Abstract
Background
Diminished growth is highly prevalent among HIV-infected children and might be improved by antiretroviral therapy (ART). We examined growth recovery in a rural Ugandan cohort of HIV-infected children randomized to lopinavir/ritonavir or non-nucleoside-reverse-transcription-inhibitor-based ART.
Methods
HIV-infected children 2 months to 6 years of age were randomized to Lopinavir/ritonavir- or non-nucleoside-reverse-transcription-inhibitor-based ART. Changes in weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) Z-scores for 24 months were evaluated using generalized linear repeated-measures models. Recovery from being underweight (WAZ<−2), stunted (HAZ<−2) and wasted (WHZ<−2) to Z-scores > −2 was also compared by arm using Kaplan-Meier survival and Cox proportional hazard modeling.
Results
A total of 129 children with median age of 3 years initiated therapy; 64 received Lopinavir/ritonavir-based and 65 non-nucleoside-reverse-transcription-inhibitor-based ART (nevirapine: 36 and efavirenz: 29). The median (IQR) difference in growth measures between baseline and 24 months for Lopinavir/ritonavir (n= 45) vs. non-nucleoside-reverse-transcription-inhibitor-based therapy (n=40) were as follows, WAZ: 0.47 (0.10, 1.62) vs. 0.53 (0.03, 1.14) (p=0.59) and HAZ: median 1.55 (0.78, 1.86) vs. 1.19 (0.62, 1.65) (p=0.23), respectively. ART regimen was not predictive of change in WAZ (beta: −0.02, 95%CI: −0.25, 0.20) or HAZ (beta: 0.05, 95%CI: −0.10, 0.19). Presence of confirmed virologic failure was not associated with growth.
Conclusions
Most ART-naive children experienced recovery of both WAZ and HAZ over the 24 months following ART-initiation, with no significant difference between those receiving Lopinavir/ritonavir vs. non-nucleoside-reverse-transcriptase-inhibitor-based ART. However, the persistence of median Z-scores below zero underscores the need for additional strategies to improve growth outcomes in HIV+ African children.
Keywords: Growth recovery, HIV infection, children, antiretroviral drugs
INTRODUCTION
Sub-optimal growth is highly prevalent among HIV-infected children, and is associated with high mortality risk (1). The impaired growth of HIV-infected children is likely the consequence of a combination of clinical and social factors including reduced caloric intake, reduced absorption and increased loss of nutrients needed for growth, altered metabolic rates, adverse socioeconomic conditions, and parental illness (2).
Antiretroviral therapy (ART) regimens have been one strategy to improve growth in HIV infected children, but different ART medications may affect growth differently. While some ART regimens have been shown to dramatically improve growth parameters (3-5), the results of two randomized trials suggested that the use of lopinavir/ritonavir (LPV/r) may lead to poorer growth outcomes among children (6, 7). Further, while ART is generally thought to improve growth, the precise impact of virologic failure on growth outcomes is not well documented. Some studies have reported robust growth responses despite failure to completely achieve virologic suppression on treatment (3, 8) while others report that virologic suppression may not be a requirement for initial growth responses in children (9, 10).
Therefore, we sought to determine if ART regimen or the presence of virologic failure were associated with differential growth recovery in the context of a clinical trial in which HIV-infected ART-naïve Ugandan children were randomized to initiate LPV/r- or non-nucleoside reverse-transcriptase inhibitor (NNRTI)-based ART.
MATERIALS AND METHODS
We evaluated children enrolled in the PROMOTE-pediatrics trial, an open-label, randomized trial in Tororo, Uganda (NCT00978068) that was conducted between 2009 to 2012. In this study, ART-naïve and -experienced HIV-infected children 2 months to 6 years of age were randomly assigned to either NNRTI -based or PI- based ART; details of the study protocol and results have been previously published (11). ART naïve children were excluded if they had received nevirapine for prevention of vertical transmission in the past 24 months. For children in the PI arm, lopinavir/ritonavir was used. For children in the NNRTI arm, NVP was used for children < 3 years of age; EFV was recommended for children ≥3 years of age, but choice of EFV vs NVP was left to provider discretion. At enrollment, children received ART, a long-lasting insecticide treated bed net, a hygienic water storage container, daily multivitamins and daily cotrimoxazole prophylaxis. Participants received all medical care at a study clinic. Children were given nutritional supplementation (Plumpy'Nut, Nutriset, Malaunay, France) at the discretion of the clinicians for severe malnutrition. Weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height-for-age (WHZ, for age < 60 months) were calculated for each visit using WHO supplied SAS macros (12). CD4 count and percentage, HIV RNA and hemoglobin levels were obtained every 12 weeks.
To evaluate growth recovery following ART initiation, we analyzed data for the 24 months following ART-initiation among ART-naive PROMOTE-pediatric enrollees using both as-treated and intention-to-treat analysis. Baseline anthropometric measures were compared between arms using Wilcoxon rank sum or Fisher's exact tests. Changes in gender-specific Z-scores for WAZ and HAZ after baseline were evaluated using Generalized Estimating Equation (GEE) models that adjusted for (selected a priori) baseline Z-score, socioeconomic status, age (< or ≥ 3 years) and confirmed virologic failure (defined as the first of 2 consecutive HIV RNA results > 400 copies/ml, 24 weeks after starting the regimen to which they had been randomized, and included as a time-varying covariate). To do this, we fitted a linear model (slope) to all time points up to month 24. The adequacy of assuming a linear relationship across time between the outcome and predictors in GEE models was assessed using a Kolmogorov-type supremum test comparing the distribution of the observed cumulative residual pattern to the distribution of computer-simulated cumulative residual patterns under a zero mean Gaussian process. We evaluated each GEE model's functional form for misspecification by using 10,000 simulated realizations. The model testing yielded p-values between 0.93 and 1.00 for all models, indicating all models were appropriately specified.
Socio-economic status (SES) was evaluated using a household assets measure that was created based on possession (or not) of a radio, telephone, television, refrigerator, motorcycle, bicycle, and car. To adjust for SES, the first two components of a principle component analysis of household assets, which accounted for 55.7% of the information contained in the asset holding variables, were used.
In addition to evaluating change over time including all children, nutritional recovery among children who were underweight (WAZ<−2), stunted (HAZ<−2) to Z-scores > −2 after 12 months and 24 months was compared between ART treatment groups using Kaplan-Meier survival plots and Cox proportional hazard modeling. WHZ was not included in longitudinal analyses because the WHO only provides WHZ reference values up to age 60 months. [The lack of standards for WHZ after 60 months (12) would have led to truncated data in the 58% (75 of 129) of children who surpassed 5 years of age during the 24 month follow up period.] We also developed an integral measure of plasma HIV RNA exposure for each individual, an area under curve (AUC) for log HIV RNA level and time up to censoring or endpoint, irrespective of treatment status or randomized regimen adherence, and included this continuous measure as a predictor in the Cox-proportional modelling of recovery for children with nutritional Z-scores < −2 at enrollment. Statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) or Stata Version 11 (StataCorp, College Station, TX, USA).
The study was approved by Makerere University School of Medicine Research and Ethics Committee, Uganda National Council for Science and Technology, and University of California, San Francisco, Committee for Human Research.
RESULTS
A total of 176 children were randomized to receive either NNRTI-based or LPV/r-based ART. Of these children, 129 were ART-naïve and initiated ART (Table 1). Children were a median age of 3 years old, with only 9 (7%) < 12 months. The majority (67%) were WHO clinical stage 1 at enrollment. All children in the PI arm initiated LPV/r (n=64). In the NNRTI arm (n=65), all children < 3 years of age received NVP (n=26); among children ≥ 3 years of age, more received EFV (n=29) than NVP (n=10). The median CD4 % was 16 (IQR 11 to 23) and HIV RNA log (copies/ml) was 5.3 (IQR 4.8 to 5.9) at baseline. The median (IQR) Z-scores did not significantly differ by arm (LPV/r vs. NNRTI) at baseline: WAZ [−1.5 (−2.4 to −0.6) vs. −1.2 (−2.3 to −0.6)], HAZ [−2.6 (−3.2 to −1.5) vs. −2.3 (−3.3 to −0.8)]. Among the 108 children ≤ 60 months; WHZ did not differ significantly between the treatment arms −0.2 (−1.1 to 0.6) vs. −0.1 (−0.9 to 0.5), p=0.97.
Table 1.
Subject characteristics at baseline
| Characteristic | LPV/r Arm (n=64*) | NNRTI Arm (n=65*) | P-value† |
|---|---|---|---|
| Age, years | 3.2 (2.3 to 4.4) | 3.1 (2.3 to 4.6) | 0.97 |
| < 3 years | 29 (45%) | 26 (40%) | 0.56 |
| Female | 32 (30%) | 35 (54%) | 0.72 |
| CD4 number, cells/μl | 582 (397 to 969) | 593 (389 to 788) | 0.89 |
| CD4 Percentage | 17 (12 to 23) | 16 (11 to 23) | 0.77 |
| HIV RNA, log(copies/ml) | 5.3 (4.8 to 5.9) | 5.3 (4.8 to 5.9) | 0.75 |
| WHO Stage | 0.82 | ||
| I | 39 (61%) | 48 (74%) | |
| II | 17 (27%) | 11 (17%) | |
| III | 2 (3%) | 1 (2%) | |
| IV | 6 (9%) | 5 (8%) | |
| WAZ | −1.5 (−2.4 to −0.6) | −1.2 (−2.3 to −0.6) | 0.67 |
| WAZ < −2^ | 24 (38%) | 22 (34%) | 0.66 |
| HAZ | −2.6 (−3.2 to −1.5) | −2.3 (−3.3 to −0.8) | 0.59 |
| HAZ < −2^ | 39 (61%) | 39 (60%) | 0.91 |
| WHZ~ | −0.2 (−1.1 to 0.6) | −0.1 (−0.9 to 0.5) | 0.97 |
| WHZ~ < −2^ | 7 (12%) | 5 (8%) | 0.48 |
Excludes the 2 children who withdrew or died before initiating study drugs.
Wilcoxon rank sum, Fisher's Exact test, or test of proportions. Values are medians (interquartile range) or number (percentage); LPV/r, ritonavir-boosted lopinavir; NNRTI, non-nucleoside-reverse-transcriptase-inhibitor. WAZ: Weight-for-age Z-score; HAZ: Height-for-age Z-score; WHZ: Weight-for-height-for-age Z-score.
Includes only the 107 of 129 who were age < 60 months at enrollment and therefore had WHO WHZ references.
Proportion (%) with Z-scores < −2 at baseline.
Of the total 129 children who initiated ART, 39 did not reach 24 months of follow-up: 7 were lost to follow up and the other 32 remained enrolled but with < 24 months of follow-up time at the time the study ended. The median (IQR) follow-up times were 23.9 months (18.4, 23.9) for the LPV/r arm and 23.8 months (17.5, 23.9) for the NNRTI arm. The 24 month confirmed virologic failure rate was 25% (16/64) in both the LPV/r arm and the NNRTI-arm (16/65; NVP: 11/25, EFV: 5/24). The proportion of failure estimated by Kaplan-Meier survival analysis at 24 months was 35% in the LPV/r Arm and 36% in the NNRTI arm (Log Rank P-value = 0.88).
In the first 24 months of follow-up, 11 children changed their ART regimen: 1 changed from NVP to LPV/r out of concern for possible Steven's Johnson Syndrome (week 3), 1 changed from NVP to EFV due to elevation of liver enzymes, 5 children switched from NNRTI to LPV/r due to virologic failure (weeks 53, 87, 91, 92, 92) and 1 child switched from LPV/r to NNRTI due to need for tuberculosis treatment (at week 58). Three children received nutritional supplementation (PI: 2, NNRTI: 1).
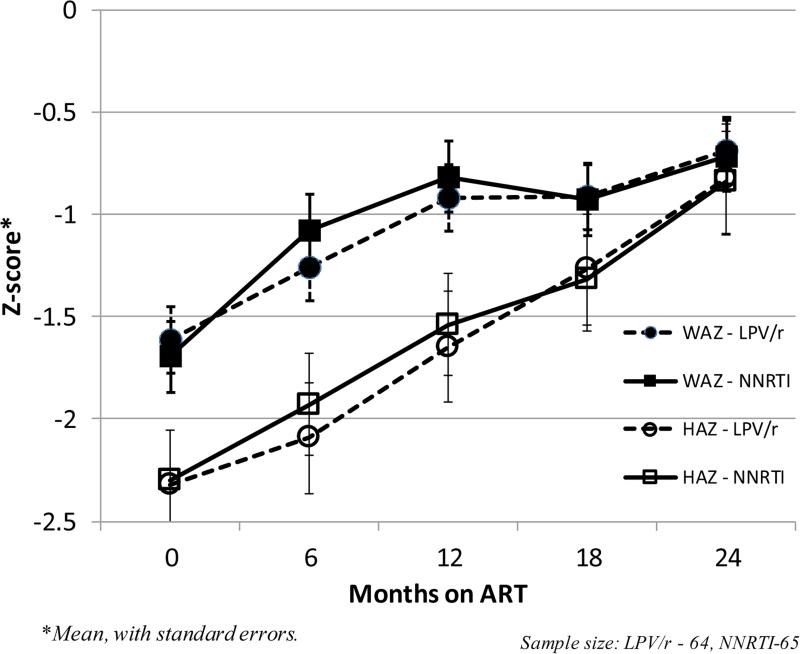
HAZ and WAZ of children in both arms increased steadily over 24 months following ART-initiation (Figure 1). The median (IQR) difference in growth measures between baseline and 24 months for Lopinavir/ritonavir (n= 45) vs. non-nucleoside-reverse-transcription-inhibitor-based therapy (n=40) were; WAZ: 0.47 (0.10, 1.62) vs. 0.53 (0.03, 1.14) (p=0.59) and HAZ: 1.55 (0.78, 1.86) vs. 1.19 (0.62, 1.65) (p=0.23), respectively. In multivariate models, ART regimen (LPV/r vs. NNRTI) was not predictive of change in WAZ or HAZ by either intention-to-treat (ITT) [WAZ, beta: −0.02, 95%CI:-0.32 to 0.29; or HAZ, beta: 0.13, 95%CI: −0.09, 0.34) or as-treated analyses (PP) [WAZ, (beta: −0.05, 95%CI: −0.35, 0.26 ) or HAZ, (beta: 0.10, 95%CI: −0.10, 0.31). In ITT modelling, age < 3 years was associated with an additional mean increase of 0.67 Z-score points in the overall change in WAZ from baseline compared to age ≥ 3 years (beta: 0.67, 95%CI: 0.35 to 0.98) but not for HAZ (beta: 0.06, 95%CI: 0.−0.16 to 0.27). SES was not a predictor of change in either WAZ or HAZ.
Figure 1.
WAZ and HAZ trajectories among HIV-infected Ugandan children < 5 y, by ART regimen (n=129).
Among children who had Z-scores < −2 at baseline and reached 24 months of follow up, the crude proportions who recovered (from below −2 SD to above −2 SD) HAZ was 89% (23/26) vs. 76% (19/25), and the proportion who recovered WAZ was 85% (17/20) vs. 79% (11/14), in the LPV/r vs. NNRTI arms respectively at 24 months. In Kaplan-Meier survival analysis, the proportion estimated to have recovered WAZ was 86% vs. 77% and the proportion who recovered HAZ was 89% vs. 76% in the LPV/r vs. NNRTI arms respectively at 24 months. Median time to recovery for HAZ did not differ between arms; it was 18.4 months (IQR 12.0, 23.8) in both the LPV/r arm and in the NNRTI arm (IQR 12.0, 24.0), p=0.45. There was likewise no significant difference in the time to recovery for WAZ in the LPV/r (6.5 months, IQR 6.2, 18.4) vs. NNRTI (9.1 months, IQR 6.2, 23.4) arms (p=0.92). In Cox Proportional Hazards Modelling of time to recovery from below to above −2 HAZ or WAZ, presence of confirmed virologic failure, HIV RNA AUC and SES were not predictive of change in either HAZ or WAZ.
DISCUSSION
In this study of growth recovery in HIV-infected children initiating either NNRTI- or LPV/r-based ART, we observed steady improvements in the HAZ and WAZ of children in both treatment arms over 24 months. This is consistent with dramatic and sustained improvement in growth responses after ART initiation, especially in younger children, previously observed in several other studies (4, 5, 10, 13, 14).
Our study did not demonstrate differential growth recovery among children receiving LPV/r- or NNRTI- based ART at 24 months of follow up, in contrast to data from other clinical trials that suggested LPV/r might lead to poorer growth at 12 months (5, 7, 15). In a cohort of infants exposed to nevirapine prophylaxis, median increases in z scores for height and weight were larger in the nevirapine group than in the LPV/r group at all study visits up to week 96, but the differences were not significant statistically (median increase in WAZ at 48wks, 0.78 vs. 0.46; p=0.46, median increase in HAZ at 48wks 0.41 vs. 0.07, p=0.15) (15). In a parallel cohort of infants not exposed to nevirapine, mean values improved for all nutritional outcomes in both treatment groups, but between-group differences were significant only for adjusted changes in WAZ (p=0.01 at week 24 and p=0.01 at week 48), BMI (p=0.02 at week 24, p=0.03 at week 48), in favor of the nevirapine arm (7). Impaired growth in children receiving LPV/r might have resulted from gastrointestinal side effects, decreased caloric intake caused by GI disturbances, or altered metabolism. However, the differences were notably small and did not endure in these trials. In the context of these findings, we believe our results suggest that children in similar settings who are prescribed LPV/r might have slight differences in growth, but that they are unlikely to be durable or clinically significant.
The mechanisms by which ART contributes to growth recovery are not clear. One might postulate that the mechanism for growth recovery following ART is suppression of viral replication, in turn leading to relief from the metabolic costs of inflammation. However, improvement in HAZ and WAZ were notably independent of virologic response in our study. Reports from other studies have shown variable association of growth with virologic suppression. One trial found that a reduction in the viral load of at least 1.5 log and increase in CD4 cells was associated with improved growth recovery(5), but other studies have shown robust growth responses despite failure to completely suppress virus during treatment (8, 10). These results suggest that even suboptimal suppression of viral replication and CD4 recovery might provide enough of a metabolic benefit to improve growth (2, 16). Growth recovery in HIV children on ART may also result from other components of care provided including improved general medical care and cotrimoxazole prophylaxis, which would reduce risk of opportunistic infections.
One concern when initiating children on ART is that nutritional status might worsen. There have been reports of severe acute malnutrition (SAM) occurring in HIV infected children within the first 12 weeks following ART initiation (17). We did not document significant worsening of nutritional status following ART initiation. In these reports, it was postulated that observed SAM probably represented a form of immune reconstitution syndrome in children with underlying chronic malnutrition and severe immunosuppression.
In our cohort, a larger number of children had HAZ scores < −2 SD than WAZ <−2 at baseline. This is a common pattern (18), also noted in HIVNET 012 which showed a 2 to 4 fold higher prevalence of HAZ < − 2 compared to WAZ (19). WAZ is considered to be a marker of recent nutritional insult, while low HAZ reflects chronic issues of health and nutrition (20). Studies have shown variable recovery of height after initiating ART (21). Kabue et al. reported improvement in HAZ at 1 year (p<0.05)(22); Mwiru et al. (2014) likewise found improvement in HAZ, but children's HAZ scores never normalized despite catch-up growth (14). Gsponer et al. (2012) found improvements in HAZ, but HAZ catch-up growth was slowest in first year of ARV treatment.
While our study has a number of important strengths, including randomized allocation of ART and close follow up, there were several limitations. It may be that the smaller sample size of our cohort and truncated follow-up time concealed small differences by arm, or that other characteristics of our cohort, such as its older age, altered the manifestation of the effects of LPV/r. Unfortunately, the study sample size and association of age with NNRTI choice in this cohort did not permit us to compare EFV to either LPV/r or NVP separately. The shorter median follow-up in our study (24 months) may have limited our ability to observe the long-term impact of virologic suppression growth and our determination of predictors of growth recovery in this population. Given that measurement of nutritional outcomes was not a primary aim of the study, nutritional supplementation was left to provider discretion and we did not document duration and quantity of supplementation. While this reflects standard local practice and adds to generalizability of our results, it limits our analytical ability to evaluate and adjust for supplementation as a factor in growth improvement. Several studies have demonstrated positive effects from supplementation, but there is a need for additional research (21).
In conclusion, our study demonstrated significant growth recovery for most HIV-infected African children initiating ART, and that children receiving LPV/r in similar settings can be expected to have comparable growth to children receiving NNRTI-based ART. However, that median nutritional Z-scores persisted below zero underscores the need for additional strategies to optimize growth outcomes in HIV positive African children.
Acknowledgments
Source of Funding
The study was funded by a grant from Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD059454 and K23HD60459) and AbbVie (formerly Abbott Laboratories) donated the lopinavir/ritonavir
Footnotes
Conflicts of Interest
The authors declare not conflicts of interest.
References
- 1.Fergusson P, Chinkhumba J, Grijalva-Eternod C, Banda T, Mkangama C, Tomkins A. Nutritional recovery in HIV-infected and HIV-uninfected children with severe acute malnutrition. Archives of disease in childhood. 2009;94(7):512–6. doi: 10.1136/adc.2008.142646. Epub 2008/11/04. doi: 10.1136/adc.2008.142646. PubMed PMID: 18977785. [DOI] [PubMed] [Google Scholar]
- 2.Rose AM, Hall CS, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Archives of disease in childhood. 2014 doi: 10.1136/archdischild-2012-303348. Epub 2014/01/11. doi: 10.1136/archdischild-2012-303348. PubMed PMID: 24406803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musoke PM, Barlow-Mosha L, Bagenda D, Mudiope P, Mubiru M, Ajuna P, et al. Response to antiretroviral therapy in HIV-infected Ugandan children exposed and not exposed to single-dose nevirapine at birth. J Acquir Immune Defic Syndr. 2009;52(5):560–8. doi: 10.1097/qai.0b013e3181b93a5a. PubMed PMID: 19950430. [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–89. doi: 10.1016/S1473-3099(08)70180-4. PubMed PMID: 18652994. [DOI] [PubMed] [Google Scholar]
- 5.Verweel G, van Rossum AM, Hartwig NG, Wolfs TF, Scherpbier HJ, de Groot R. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109(2):E25. doi: 10.1542/peds.109.2.e25. Epub 2002/02/05. PubMed PMID: 11826235. [DOI] [PubMed] [Google Scholar]
- 6.Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, Gibb DM, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11(4):273–83. doi: 10.1016/S1473-3099(10)70313-3. Epub 2011/02/04. doi: S1473-3099(10)70313-3 [pii] 10.1016/S1473-3099(10)70313-3. PubMed PMID: 21288774; PubMed Central PMCID: PMC3111069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366(25):2380–9. doi: 10.1056/NEJMoa1113249. Epub 2012/06/22. doi: 10.1056/NEJMoa1113249. PubMed PMID: 22716976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaffari G, Passalacqua DJ, Caicedo JL, Goodenow MM, Sleasman JW. Two-year clinical and immune outcomes in human immunodeficiency virus-infected children who reconstitute CD4 T cells without control of viral replication after combination antiretroviral therapy. Pediatrics. 2004;114(5):e604–11. doi: 10.1542/peds.2004-0274. PubMed PMID: 15492356. [DOI] [PubMed] [Google Scholar]
- 9.Nachman SA, Lindsey JC, Moye J, Stanley KE, Johnson GM, Krogstad PA, et al. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24(4):352–7. doi: 10.1097/01.inf.0000157095.75081.43. Epub 2005/04/09. doi: 00006454-200504000-00011 [pii]. PubMed PMID: 15818296. [DOI] [PubMed] [Google Scholar]
- 10.Musoke PM, Mudiope P, Barlow-Mosha LN, Ajuna P, Bagenda D, Mubiru MM, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. doi: 10.1186/1471-2431-10-56. Epub 2010/08/10. doi: 1471-2431-10-56 [pii] 10.1186/1471-2431-10-56. PubMed PMID: 20691045; PubMed Central PMCID: PMC2923128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achan J, Kakuru A, Ikilezi G, Ruel T, Clark TD, Nsanzabana C, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012;367(22):2110–8. doi: 10.1056/NEJMoa1200501. Epub 2012/11/30. doi: 10.1056/NEJMoa1200501. PubMed PMID: 23190222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization [2015 May 5];The WHO Child Growth Standards. http://www.who.int/childgrowth/standards/en/
- 13.Kekitiinwa A, Lee KJ, Walker AS, Maganda A, Doerholt K, Kitaka SB, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49(4):384–92. doi: 10.1097/QAI.0b013e31818cdef5. doi: 10.1097/QAI.0b013e31818cdef5. PubMed PMID: 18931630. [DOI] [PubMed] [Google Scholar]
- 14.Mwiru RS, Spiegelman D, Duggan C, Seage GR, 3rd, Semu H, Chalamilla G, et al. Growth among HIV-infected children receiving antiretroviral therapy in Dar es Salaam, Tanzania. J Trop Pediatr. 2014;60(3):179–88. doi: 10.1093/tropej/fmt104. doi: 10.1093/tropej/fmt104. PubMed PMID: 24393831; PubMed Central PMCID: PMCPMC4040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363(16):1510–20. doi: 10.1056/NEJMoa1000931. PubMed PMID: 20942667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose AM, Hall CS, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Arch Dis Child. 2014 doi: 10.1136/archdischild-2012-303348. Epub 2014/01/11. doi: archdischild-2012-303348 [pii] 10.1136/archdischild-2012-303348. PubMed PMID: 24406803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prendergast A, Bwakura-Dangarembizi MF, Cook AD, Bakeera-Kitaka S, Natukunda E, Nahirya Ntege P, et al. Hospitalization for severe malnutrition among HIV-infected children starting antiretroviral therapy. AIDS. 2011;25(7):951–6. doi: 10.1097/QAD.0b013e328345e56b. Epub 2011/04/14. doi: 10.1097/QAD.0b013e328345e56b 00002030-201104240-00008 [pii]. PubMed PMID: 21487251. [DOI] [PubMed] [Google Scholar]
- 18.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–80. doi: 10.1542/peds.2009-1519. doi: 10.1542/peds.2009-1519. PubMed PMID: 20156903. [DOI] [PubMed] [Google Scholar]
- 19.Owor M, Mwatha A, Donnell D, Musoke P, Mmiro F, Allen M, et al. Long-term follow-up of children in the HIVNET 012 perinatal HIV prevention trial: five-year growth and survival. J Acquir Immune Defic Syndr. 2013;64(5):464–71. doi: 10.1097/QAI.0000000000000015. doi: 10.1097/QAI.0000000000000015. PubMed PMID: 24121753; PubMed Central PMCID: PMC4172334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization [2015 May 5];Global Database on Child Growth and Malnutrition. http://www.who.int/nutgrowthdb/about/introduction/en/index2.html.
- 21.McGrath CJ, Diener L, Richardson BA, Peacock-Chambers E, John-Stewart GC. Growth reconstitution following antiretroviral therapy and nutritional supplementation: systematic review and meta-analysis. AIDS. 2015;29(15):2009–23. doi: 10.1097/QAD.0000000000000783. doi: 10.1097/QAD.0000000000000783. PubMed PMID: 26355573; PubMed Central PMCID: PMCPMC4579534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22(3):245–51. doi: 10.1089/apc.2007.0049. doi: 10.1089/apc.2007.0049. PubMed PMID: 18298315. [DOI] [PubMed] [Google Scholar]