EVOLUTION OF CATHETER-BASED STRUCTURAL INTERVENTIONS HAS GIVEN PATIENTS LESS INVASIVE alternatives to surgery; however, the current generation of transcatheter heart valves (THV) are not specifically designed for mitral position implantation and have intrinsic geometry that may make mitral implantation suboptimal. Operators are faced with unique challenges with valve deliverability, embolism, and notably left ventricular outflow tract (LVOT) obstruction. Therefore, understanding the suitability of prosthesis delivery and implantation individualized to each heart is of paramount importance.
Successful transcatheter mitral valve replacement (TMVR) depends on accurate sizing of the mitral annulus (Figure 1) and avoidance of LVOT obstruction. Incorporation of computer-aided design and generation of 3-dimensional-printed heart models allows for ex vivo device bench testing in patient-specific anatomy (Figures 1 and 2). Modeling of proposed THV at different angles/depths of deployment into the LV allows estimation of LVOT obstruction of neo-LVOT/LVOT (Figure 3). We now aim to describe the utility of cardiac computed tomography (Table 1) and ex vivo THV fit testing with 3-dimensional models to predict LVOT obstruction in TMVR.
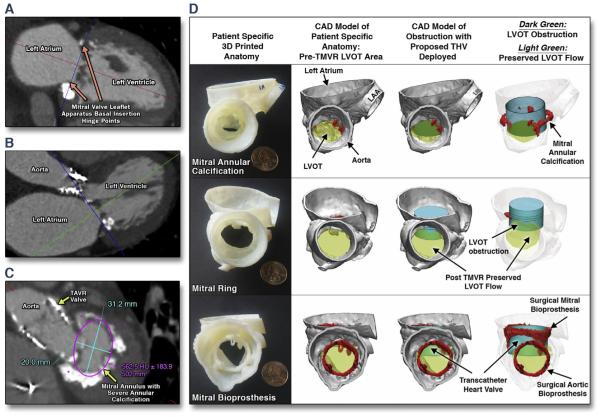
FIGURE 1. CT Guided Sizing of the Mitral Annulus Landing Zone and Fit-Testing CAD-Generated THV into Patient-Specific Mitral Anatomy to Predict LVOT Obstruction.
Pre-procedural imaging utilizes a contrast-enhanced, retrospectively electrocardiogram-gated computed tomography (CT) angiography acquisition without application of electrocardiogram-dose modulation (Table 1). Four-dimensional cine clips of the mitral annulus (MA) motion are generated. The MA plane is defined as the basal-most insertion of the mitral leaflets, characterized by a hinge-point motion of the leaflet base during the mid-to-late diastolic phase of the cardiac cycle, or in the case of a degenerative mitral ring/bioprosthesis, by prosthesis-artifact on CT. Using the double-oblique method, crosshairs are aligned to the MA plane in sagittal and coronal cross-sections (A and B) allowing for MA dimensions to be obtained on axial thin sections (C). Once the proposed THV size is identified, computer-aided design (CAD)-generated Sapien, Sapien XT (SXT) and Sapien 3 valves (Edwards Lifesciences, Irvine, California) are then virtually tested on-screen and again physically in the patient’s 3DP anatomy to confirm sizing and estimate risk of LVOT obstruction (D). For difficult to-conceptualize computer-based 3D imaging anatomy, 3DP THV models were implanted into the 3DP patient-specific systolic left ventricular outflow tract (LVOT) model to help the structural heart team visualize the predicted LVOT obstruction to anatomical scale. HU = Hounsfield unit(s); TAVR = transcatheter aortic valve replacement; 3D = 3-dimensional; 3DP = 3-dimensionally printed; TMVR = transcatheter mitral valve replacement.
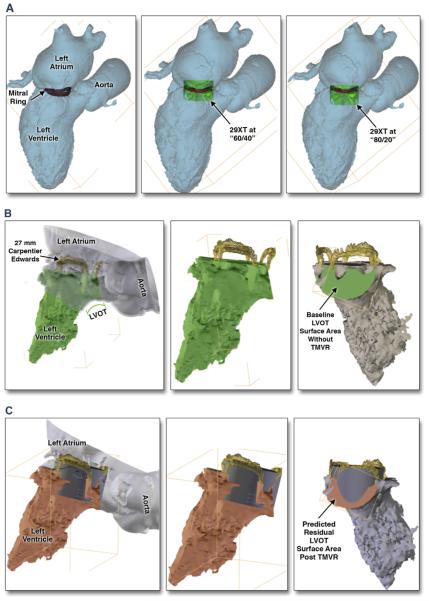
FIGURE 2. LVOT CAD Prediction Modeling Algorithm.
The blood volume of the mid-to-late systolic phase demonstrating the smallest LVOT surface area between the basal anteroseptal wall of the LV and the most inferior/ventricular hinge of the anterior mitral leaflet or surgical frame strut is segmented out for 3D modeling. CAD models are generated with the proposed THV deployed in different LV landing zones (60% LV/40% atrial to 80% LV/20% atrial) within the patient’s MA/ring (A). In the setting of surgical bioprosthesis, modeling is performed with 0% defined within the confines of the inferior border of the surgical prosthesis strut, and 10% as inferior to the surgical prosthesis plane into the LV. The post-TMVR residual LVOT, “neo-LVOT,” surface area is then calculated for proposed THV by various depths of LV deployment and angulations of THV delivery system toward/away from aortic outflow tract (B and C). In order to predict percentage of LVOT obstruction, the ratio of neo-LVOT to native LVOT surface area is calculated: (native LVOT area – neo LVOT area)/native LVOT area. Abbreviations as in Figure 1.
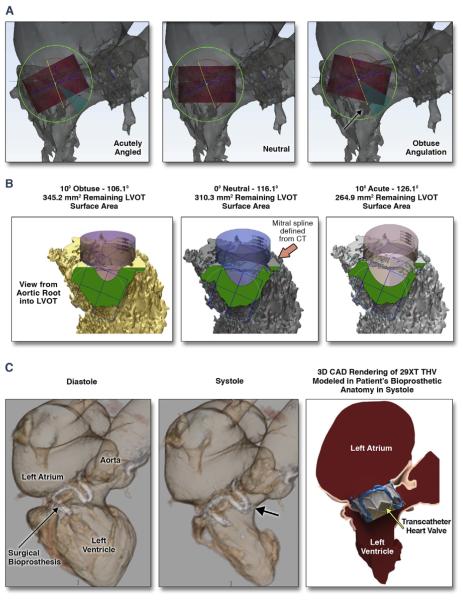
FIGURE 3. CT, Computer-Aided-Design, and 3D Print Analysis of the LVOT to Characterize and Quantify the Residual Post-TMVR “Neo-LVOT” Area Overcomes the Limitations of Traditional 2D Imaging Planes.
Preliminary data utilizing 2-dimensional (2D) echo imaging correlated acute angulation of the mitral aorta-outflow-angle (mAOA) with higher risk of LVOT obstruction compared with that of more obtuse mAOA. On the basis of our single-center experience, the association between mAOA and risk of LVOT obstruction was not reproducible (A and B). LVOT obstruction is not solely dependent on mAOA. Personalization and determination of feasibility of TMVR in patient-specific anatomy may not be accounted for if relying on 2D measurements of a 3D anatomy (A). CT and CAD 3DP analysis of the “neo-LVOT” surface area overcomes the limitations of traditional 2D imaging planes. THV implantation in the mitral position results in permanent anterior motion/displacement of the surgical/native anterior mitral leaflet. Both THV frame and permanent anterior motion/displacement can result in severe LVOT obstruction (C). The importance of understanding the application of this technology is the ability to test devices in patient-specific cardiac anatomy ex vivo for LVOT encroachment. Abbreviations as in Figure 1.
TABLE 1.
TMVR CT Scanning Protocol
| Scan Type | Gated CTA Chest |
|---|---|
| Field of view | Start scan acquisition: above the lung apices |
| Complete scan acquisition: at the bottom of rib cage | |
| Algorithm | Standard |
| Scan helical thickness | 0.625 mm |
| 0.16:0.36 | |
| 0.18:0.36 | |
| Pitch:gantry rotation speed,s* | 0.20:0.36 |
| 0.22:0.36 | |
| 0.24:0.36 | |
| Kilovolts | 100 kV if BMI <30 |
| 120 kV if BMI >30 | |
| Milliamp | ECG-modulated mA |
| Minimum of 200 to maximum of 600 cardiac RR 40–80 | |
| Adaptive statistical iterative reconstruction | 30% |
| Recon 2 (for pertinent radio logical findings) | 2.5 mm |
| (75% of cardiac cycle) | |
| Recon 3 (for mitral annulus sizing and LVOT evaluation) | 1.25 mm |
| (5%–95% of cardiac cycle by 10% increments for 4D cine loop of dynamic mitral annulus motion and LVOT evaluation) |
|
| Recon 4 (additional LVOT evaluation as needed) | 0.625 mm |
| (25%–55% by 10% increments) | |
| Prep delay | SmartPrep at level of left atrium, trigger at 120 HU |
| Injection rate | 4cc/s |
| Injection volume | 80 cc |
| Auto transfer | PACS |
| Enter “gated mitral” in exam description | |
| Set maximum mA at phases 35–80 and r-peak center to 75 | |
| Injector Protocol | |
| 4 cc/s 80 cc contrast | |
| 3 cc/s 30 cc saline |
Pitch:speed should be adjusted according to the heart rate gating algorithm provided by different vendors as recommended for routine cardiac gating acquisition protocols. 4D = 4-dimensional; BMI = body mass index; CT = computed tomography; CTA = computed tomography angiography; ECG = electrocardiography; HU = Hounsfield unit(s); LVOT = left ventricular outflow tract; PACS = Picture Archiving Communication System; TMVR = transcatheter mitral valve replacement.
ACKNOWLEDGMENTS
The authors thank Dr. Scott Dulchavsky, Mark Coticchia, and Lindsay Klee of the Henry Ford Innovation Institute for their support toward clinical 3-dimensional printing.
Footnotes
Drs. Wang, O’Neill, Mr. Myers and Forbes are co-inventors on a patent application, assigned to their employer Henry Ford Health System, on software to predict LVOTO. Dr. Greenbaum is a proctor for Edwards Lifesciences and St. Jude Medical. Dr. Guerrero has received a research grant from and is a proctor for Edwards Lifesciences. Dr. Paone is a consultant and proctor for Edwards Lifesciences. Dr. O’Neill is a consultant for Edwards Lifesciences, Medtronic, and St. Jude Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.