598) were found to account differently for the risk of EARR. In a study from Spain there was no statistical association with the IL1A gene, although subjects homozygous for the T-allele of the IL1RN gene were 6.7 times more likely to be affected by EARR.[29] Similarly, a significant association was observed in a sample from the Czech Republic between a variable number tandem repeat (VNTR) in the IL1RN gene and EARR. Interestingly there was a predisposition for EARR in females heterozygous or homozygous for the second allele.[30]
There is disagreement in the literature regarding the variation of IL1ra protein levels due to IL1RN gene polymorphisms.[31–33] The IL1RN +2018T>C polymorphism is in complete linkage disequilibrium with a penta-allelic 86 bp VNTR polymorphism in intron 2 of the gene, and is strongly linked to increased production of IL1Ra.[31] The IL1RN +2018T>C SNP showing a strong association with EARR during orthodontic strain could be consistent with an increase in IL1Ra protein levels, as previous in vitro and in vivo studies have found. Any cytokine level variation in the IL1Ra/IL-1β axis might have influence not only on bone modeling/remodeling, but also on the subsequent increase in radicular stress during orthodontic tooth movement. Again, these results point to the potentially central role of this cluster of genes in the EARR process, and their effect on the process of alveolar bone resorption and density during orthodontic tooth movement.
In the same vein, recent studies using different experimental approaches and study designs have provided further data about variations in candidate genes—usually single nucleotide polymorphisms (SNPs)—that influence the process of EARR development secondary to orthodontic forces.[16, 34, 29] An age and sex-matched case-control study comparing orthodontic treatment factors and genotyping results for 134 unrelated Caucasian subjects described a marginally significant association with EARR in the purinergic receptor P2X, ligand-gated ion channel 7 (P2RX7) gene when no other cofactors were included, specifically, between individual carriers of two T alleles in P2RX7 at SNP rs208294 [CC&CT vs. TT], while other P2RX7 variants (rs1718119 and rs2230912) were not be associated with EARR.[35] This conflicts with results reported by Pereira et al. in a sample of Portuguese origin, who found a statistically significant association between subjects with the P2RX7 gene (GG genotype) rs1718119 variant and an increased risk of being affected by EARR concurrent with orthodontia.[36]
The P2RX7 receptor is commonly found on the surface of macrophages involved in the response to orthodontic loading. Specifically, necrotic tissue deriving from apoptotic cells leads to the release of ATP from the damaged cells and it’s binding to the P2×7 receptor. Activation of this receptor leads in turn to autocrine and paracrine cell stimulation with overexpression of IL-1 cytokines and other inflammatory-related molecules. The released molecules all function as a chemotactic stimulus of the phagocytic cellular system responsible for eliminating necrotic tissue, so allowing tooth movement to take place. This, in turn, is directly associated with the alveolar bone remodeling process after applying orthodontic strain.
Apart from the IL1 gene cluster and associated pathways, other potentially predisposing genetic variants have been analyzed. The regions coding for TNFRSF11B (OPG) and TNFRSF11A (RANK) rs3102735, rs1805034, respectively, for example, were not associated with EARR in orthodontics in that study.[36] Nevertheless, other authors genotyped single nucleotide polymorphism rs2073618 in the TNFRSF11B (OPG) gene in orthodontically treated patients and concluded that subjects carrying the C allele at this position on the TNFRSF11B gene were 1.9 times more likely to be affected by EARR than those not carrying the C allele (p = 0.02).[37] Furthermore, subjects homozygous for the C allele, that is, carriers of CC alleles, were found to be 2.8 times more likely to be affected than those with GG or GC genotypes. The hypothesis about the influence of the OPG/RANK/RANKL pathway on the EARR process is also consistent with findings from knockout mouse models, in which a dramatic increase in EARR concurrent with orthodontic forces was observed when there was an imbalance in OPG/RANKL, suggesting that the OPG gene, or perhaps certain variations in its regulation/function, may be influencing EARR in some way.
In this connection, under permissive levels of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), the transformation of odontoblasts or progenitors into resorbing odontoclasts was described in cells derived from dental papilla that are mainly found in the apical area, which is known to be a target for EARR. The authors suggested that RANKL is directly involved in inducing odontoblast-like cells to differentiate into odontoclast-like cells or to function as odontoclasts. [38] Other predisposing genetic markers have been found in the vitamin D receptor gene. [39] These variants have been associated with homeostatic imbalances of bone that could affect the pathological process of root resorption. This variation has been associated with affording some protection (for carriers of the C allele) against EARR during orthodontic treatment.
Finally, a hypothetical alternative or simultaneous regulatory pathway of EARR was recently explored and studied, focusing on the possible role of two specific epigenetic regulators in EARR during orthodontic treatment.[40] The main role of histone deacetylases (HDACs) is to regulate transcription by removing acetyl groups from lysine residues in histone tails.[41] A recent meta-analysis found that specific HDAC gene variants are associated with bone remodeling disorders.[4] In this study, carriers of two copies of the least frequent allele of the rs228769 SNP in the HDAC5 gene were significantly predisposed to develop EARR, compared to other genotypes.
To explain these findings, it should be borne in mind that variants in the intronic region of HDAC5 were previously found to be highly associated with alterations in the bone mineral remodeling process, where HDAC5 is needed for TGF-β-mediated osteoblast differentiation, which occurs when Smad3 inhibits the function of Runx2.[42] More closely connected to dental development, however, altered pulp cell differentiation was observed with accelerated mineralization when two HDAC inhibitors were administered.[43] More importantly, recent evidence, which surprisingly contrasts with these results, has contributed the insights that HDAC5 negatively regulates sclerostin levels secreted by osteocytes in vitro and in vivo, and that sclerostin is an inhibitor of bone formation by osteoblasts.[44] Therefore, HDAC5 itself may indirectly be an enhancer of alveolar bone mineral density.
A suggested explanatory hypothesis would be that the HDAC5 genetic variant genotyped in this study induced a post-translational epigenetic modification in the sclerostin/MEF-2 pathway, and hence a different dental root-alveolar bone scenario.[45] The osteocyte, regarded as the key regulatory cell in orthodontic tooth movement, would have a different gene expression pattern in orthodontic tooth movement induced in subjects homozygous for the rare allele G that would lead to the normal bone modeling/remodeling process being impaired. Meanwhile, odontoblast-like cells may respond in a contrary way, with reduced reparative capabilities on dentin and root surfaces. This may predispose to increased EARR secondary to orthodontic forces.
A study in a Chinese Han sample found that while sex, and the amount of root movement were not risk factors for EARR, variation in IL-6 rs1800796 is a risk factor for EARR.[46] This last study is a good example of how the relative frequency of polymorphic alleles for a specific SNP among different ethnic groups may affect the outcome of studies and hamper their comparison. In summary support for treatment related risk factors has varied amongst studies, with a longer of treatment being associated with increased likelihood of EARR being the most consistent.
It is likely that the genetic factors that influence EARR are heterogeneous, with different mechanisms in affected persons, or even site-specific responses in the same person (Table 1).[47] It has been put forth that incorporating this type of single gene variant into clinical practice is of benefit, although as would be expected any single genetic factor that is associated with what is in most patients a complex trait, the benefit is questionable.[48–50]
Table.
Single Nucleotide Polymorphisms Studied in Conjunction with EARR.
| Single Nucleotide Polymorphisms Studied in Conjunction with EARR | ||||||
|---|---|---|---|---|---|---|
| Chr | Gene | Marker | Type of Marker | Population tested | Results | Refs |
| 2q14 .1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
35 White American families from Indiana, USA Families having at least 2 siblings who had received full-banded comprehensive orthodontic treatment; 2.82 years (±1.09 stdev) average length of treatment |
No statistical association | [16] |
| 2q14.1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
45 sporadic EARR patients including two sibling pairs with EARR 44 controls All subjects from Aachen, Germany |
The rs1800587 2/2 [T/T] genotype was associated with EARR compared to the control group (p < 0.032) |
[68] |
| 2q14 .1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
54 Caucasian orthodontic patients from Seville, Spain 25 individuals with EARR > 2 mm 29 Controls |
No statistical association p= 0.09 |
[29] |
| 2q14.1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
73 root filled teeth (35 with EARR > 2mm and 38 non-EARR teeth) 73 vital control teeth (30 with EARR > 2 mm and 43 non-EARR teeth) All patients were from Seville, Spain and had undergone root canal treatment in a maxillary premolar but not in the contra-lateral tooth and had received comprehensive orthodontic treatment (straight-wire technique) at least 1 year later. |
No statistical association of EARR with non-vital endodontically-treated teeth |
[69] |
| 2q14.1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
93 orthodontic patients from Seville, Spain 39 individuals with >2 mm of EARR on root-filled teeth after orthodontic treatment 54 Controls |
No statistical association of EARR with non-vital endodontically-treated teeth |
[70] |
| 2q14 .1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
32 individuals with EARR of maxillary incisors (age 15.0 ± 4.1 years) 74 controls (age 15.2 ± 5.3 years) All individuals were white from Brno, Czech Republic; treated with fixed appliances |
No statistical association | [30] |
| 2q14 .1 |
Interleukin-1α (IL-1α; OMIM #147760) |
rs1800587 (−889 C/T) |
Promoter variation |
67 biologically unrelated Caucasians with moderate to severe EARR of maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953; C/T rev) *Allele 2 (T) associated with more IL-1 production (See a, b, c) |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
35 White American families in Indiana, USA Families having at least 2 siblings who had received full-banded comprehensive orthodontic treatment 2.82 years (±1.09 stdev) average length of treatment |
rs1143634 was linked to the EARR phenotype (p= 0.0003) Allele-1/Allele-1 (C/C) homozygous individuals showed a 5.6 fold increase risk of EARR > 2mm (95% CI 1.9–21.2) compared to C/T and T/T individuals |
[16] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
61 orthodontic patients from San Paulo, Brazil 23 affected individuals 38 control individuals EARR in the central and lateral maxillary incisors in the post-treatment period |
Allele 1 (C) predisposed the subjects to EARR (OR = 4.0) |
[34] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
45 sporadic EARR patients including two sibling pairs with EARR 49 controls All subjects from Aachen, Germany |
No statistical association Allele-1 (C) occurred slightly less often in the EARR affected individuals compared to controls |
[68] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
54 Caucasian orthodontic patients from Seville, Spain 25 individuals with EARR > 2 mm 29 Controls |
Allele-1/Allele-1 (CC) homozygous individuals had an increased risk of post- orthodontic EARR (OR 3.48; p=0.027; 95%CI=1.12–10.72) |
[29] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
73 root filled teeth (35 with EARR > 2mm and 38 non-EARR teeth) 73 vital control teeth (30 with EARR > 2 mm and 43 non-EARR teeth) All patients were from Seville, Spain and had undergone root canal treatment in a maxillary premolar but not in the contra-lateral tooth and had received comprehensive orthodontic treatment (straight-wire technique) at least 1 year later. |
Homozygous subjects 2/2 [TT] had 2X the risk of post- orthodontic EARR in non-vital, endodontically-treated teeth [OR, 2.032 (p= 0.031); 95%CI=1.99–14.77] when compared to control teeth with vital pulp. There was an increased predisposition of EARR for control teeth with vital pulp and control root-filled teeth when the subject is homozygous for Allele-1 (C) [OR, 5.05 (p= 0.002)] and [OR, 2.77 (p= 0.037)], respectively. |
[69] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
93 orthodontic patients from Seville, Spain 39 individuals with >2 mm of EARR on root-filled teeth after orthodontic treatment 54 Controls |
Homozygous subjects (2/2[TT]) and (1/1[CC]), had an increased risk of post- orthodontic EARR in non-vital, endondontically-treated teeth (odds ratio = 11.59; p=0.006; 95%CI= 1.36–98.64) and (odds ratio = 2.54; p=0.035; 95%CI=1.05–6.12), respectively |
[70] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
32 individuals with EARR of maxillary incisors (age 15.0 ± 4.1 years) 74 controls (age 15.2 ± 5.3 years) All individuals were white from Brno, Czech Republic; treated with fixed appliances |
No statistical association | [30] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
195 total patients from Coimbra, Portugal (72 males and 123 female patients) 17.24 + 6.8 years old (average age + stdev); Average treatment time was 36 ± 10 months Portuguese Caucasian origin with completed comprehensive orthodontic treatment (straight-wire technique); Six teeth were assessed: the four maxillary incisors and the two maxillary canines |
No statistical association | [36] [51] |
| 2q14 .1 |
Interleukin -1β (IL-1β; OMIM #147720) |
rs1143634 (+3954/ +3953 C/T) *See note above |
Synonymous variant in Exon 5 TTC→TTT Phe105Phe |
67 biologically unrelated Caucasians with moderate to severe EARR of maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 2q14.1–2 |
Interleukin-1 Receptor Antagonist (IL-1RN; OMIM #147679) Aliases: IL-1ra, IL-1RA |
rs315952 (+390 T/C) |
Synonymous variant in Exon 4 AGT→AGC Ser130Ser |
195 total patients from Coimbra, Portugal (72 males and 123 female patients) 17.24 + 6.8 years old (average age + stdev); Average treatment time was 36 ± 10 months Portuguese Caucasian origin with completed comprehensive orthodontic treatment (straight-wire technique); Six teeth were assessed: the four maxillary incisors and the two maxillary canines |
No statistical association | [51] |
| 2q14.1–2 |
Interleukin-1 Receptor Antagonist (IL-1RN; OMIM #147679) Aliases: IL-1ra, IL-1RA |
rs419598 (C/T fwd) |
Synonymous variant GCT→GCC Ala→Ala |
54 Caucasian orthodontic patients from Seville, Spain 25 individuals with EARR > 2 mm 29 Controls |
Homozygous [1/1(TT)] more likely to be affected with EARR (OR: 6.75; p= 0.001; 95%CI=2.04–22.27). |
[29] |
| 2q14.1–2 |
Interleukin-1 Receptor Antagonist (IL-1RN; OMIM #147679) Aliases: IL-1ra, IL-1RA |
rs419598 (C/T fwd) |
Synonymous variant GCT→GCC Ala→Ala |
93 orthodontic patients from Seville, Spain 39 individuals with >2mm EARR following orthodontic treatment on root-filled teeth 54 individuals with no EARR or EARR ≤ 2mm following orthodontic treatment on root-filled teeth |
Subjects homozygous [1/1(TT)] for rs419598 were at increased risk of EARR in root-filled teeth (OR:10.85; p=0.001;CI:95%=3.97 – 29.6) |
[71] |
| 2q14.1–2 |
Interleukin-1 Receptor Antagonist (IL-1RN; OMIM #147679) Aliases: IL-1ra, IL-1RA |
rs419598 (C/T fwd) |
Synonymous variant GCT→GCC Ala→Ala |
Case-Control Study; 67 biologically unrelated Caucasians with moderate to severe EARR of maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 2q14.1–2 |
Interleukin-1 Receptor Antagonist (IL-1RN; OMIM #147679) Aliases: IL-1ra, IL-1RA |
86-bp Variable Number Tandem Repeat (VNTR) in 2nd intron |
Short allele 2 repeats (240bp) Long alleles 3 (326 bp), 4 (412bp), 5 (498bp), and 6 (584bp) repeats |
32 individuals with EARR of maxillary incisors (age 15.0 ± 4.1 years) 74 controls (age 15.2 ± 5.3 years) All individuals were white from Brno, Czech Republic; treated with fixed appliances |
IL1RN*12, *22 genotypes and the short (*2) allele were significantly associated with EARR in the subgroup of girls (p= 0.04 and p= 0.02, p= 0.02, respectively) |
[30] |
| 2q14.1–2 |
Interleukin-1 Receptor Antagonist (IL-1RN; OMIM #147679) Aliases: IL-1ra, IL-1RA |
rs419598 (C/T fwd) |
Synonymous variant GCT→GCC Ala→Ala |
174 orthodontic patients within a Chinese Han population Left maxillary central incisors monitored for EARR in 3D during straight wire orthodontic treatment; Age range 12–34 years; average 20.55 ± 6.54 months in treatment |
No statistical association | [46] |
| Xq28 |
Interleukin-1 receptor- activated protein kinase (IRAK1; OMIM #300283) |
rs1059703 (+6434; C/T rev) |
Non- synonymous Variant in Exon 12 TCG→TTG Ser532Leu |
195 total patients from Coimbra, Portugal (72 males and 123 female patients) 17.24 + 6.8 years old (average age + stdev); Average treatment time was 36 ± 10 months Portuguese Caucasian origin with completed comprehensive orthodontic treatment (straight-wire technique); Six teeth were assessed: the four maxillary incisors and the two maxillary canines |
Homozygosity and hemizygosity for the C-allele was protective for EARR (p = 0.018) |
[51] |
| 7p15.3 |
Interleukin-6 (IL-6; OMIM #147620) |
rs1800796 (−634 C/G) |
Promoter variation |
174 orthodontic patients within a Chinese Han population Left maxillary central incisors monitored for EARR in 3D during straight wire orthodontic treatment; Age range 12–34 years; average 20.55 ± 6.54 months in treatment |
GC individuals had an increased risk of EARR |
[46] |
| 6p12.2 |
Interleukin-17A (IL-17A; OMIM # 603149) |
rs2275913 (−197 G/A) |
Promoter variation |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys: 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No statistical association | [72] |
| 11q22.3 |
Caspase-1 (CASP1; OMIM #147678); Alias Interleukin-1 Beta Converting Enzyme (ICE) |
rs530537 (A/G rev) |
Intronic variant |
Case-Control Study; 67 biologically unrelated Caucasians with moderate to severe EARR of maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 11q22.3 |
Caspase-1 (CASP1; OMIM #147678); Alias Interleukin-1 Beta Converting Enzyme (ICE) |
rs580253 (C/T rev) |
Synonymous variant CTA→TTA Leu→Leu |
67 biologically unrelated Caucasians with moderate to severe EARR of maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 11q22.3 |
Caspase-1 (CASP1; OMIM #147678); Alias Interleukin-1 Beta Converting Enzyme (ICE) |
rs554344 (C/G fwd) |
Near 3’ end of the gene |
67 biologically unrelated Caucasians with moderate to severe EARR of maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 12q24.31 |
Purinergic- receptor-P2X, ligand-gated ion channel 7 (P2RX7; OMIM #602566) |
rs208294 (+489 C/T) |
Non- Synonymous Variant CAT→TAT His155Tyr Gain-of-function |
67 biologically unrelated Caucasians with moderate to severe EARR of the maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
Individuals with CC&CT were at higher risk for EARR than individuals with TT (p=0.002) |
[35] |
| 12q24.31 |
Purinergic- receptor-P2X, ligand-gated ion channel 7 (P2RX7; OMIM #602566) |
rs208294 (+489 C/T) |
Non- Synonymous Variant CAT→TAT His155Tyr Gain-of-function |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys: 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No association with individual SNP Association seen with rs208294 + rs1718119 haplotype and EARR |
[72] |
| 12q24.31 |
Purinergic- receptor-P2X, ligand-gated ion channel 7 (P2RX7; OMIM #602566) |
rs1718119 (+1068 G/A fwd) |
Non- Synonymous Variant in Exon 11 GCT→ACT Ala348Thr Gain-of-function |
195 total patients from Coimbra, Portugal (72 males and 123 female patients) 17.24 + 6.8 years old (average age + stdev); Average treatment time was 36 ± 10 months Portuguese Caucasian origin with completed comprehensive orthodontic treatment (straight-wire technique); Six teeth were assessed: the four maxillary incisors and the two maxillary canines |
The GG genotype for rs1718119 was significantly associated with EARR (p<0.01) |
[36] |
| 12q24.31 |
Purinergic- receptor-P2X, ligand-gated ion channel 7 (P2RX7; OMIM #602566) |
rs1718119 (+1068 G/A fwd) |
Non- Synonymous Variant in Exon 11 GCT→ACT Ala348Thr Gain-of-function |
67 biologically unrelated Caucasians with moderate to severe EARR of the maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 12q24.31 |
Purinergic- receptor-P2X, ligand-gated ion channel 7 (P2RX7; OMIM #602566) |
rs1718119 (+1068 G/A fwd) |
Non- Synonymous Variant in Exon 11 GCT→ACT Ala348Thr Gain-of-function |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys: 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No association with individual SNP Association seen with rs208294 + rs1718119 haplotype and EARR |
[72] |
| 12q24.31 |
Purinergic- receptor-P2X, ligand-gated ion channel 7 (P2RX7; OMIM #602566) |
rs2230912 (A/G) |
Non- Synonymous Variant in Exon 13 CAG→CGG Gln460Arg |
67 biologically unrelated Caucasians with moderate to severe EARR of the maxillary incisors; 38 females and 29 males 67 age and sex-matched biologically unrelated Caucasian controls All subjects from Northern Indiana, USA received standard orthodontic treatment |
No statistical association | [35] |
| 18q21.33 |
Tumor Necrosis Factor Receptor Superfamily Member 11a (TNFRSF11A; OMIM #603499) Alias: Receptor Activator of NF-KB (RANK) |
D18S64 D18S64 is tightly linked to TNFRSF11A |
Microsatellite |
38 American Caucasian families with a total of 79 siblings who completed comprehensive orthodontic treatment. Evaluated EARR in the maxillary central incisors, the mandibular central incisors, and the mesial and distal roots of the mandibular first molars |
Linkage of maxillary central incisor EARR to microsatellite marker D18S64 (LOD = 2.5; p = 0.02) |
[73] |
| 18q21.33 |
Tumor Necrosis Factor Receptor Superfamily Member 11a (TNFRSF11A; OMIM #603499) Alias: Receptor Activator of NF-KB (RANK) |
rs1805034 (C/T fwd) |
Non- synonymous variant in Exon 6 GCG→GTG Val192Ala |
195 total patients from Coimbra, Portugal (72 males and 123 female patients) 17.24 + 6.8 years old (average age + stdev); Average treatment time was 36 ± 10 months Portuguese Caucasian origin with completed comprehensive orthodontic treatment (straight-wire technique); Six teeth were assessed: the four maxillary incisors and the two maxillary canines |
No statistical association | [36] |
| 8q24.12 |
Tumor Necrosis Factor Receptor Superfamily Member 11b (TNFRSF11B; OMIM #602643) Alias: (OPG) Osteoprotegerin |
rs2073618 (+1181 C/G) |
Non- Synonymous Variant AAC→AAG Asn3Lys |
135 Caucasian subjects were studied from Indiana, USA; EARR >2 mm in at least one maxillary central incisor Mean age of the patients pre-treatment was 14.6 ±6.9 years (stdev); average treatment time was 1.6 ± 0.5 years (stdev) |
G allele was associated with EARR (p= 0.003) |
[6] |
| 8q24.12 |
Tumor Necrosis Factor Receptor Superfamily Member 11b (TNFRSF11B; OMIM #602643) Alias: (OPG) Osteoprotegerin |
rs3102735 (−163 A/G rev strand) |
Promoter variation |
195 total patients from Coimbra, Portugal (72 males and 123 female patients) 17.24 + 6.8 years old (average age + stdev); Average treatment time was 36 ± 10 months Portuguese Caucasian origin with completed comprehensive orthodontic treatment (straight-wire technique); Six teeth were assessed: the four maxillary incisors and the two maxillary canines |
No statistical association | [36] |
| 8q24.12 |
Tumor Necrosis Factor Receptor Superfamily Member 11b (TNFRSF11B; OMIM #602643) Alias: (OPG) Osteoprotegerin |
rs3102735 (−163 C/T fwd strand) |
Promoter variation |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys: 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No statistical association | [72] |
| 8q24.12 |
Tumor Necrosis Factor Receptor Superfamily Member 11b (TNFRSF11B; OMIM #602643) Alias: (OPG) Osteoprotegerin |
rs2073618 (+1181 C/G) |
Non- Synonymous Variant AAC→AAG Asn3Lys |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys : 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No statistical association | [72] |
| 6p21.33 |
Tumor Necrosis Factor alpha (TNFα; OMIM #191160) |
rs1800629 (−308 G/A) |
Promoter variation |
38 American Caucasian families with a total of 79 siblings who completed comprehensive orthodontic treatment. Evaluated EARR in the maxillary central incisors, the mandibular central incisors, and the mesial and distal roots of the mandibular first molars |
No linkage identified | [73] |
| 1p36.12 |
Tissue- NonSpecific ALkaline Phosphatase (TNSALP; OMIM #171760) Alias: Alkaline phosphatase, tissue- nonspecific isozyme (ALPL) |
AL215L |
38 American Caucasian families with a total of 79 siblings who completed comprehensive orthodontic treatment Evaluated EARR in the maxillary central incisors, the mandibular central incisors, and the mesial and distal roots of the mandibular first molars |
No linkage identified | [73] | |
| 4q22.1 |
Osteopontin (OPN) Aliases: bone sialoprotein I (BSP-1/BNSP), secreted phosphoprotein 1 (SPP1, OMIM #166490) |
rs11730582 (−443T/C) |
5'near gene |
87 Caucasian orthodontic patients from Seville, Spain 37 with EARR (>2 mm) 50 Controls Mean length of orthodontic treatment 27.5 ± 8.3 months (stdev); EARR measured at the completion of orthodontic treatment |
Allele-2/Allele-2 [CC] increased risk of EARR (OR:11.68; p<0.039; 95%CI = 1.12–121.06) compared with other genotypes; Allele-1 [A] is protective for EARR (OR: 0.20; p=0.025; 95%CI= 0.05–0.81) |
[74] |
| 4q22.1 |
Osteopontin (OPN) Aliases: bone sialoprotein I (BSP-1/BNSP), secreted phosphoprotein 1 (SPP1, OMIM #166490) |
rs11730582 (–443T/C) |
5′near gene |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys : 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No statistical association | [72] |
| 4q22.1 |
Osteopontin (OPN) Aliases: bone sialoprotein I (BSP-1/BNSP), secreted phosphoprotein 1 (SPP1, OMIM #166490) |
rs9138 (+1239A/C) |
3′ UTR |
87 Caucasian orthodontic patients from Seville, Spain 37 with EARR (>2 mm) 50 Controls Mean length of orthodontic treatment 27.5 ± 8.3 months (stdev); EARR measured at the completion of orthodontic treatment |
Allele-2/Allele-2 [CC] at increased risk of post- orthodontic EARR (OR: 4.10; p= 0.045; 95%CI = 1.03–16.35) Allele-1 [T] is protective for EARR (OR: 0.035; p=0.035’ 95%CI= 0.062–0.90) |
[74] |
| 4q22.1 |
Osteopontin (OPN) also known as bone sialoprotein I (BSP-1 or BNSP), secreted phosphoprotein 1 (SPP1, OMIM #166490) |
rs9138 (+1239 A/C) |
3′ UTR |
Case-control retrospective study 99 Caucasian children of Czech origin 15.0 ± 4.7 (mean age ± standard deviation) 30 individuals with EARR of maxillary incisors(11 boys : 19 girls) 69 unrelated controls (26 boys: 43 girls) |
No statistical association | [72] |
| 12q13.11 |
Vitamin D Receptor (VDR; OMIM #601769) |
rs731236 (C/T; also termed TaqI) |
Synonymous variant in Exon 1 AAT→ ATC Ile→Ile |
377 Brazilian Orthodontic Patients Diagnosed with Class II Div 1 160 individuals with EARR ≤ 1.43 179 individuals with EARR > 1.43 38 untreated individuals Mean age, 14.9 years (range, 8–21 years); EARR measured after the first 6 months of treatment |
CC+CT versus TT C allele was protective for EARR (p= 0.091; OR=0.29; 95%CI = 0.07–1.23) |
[39] |
References related to rs1143634 influencing IL-1B production: (a) Pociot F, Molvig J, Wogensen LW, Orsaae H, Nerup J. A Taq I polymorphism in the human interleukin-1 (IL-1) gene correlates with IL-1 secretion in vitro. Eur J Clin Invest 1992; 22:396–402. (b) Iwasaki LR, Gibson CS, Crouch LD, Marx DB, Pandey JP, Nickel JC. Speed of tooth movement is related to stress and IL-1 gene polymorphisms. Am J Orthod Dentofacial Orthop 2006; 130. 698.e1–9, and (c) Latkovski G, Licis N, Kalnins U. C-reactive protein levels and common polymorphism of the interleukin-1 gene cluster and interleukin-6 gene in patients with coronary heart disease. Eur J Immunogenet 2004; 31:207–13.
Abbreviations: OR=odds ratio; CI=confidence interval
Treatment Related Factors and Genetics Factors Influencing Human EARR
The occurrence of EARR concurrent with orthodontics is a complex trait contributed to by treatment related and genetic factors. For example, 30% of the EARR variability in one study from Portugal was explained by variation in or the presence of treatment duration, use of a Hyrax appliance for palatal expansion, closure of premolar extraction spaces, sex, and the P2RX7 gene rs1718119 SNP, while age, overjet, tongue thrust, skeletal Class II, and other genetic polymorphisms made minor contributions.[36] A follow-up study found that sex, length of treatment, closure of premolar extraction space, use of a Hyrax appliance, and homozygosity/hemizygosity for variant C from the IRAK1 gene were associated with EARR concurrent with orthodontia; the later IRAK polymorphism being a protective factor.[51]
Likewise, in a study already mentioned for its association with P2RX7 in the previous section treatment factors such as the influence of treatment duration, numerous cephalometric measurements (pretreatment values and post-treatment change in values), extraction of maxillary premolars, as well as genotypes for multiple DNA polymorphisms were investigated for their influence on EARR. It was found that a longer length of treatment and specific genotypes for P2RX7 SNP rs208294 together explained 25% of the total variation associated with EARR concurrent with orthodontics in a North American Caucasian sample.[35] The genetic findings in this study and the one from Portugal are particularly interesting since the protein products of the P2RX7 and IRAK genes are involved in the IL1B pathway.
Osteoporosis, tooth movement and EARR in orthodontics
Transient or chronic imbalances in bone remodeling, caused by cellular over-activation or inhibition of the effector cells, osteoclasts or osteoblasts, respectively, affect alveolar bone density in the case of the long bones. In this respect, the sex hormones act as potent regulators of bone metabolism, to the extent that the balance and rate of bone remodeling are even affected by an estrus cycle-dependent variation described in orthodontic tooth movement.[52] Tooth movement was described to be some 30% greater in estrus than in pro-estrus (p<0.05), with a negative correlation observed between estradiol levels and pyridinoline, TRAP activity and also IL-1 and interleukin-6 (IL-6), which suggests that estrus cycle-dependent variations in tooth movement occur through their effects on bone resorption.[53, 54]
In this respect, the effect of osteoporosis on orthodontic tooth movement has been extensively described in the literature at different levels. Estrogen withdrawal plays a critical role in the pathogenesis of post-menopausal osteoporosis, accelerating bone remodeling with a completely negative calcium balance.[55, 56] Diminished levels of estrogen correlate with unbalanced production of tumor necrosis factor α (TNF-α), receptor activator of nuclear factor κB ligand (RANKL), and IL-6. The cellular effects of estrogen are triggered by α and beta estrogen receptors (Erα, ERβ), localized in osteoblasts and osteoclasts.[57, 56] Selective deletion of osteoclast-α receptors results in the increased proliferation and rate of survival of osteoclasts, leading to a dramatic loss of trabecular bone mass, while the constitutively active ERα in osteoblasts has the opposite effect, inducing the upregulation of osteoprotegerin (OPG) and IL-6 subsequently increasing bone mineral density in the femur.[57, 58]
The knockout mouse model of ERα (ERKOα) showed a critical reduction in bone volume fraction and bone density irrespective of sex, indicating that ERα plays a central role in controlling bone turnover.[58] Erα- deficient animals exhibit clearly impaired cellular apoptosis processes, with a simultaneous increase/decrease in osteoclast/osteoblast numbers, which leads to an uncoupled bone remodeling process favoring catabolic activity.[59–61] Moreover, the pro-resorptive phenotype observed in homozygous specimens with selective deletion of Erα has been associated with increased production of IL-33 as an ineffective counter-regulatory effect, and increased levels of TNF-α and IL-1β in the periodontium.[62] Similarly, under OTM, ERαKO mice show an increase in the RANKL/OPG ratio, RANK, IL-6, and ST2, as well as decreased expression of dentin matrix acidic phosphoprotein and alkaline phosphatase in periodontal tissue.[63] Estrogen deficiency affects the rate of alveolar bone remodeling overall and secondarily the rate of experimental tooth movement, as shown in ovariectomized (OVX) rats undergoing lateral orthodontic tooth movement, where OVX specimens showed significantly (p<.001) increased rates of experimental tooth movement.[64, 56]
The occurrence and severity of root resorption during orthodontic tooth movement in experimentally induced osteoporosis was also evaluated by some authors after 28 days of force application, analyzing root resorption craters, depth, and volume using electron and laser scanning microscopes. Specifically, and most importantly, distal roots, those on the opposite side of the direction of tooth movement, were found to have resorption craters that were deeper and of greater volume than those observed in control animals in the cervical, middle, and apical thirds. The tooth movement was therefore not only faster in the experimentally induced osteoporosis group, but the severity of the root resorption was also found to be greater compared to the control group in those roots.[65] These results have however been questioned by other studies, which reported a similar acceleration in the tooth movement rate during orthodontic loading, but found a reduced incidence of root resorption when assessed by histomorphometry in the roots near the alveolar bone and in the direction of the tooth movement.[66]
Conclusion
Although EARR concurrent with orthodontic force is a complex trait, with multiple factors involving the reaction of the dental root, PDL, and alveolar bone to the force induced strain on the root, it is clear that how all of these factors affect alveolar bone density has an effect on the degree and duration of strain on the dental root, leading to a cascade of resorption of the dental root. The combination of factors that may result in this complex trait appear to vary sample to sample, and likely vary individual to individual, making a precise prediction of the occurrence of EARR unlikely, although with sufficient study a relatively qualitative high, medium or low risk may someone day be possible to determine, although these would not be absolutes.
Acknowledgments
This study PI13/00310 was supported by the Instituto de Salud Carlos III (Plan Estatal de I+D+i 2013–2016), and co-financed by the European Development Regional Fund “A way to
 achieve Europe” (ERDF)
achieve Europe” (ERDF)USA NIH P30GM110788 COBRE III (LAM, JKH), and the University of Kentucky E. Preston Hicks Endowed Professorship (JKH).
Conflict of Interest
Alejandro Iglesias-Linares reports grants from Instituto de Salud Carlos III & ERDF during the conduct of the study. Lorri Morford reports grants from National Institutes of Health, during the conduct of the study. Jim Hartsfield reports grants from National Institutes of Health, during the conduct of the study.
Footnotes
Compliance with Ethical Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Alejandro Iglesias-Linares, Department of Orthodontics, Complutense University of Madrid, Plaza Ramon y Cajal sn, Phone: +34636705246, aleigl01@ucm.es.
Lorri Ann Morford, University of Kentucky Center for the Biologic Basis of Oral/Systemic Diseases, 1095 Veterans Administration Drive, HSRB Room 414, Lexington, KY 40536-0305 USA, Phone: 859-323-2595 Fax: 859-257-6566, Lorri.Morford@uky.edu.
James Kennedy Hartsfield, Jr., University of Kentucky Center for the Biologic Basis of Oral/Systemic Diseases, 1095 Veterans Administration Drive, HSRB Room 414, Lexington, KY 40536-0305 USA, Phone: 859-323-0296 Fax: 859-257-6566, James.Hartsfield@uky.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links Between the Microbiome and Bone. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver CM. Diet, gut microbiome, and bone health. Current Osteoporosis Reports. 2015;13(2):125–130. doi: 10.1007/s11914-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takaishi Y, Arita S, Honda M, Sugishita T, Kamada A, Ikeo T, et al. Assessment of alveolar bone mineral density as a predictor of lumbar fracture probability. Adv Ther. 2013;30(5):487–502. doi: 10.1007/s12325-013-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weltman B, Vig KW, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010;137(4):462–476. doi: 10.1016/j.ajodo.2009.06.021. discussion 12A. [DOI] [PubMed] [Google Scholar]
- 6.Hartsfield JK. Pathways in external apical root resorption associated with orthodontia. Orthod Craniofac Res. 2009;12(3):236–242. doi: 10.1111/j.1601-6343.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 8.Roberts WE, Roberts JA, Epker BN, Burr DB, Hartsfield JK, editors. Semin Orthod. Elsevier; 2006. Remodeling of mineralized tissues, part, I: the Frost legacy. [Google Scholar]
- 9.Roberts WE. Bone physiology of tooth movement, ankylosis, and osseointegration. Semin Orthod. 2000;6:173–182. [Google Scholar]
- 10.Roberts WE, Epker BN, Burr DB, Hartsfield JK, Roberts JA, editors. Semin Orthod. Elsevier; 2006. Remodeling of mineralized tissues, part, II: control and pathophysiology. [Google Scholar]
- 11.Roberts WE, Smith RK, Zilberman Y, Mozsary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod. 1984;86(2):95–111. doi: 10.1016/0002-9416(84)90301-4. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida N, Koga Y, Peng CL, Tanaka E, Kobayashi K. In vivo measurement of the elastic modulus of the human periodontal ligament. Med Eng Phys. 2001;23(8):567–572. doi: 10.1016/s1350-4533(01)00073-x. [DOI] [PubMed] [Google Scholar]
- 13.Poppe M, Bourauel C, Jager A. Determination of the elasticity parameters of the human periodontal ligament and the location of the center of resistance of single-rooted teeth a study of autopsy specimens and their conversion into finite element models. J Orofac Orthop. 2002;63(5):358–370. doi: 10.1007/s00056-002-0067-8. [DOI] [PubMed] [Google Scholar]
- 14.Goldie RS, King GJ. Root resorption and tooth movement in orthodontically treated, calcium-deficient, and lactating rats. Am J Orthod. 1984;85(5):424–430. doi: 10.1016/0002-9416(84)90163-5. [DOI] [PubMed] [Google Scholar]
- 15.Katona TR, Paydar NH, Akay HU, Roberts WE. Stress analysis of bone modeling response to rat molar orthodontics. J Biomech. 1995;28(1):27–38. doi: 10.1016/0021-9290(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 16.Al-Qawasmi RA, Hartsfield JK, Jr, Everett ET, Flury L, Liu L, Foroud TM, et al. Genetic predisposition to external apical root resorption. Am J Orthod Dentofacial Orthop. 2003;123(3):242–252. doi: 10.1067/mod.2003.42. [DOI] [PubMed] [Google Scholar]
- 17.Srivicharnkul P, Kharbanda OP, Swain MV, Petocz P, Darendeliler MA. Physical properties of root cementum: Part 3. Hardness and elastic modulus after application of light and heavy forces. Am J Orthod Dentofacial Orthop. 2005;127(2):168–176. doi: 10.1016/j.ajodo.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Viecilli RF, Katona TR, Chen J, Hartsfield JK, Jr, Roberts WE. Three-dimensional mechanical environment of orthodontic tooth movement and root resorption. Am J Orthod Dentofacial Orthop. 2008;133(6):791 e11–791 e26. doi: 10.1016/j.ajodo.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Field C, Ichim I, Swain MV, Chan E, Darendeliler MA, Li W, et al. Mechanical responses to orthodontic loading: a 3-dimensional finite element multi-tooth model. Am J Orthod Dentofacial Orthop. 2009;135(2):174–181. doi: 10.1016/j.ajodo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Vikram NR, Senthil Kumar KS, Nagachandran KS, Hashir YM. Apical stress distribution on maxillary central incisor during various orthodontic tooth movements by varying cemental and two different periodontal ligament thicknesses: a FEM study. Indian J Dent Res. 2012;23(2):213–220. doi: 10.4103/0970-9290.100429. [DOI] [PubMed] [Google Scholar]
- 21.Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. 2001;119(3):307–312. doi: 10.1067/mod.2001.110809. [DOI] [PubMed] [Google Scholar]
- 22.Sharp L, Anderson S, Sammon P, Klemenz L, Cohen D, Drummond J. Local and systemic effects of IL-1 on interradicular alveolar bone. J Dent Res. 1991;70:596. [Google Scholar]
- 23.King G, Fischlschweiger W. The effect of force magnitude on extractable bone resorptive activity and cemental cratering in orthodontic tooth movement. J Dent Res. 1982;61(6):775–779. doi: 10.1177/00220345820610062501. [DOI] [PubMed] [Google Scholar]
- 24.Endres S, Cannon JG, Ghorbani R, Dempsey RA, Sisson SD, Lonnemann G, et al. In vitro production of IL 1β, IL 1α, TNF and IL 2 in healthy subjects: distribution, effect of cyclooxygenase inhibition and evidence of independent gene regulation. Eur J Immunol. 1989;19(12):2327–2333. doi: 10.1002/eji.1830191222. [DOI] [PubMed] [Google Scholar]
- 25.Pociot F, Mølvig J, Wogensen L, Worsaae H, Nerup J. A Taql polymorphism in the human interleukin-1β (IL-1β) gene correlates with IL-1β secretion in vitro. Eur J of Clin Invest. 1992;22(6):396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 26.Di Giovine F, Cork M, Crane A, Mee J, Duff G. Novel genetic association of an IL-1B gene variation a+ 3953 with IL-1B protein production and psoriasis. Cytokine. 1995;7(6):606. [Google Scholar]
- 27.Kornman KS, Crane A, Wang HY, Giovlne FSd, Newman MG, Pirk FW, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodont. 1997;24(1):72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Offenbacher S, Lόpez N, Chen D, Wang HY, Rogus J, et al. Association of interleukin-1 gene variations with moderate to severe chronic periodontitis in multiple ethnicities. J Periodontal Res. 2015;50(1):52–61. doi: 10.1111/jre.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglesias-Linares A, Yanez-Vico R, Ballesta-Mudarra S, Ortiz-Ariza E, Ortega-Rivera H, Mendoza-Mendoza A, et al. Postorthodontic external root resorption is associated with IL1 receptor antagonist gene variations. Oral Dis. 2012;18(2):198–205. doi: 10.1111/j.1601-0825.2011.01865.x. [DOI] [PubMed] [Google Scholar]
- 30.Linhartova P, Cernochova P, Izakovicova Holla L. IL1 gene polymorphisms in relation to external apical root resorption concurrent with orthodontia. Oral Dis. 2013;19(3):262–270. doi: 10.1111/j.1601-0825.2012.01973.x. [DOI] [PubMed] [Google Scholar]
- 31.Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28(8):2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Danis V, Millington M, Hyland V, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99(2):303–310. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vamvakopoulos J, Green C, Metcalfe S. Genetic control of IL-1β bioactivity through differential regulation of the IL-1 receptor antagonist. Eur J Immunol. 2002;32(10):2988–2996. doi: 10.1002/1521-4141(2002010)32:10<2988::AID-IMMU2988>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Bastos Lages EM, Drummond AF, Pretti H, Costa FO, Lages EJ, Gontijo AI, et al. Association of functional gene polymorphism IL-1beta in patients with external apical root resorption. Am J Orthod Dentofacial Orthop. 2009;136(4):542–546. doi: 10.1016/j.ajodo.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 35. Sharab LY, Morford LA, Dempsey J, Falcao-Alencar G, Mason A, Jacobson E, et al. Genetic and treatment-related risk factors associated with external apical root resorption (EARR) concurrent with orthodontia. Orthod Craniofac Res. 2015;18(Suppl 1):71–82. doi: 10.1111/ocr.12078. Paper combines analysis of multiple treatment and genetic factors.
- 36. Pereira S, Lavado N, Nogueira L, Lopez M, Abreu J, Silva H. Polymorphisms of genes encoding P2X7R, IL-1B, OPG and RANK in orthodontic-induced apical root resorption. Oral Dis. 2014;20(7):659–667. doi: 10.1111/odi.12185. Paper analyzes multiple genetic markers in two pathways.
- 37.Shank S, Shank K, Caudill R, Foroud T, Wetherill L, Weaver M, et al. Evaluation of SNPs in orthodontic patients with root resorption. J Dent Res. 2007;86 (Spec Iss A, abstract #1922) [Google Scholar]
- 38.Duan X, Yang T, Zhang Y, Wen X, Xue Y, Zhou M. Odontoblast-like MDPC-23 cells function as odontoclasts with RANKL/M-CSF induction. Arch Oral Biol. 2013;58(3):272–278. doi: 10.1016/j.archoralbio.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Fontana ML, de Souza CM, Bernardino JF, Hoette F, Hoette ML, Thum L, et al. Association analysis of clinical aspects and vitamin D receptor gene polymorphism with external apical root resorption in orthodontic patients. Am J Orthod Dentofacial Orthop. 2012;142(3):339–347. doi: 10.1016/j.ajodo.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 40. Iglesias-Linares A, Yanez-Vico R, Moreno-Fernandez A, Bueso Madrid D, Solano-Reina E, Mendoza-Mendoza M, et al. Genetic variants of epigenetic regulators (HDACs) influence predisposition to orthodontic EARR. J Dent Res. 2015;94 (Spec Iss A, abstract #2121921) This abstract is the first indication that epigenetics can affect EARR.
- 41.López JE, Sullivan ED, Fierke CA. Metal-dependent Deacetylases: Cancer and Epigenetic Regulators. ACS Chem Biol. 2016;11(3):706–716. doi: 10.1021/acschembio.5b01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. Embo J. 2005;24(14):2543–2555. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan HF, Smith AJ, Fleming GJ, Cooper PR. Histone deacetylase inhibitors induced differentiation and accelerated mineralization of pulp-derived cells. J Endod. 2012;38(3):339–345. doi: 10.1016/j.joen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, et al. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res. 2015;30(3):400–411. doi: 10.1002/jbmr.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishiyama Y, Matsumoto T, Lee J-W, Saitou T, Imamura T, Moriyama K, et al. Changes in the spatial distribution of sclerostin in the osteocytic lacuno-canalicular system in alveolar bone due to orthodontic forces, as detected on multimodal confocal fluorescence imaging analyses. Arch Oral Biol. 2015;60(1):45–54. doi: 10.1016/j.archoralbio.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y, He S, Gu T, Liu Y, Chen S. Genetic and clinical risk factors of root resorption associated with orthodontic treatment. Am J Orthod Dentofacial Orthop. 2016;150(2):283–289. doi: 10.1016/j.ajodo.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 47.Aminoshariae A, Aminoshariae A, Valiathan M, Kulild JC. Association of genetic polymorphism and external apical root resorption: A systematic review. The Angle Orthodontist. 2016 doi: 10.2319/011916-50.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun TM, Doucette-Stamm L, Duff GW, Kornman KS, Giannobile WV. Counterpoint: Risk factors, including genetic information, add value in stratifying patients for optimal preventive dental care. The Journal of the American Dental Association. 2015;146(3):174–178. doi: 10.1016/j.adaj.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 49.Diehl SR, Kuo F, Hart TC. Interleukin 1 genetic tests provide no support for reduction of preventive dental care. The Journal of the American Dental Association. 2015;146(3):164.e4–173.e4. doi: 10.1016/j.adaj.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Hartsfield JK., Jr . Personalized orthodontics: Limitations and possibilities in orthodontic practice. In: Krishnan V, Davidovitch Z, editors. Biological Mechanisms of Tooth Movement. Wiley; 2015. pp. 164–172. [Google Scholar]
- 51. Pereira S, Nogueira L, Canova F, Lopez M, Silva HC. IRAK1 variant is protective for orthodontic-induced external apical root resorption. Oral Dis. 2016 doi: 10.1111/odi.12514. in press. This paper available online combines analysis of multiple treatment and genetic factors.
- 52.Haruyama N, Igarashi K, Saeki S, Otsuka-Isoya M, Shinoda H, Mitani H. Estrous-cycle-dependent variation in orthodontic tooth movement. J Dent Res. 2002;81(6):406–410. doi: 10.1177/154405910208100610. [DOI] [PubMed] [Google Scholar]
- 53.Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227(4691):1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- 54.Angstwurm MW, Gärtner R, Ziegler-Heitbrock HL. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine. 1997;9(5):370–374. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- 55.Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrin. 2013;9(12):699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macari S, Duffles LF, Queiroz-Junior CM, Madeira MF, Dias GJ, Teixeira MM, et al. Oestrogen regulates bone resorption and cytokine production in the maxillae of female mice. Arch Oral Biol. 2015;60(2):333–341. doi: 10.1016/j.archoralbio.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda K, Tsukui T, Horie-Inoue K, Inoue S. Conditional expression of constitutively active estrogen receptor alpha in osteoblasts increases bone mineral density in mice. FEBS Lett. 2011;585(9):1303–1309. doi: 10.1016/j.febslet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 59.Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 2004;45(4):455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- 60.Imai Y, Youn MY, Kondoh S, Nakamura T, Kouzmenko A, Matsumoto T, et al. Estrogens maintain bone mass by regulating expression of genes controlling function and life span in mature osteoclasts. Ann NY Acad Sci. 2009;1173(s1):E31–E39. doi: 10.1111/j.1749-6632.2009.04954.x. [DOI] [PubMed] [Google Scholar]
- 61.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Canc Res. 2009;11(3):1. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keller J, Catala-Lehnen P, Wintges K, Schulze J, Bickert T, Ito W, et al. Transgenic over-expression of interleukin-33 in osteoblasts results in decreased osteoclastogenesis. Biochem Biophys Res Commun. 2012;417(1):217–222. doi: 10.1016/j.bbrc.2011.11.088. [DOI] [PubMed] [Google Scholar]
- 63.Lima IL, Macari S, Madeira MF, Rodrigues LF, Colavite PM, Garlet GP, et al. Osteoprotective effects of IL-33/ST2 link to osteoclast apoptosis. Am J Pathol. 2015;185(12):3338–3348. doi: 10.1016/j.ajpath.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Yamashiro T, Takano-Yamamoto T. Influences of ovariectomy on experimental tooth movement in the rat. J Dent Res. 2001;80(9):1858–1861. doi: 10.1177/00220345010800091701. [DOI] [PubMed] [Google Scholar]
- 65.Sirisoontorn I, Hotokezaka H, Hashimoto M, Gonzales C, Luppanapornlarp S, Darendeliler MA, et al. Tooth movement and root resorption; the effect of ovariectomy on orthodontic force application in rats. Angle Orthod. 2011;81(4):570–577. doi: 10.2319/101710-607.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seifi M, Ezzati B, Saedi S, Hedayati M. The Effect of Ovariectomy and Orchiectomy on Orthodontic Tooth Movement and Root Resorption in Wistar Rats. J Dent (Shiraz) 2015;16(4):302–309. [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver C, Gordon C, Janz K, Kalkwarf H, Lappe J, Lewis R, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporosis Internat. 2016;27(4):1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gülden N, Eggermann T, Zerres K, Beer M, Meinelt A, Diedrich P. Interleukin-1 polymorphisms in relation to external apical root resorption (EARR) J Orofacial Orthop/Fortschritte der Kieferorthopädie. 2009;70(1):20–38. doi: 10.1007/s00056-009-8808-6. [DOI] [PubMed] [Google Scholar]
- 69.Iglesias-Linares A, Yañez-Vico R, Ballesta S, Ortiz-Ariza E, Mendoza-Mendoza A, Perea E, et al. Interleukin 1 gene cluster SNPs (rs1800587, rs1143634) influences post-orthodontic root resorption in endodontic and their contralateral vital control teeth differently. International endodontic journal. 2012;45(11):1018–1026. doi: 10.1111/j.1365-2591.2012.02065.x. [DOI] [PubMed] [Google Scholar]
- 70.Iglesias-Linares A, Yañez-Vico R-M, Ortiz-Ariza E, Ballesta S, Mendoza-Mendoza A, Perea E, et al. Postorthodontic external root resorption in root-filled teeth is influenced by interleukin-1β polymorphism. J Endod. 2012;38(3):283–287. doi: 10.1016/j.joen.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 71.Iglesias-Linares A, Yañez-Vico RM, Ballesta-Mudarra S, Ortiz-Ariza E, Mendoza-Mendoza A, Perea-Pérez E, et al. Interleukin 1 receptor antagonist (IL1RN) genetic variations condition post-orthodontic external root resorption in endodontically-treated teeth. Histol Histopathol. 2013;28:767–773. doi: 10.14670/HH-28.767. [DOI] [PubMed] [Google Scholar]
- 72.Linhartova PB, Cernochova P, Kastovsky J, Vrankova Z, Sirotkova M, Izakovicova Holla L. Genetic determinants and post-orthodontic external apical root resorption in Czech children. Oral Dis. 2016 doi: 10.1111/odi.12564. in press [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Al-Qawasmi RA, Hartsfield JK, Jr, Everett ET, Flury L, Liu L, Foroud TM, Macri JV, Roberts WE, et al. Genetic predisposition to external apical root resorption in orthodontic patients: linkage of chromosome-18 marker. J Dent Res. 2003;82(5):356–360. doi: 10.1177/154405910308200506. [DOI] [PubMed] [Google Scholar]
- 74.Iglesias-Linares A, Yañez-Vico RM, Moreno-Fernández AM, Mendoza-Mendoza A, Orce-Romero A, Solano-Reina E. Osteopontin gene SNPs (rs9138, rs11730582) mediate susceptibility to external root resorption in orthodontic patients. Oral Dis. 2014;20(3):307–312. doi: 10.1111/odi.12114. [DOI] [PubMed] [Google Scholar]