Abstract
Loss-of-function germline mutations in the fumarase (FH)-gene of the Krebs cycle characterize hereditary leiomyomatosis and renal cell cancer (HLRCC)-syndrome. Fumarase (FH)-deficiency can be diagnosed by the loss of immunohistochemical expression. In this study, we investigated the occurrence and clinicopathologic features of FH-deficient uterine smooth muscle tumors (SMTs). A total of 1583 uterine and 157 non-uterine SMTs were examined using a polyclonal FH antibody and automated immunohistochemistry, and 86 uterine leiomyomas with an FH loss were identified. The frequencies of FH-deficiency for subcohorts of uterine SMTs were 1.6% for unselected non-atypical leiomyomas, 1.8% for cellular leiomyomas, 37.3% for atypical leiomyomas, and 0% for leiomyosarcomas. One extrauterine, retroperitoneal ER-positive leiomyoma, was also FH-deficient. The patient age of FH-deficient uterine leiomyomas was 20-52 years (median, 38 years). Grossly these tumors were often soft and amorphous resembling a fibrothecoma. Histologically the FH-deficient non-atypical leiomyomas lacked cellular packeting and distinct collagenous zones and showed chain-like or palisading nuclear arrangements, prominent staghorn-shaped blood vessels, oval nuclei with no or at most, mild atypia, small eosinophilic nucleoli, and a low mitotic rate (0-1/10 HPFs). The FH-deficient atypical leiomyomas had nuclear atypia often manifesting as multinucleation, prominent eosinophilic nucleoli, and mitotic activity up to 7/10 HPFs, with atypical mitoses seen in 32% of cases. However, similar histologic changes were seen in some non FH-deficient atypical leiomyomas. Loss-of-function FH-gene mutations including 5 whole gene deletions and 3 frameshift mutations were identified in 8 of 16 FH-deficient non-atypical leiomyomas using multiplex ligation-dependent probe amplification and Sanger sequencing, respectively. Follow-up data on patients with FH-deficient atypical uterine leiomyomas revealed 19 patients alive (median follow-up 27 years) and 5 patients dead. Deaths were 9-30 years after surgery at median age of 72 years; causes of death could not be determined. These results indicate that FH-deficient uterine leiomyomas occur with a high frequency among atypical leiomyomas and infrequently in non-atypical leiomyomas and are often histologically distinctive. They seem to have a low biologic potential and lack any significant association with leiomyosarcoma.
Keywords: leiomyoma, leiomyosarcoma, renal cell cancer, FH, immunohistochemistry
INTRODUCTION
Bi-allelic inactivation of the fumarase/fumarate hydratase gene that encodes tricarboxylic acid cycle enzyme fumarase (FH) characterizes tumors associated with hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome. In general, an underlying germline mutation combined with allelic imbalance at FH locus (1q43) in tumor tissue, could be identified in affected individuals, characteristic of classic Knudson's hypothesis of inactivation of tumor suppressor genes. This syndrome follows an autosomal-dominant pattern of inheritance. 1-4
Individuals affected by FH-gene germline mutations are prone to a triad of tumors including multiple pilar leiomyomas of skin, early onset uterine leiomyomas, and renal cell carcinomas often showing papillary high-grade histology. While most patients have leiomyomas, only 15-20% of them develop a kidney cancer.1 Recurrent histologic patterns have been reported in uterine leiomyomas associated with the syndrome. These include increased cellularity, focal nuclear atypia including multinucleation, cells with prominent nucleoli, and cytoplasmic eosinophilic inclusions. Non-atypical examples have also been reported recently. 5-8
Loss of FH protein in the renal carcinoma cells has been considered a diagnostic feature of HLRCC syndrome. 9-11 Immunohistochemical loss of FH has also been demonstrated in uterine and cutaneous leiomyomas. 7,8,12 However, more recently it has become apparent that FH loss could also be a somatic event in uterine leiomyomas and might not exclusively implicate the HLRCC syndrome. 8,13
In this study, we evaluated immunohistochemically a large number of tumors in the spectrum of uterine and other smooth muscle tumors for FH-loss to better define the occurrence of FH losses in various uterine and other SMTs and also examine the histological profile and prognosis of our patient cohort of FH-deficient uterine smooth muscle tumors.
MATERIALS AND METHODS
Study material
Histologically defined subcohorts of uterine smooth muscle tumors (a total of 1583 cases) were immunohistochemically studied for fumarase (FH) expression. These groups were defined according to Bell et al. 15 They included: 1) atypical leiomyomas (n = 182), all of which had significant nuclear atypia but fell short of leiomyosarcoma by low mitotic activity (<10/10 HPFs, without having coagulative tumor necrosis). 2) Cellular leiomyomas (n = 57) were highly cellular, variably mitotically active spindle cell smooth muscle tumors without significant nuclear atypia. 3) Epithelioid leiomyomas were smooth muscle tumors with distinctly epithelioid cytology/epithelioid component (n = 61). 4) Leiomyosarcomas (n = 226) included 159 tumors with significant nuclear atypia and mitotic rate ≥ 10/10 HPFs. Another group (n = 67) was ascertained as leiomyosarcoma based on significant nuclear atypia and presence coagulative tumor necrosis, despite mitotic activity < 10/10 HPFs. The above tumors were retrieved from the Armed Forces Institute of Pathology from 1970-1996. In addition, 1058 unselected, near consecutive uterine leiomyomas (excluding only totally necrotic or calcified tumors) were collected from European and North American sources in the period 2011-2015, examined grossly, and sampled for the study. These tumors contained only minor or focal atypia at the most. Selected categories of non-uterine smooth muscle tumors included retroperitoneal ER-positive leiomyoma in women (n = 40), angioleiomyoma (n = 33), glomus tumor (n = 23), and leiomyosarcoma of trunk or extremities (n = 30), and retroperitoneum (n = 31). Cutaneous leiomyomas were excluded from the study.
FH-deficient atypical leiomyomas were also histologically compared with non FH-deficient atypical leiomyomas. The presence of the following features was recorded: sharp vs. infiltrative microscopic border, fascicle formation and packeting of fascicles by collagenous bands, staghorn vessels, cytoplasmic eosinophilic hyaline globules, enlarged eosinophilic nucleoli, mitotic rate per 10 HPFs, and the presence of atypical mitoses.
Immunohistochemical methods
All smooth muscle tumors studied for FH expression were arranged in multitissue blocks each containing 30-65 cases. The sample size was estimated to exceed the size of a 0.6 mm2 punch array sample by a factor of 5-15.
The primary antibody was a polyclonal anti-FH antibody (GeneTex, # GTX109877, diluted 1:500). All immunostaining was performed with Leica Bond-Max automation. Application of primary antibody (30 min) was preceded by a high-pH epitope retrieval buffer (Leica ar9640 buffer) for 25 min. The primary antibody was incubated for 30 min, polymer for 15 min, and post-polymer for 15 minutes. The color was developed with diaminobenzidine, followed by a light hematoxylin counterstain.
FH immunohistochemistry was initially evaluated with 18 specimens from known hereditary leiomyomatosis and renal cancer (HLRCC) syndrome patients. These included 7 uterine leiomyomas and 11 renal cell cancers, all of which demonstrated immunohistochemical loss of FH expression tumor cells with retention of expression in non-neoplastic components such as blood vessel walls, lymphoid cells, epithelia, and normal smooth muscle. In the subsequent study cases, fumarase deficiency was defined as absence of staining in the tumor cells while observing positivity in the non-neoplastic elements.
Molecular genetic studies
Formalin-fixed paraffin-embedded tumor samples from non-atypical FH-deficient uterine leiomyomas (n = 16) were available for DNA extraction. Five to ten 5μm sections were deparaffinized with xylene, washed twice in ethanol, lyophilized, and incubated with proteinase K (10μg/μl, Roche Diagnostics, Indianapolis, IN) in Hirt-Buffer at 55°C for 24 hours. DNA was recovered using the Maxwell®16 robotic system and DNA IQ™ Casework Pro Kit (Promega, Madison, WI).
Multiplex Ligation-Dependent Probe Amplification (MLPA) assay developed by MRC-Holland (Amsterdam, Netherlands) was used to evaluate the integrity of the FH gene. The reagents including SALSA MLPA probe mix for assessing losses in the FH-gene, and MLPA protocol, were provided by the manufacturer. Data were analyzed with the Coffalyser.Net software available on line at www.mlpa.com. MLPA ratios < 0.7 defined the loss of a gene copy number (deletion), while ≥ 0.7 < 1.3 and ≥ 1.3 defined normal or increased number of copies, respectively.
FH-gene coding sequences were PCR-amplified with AmpliTaq Gold® DNA polymerase (Applied Biosystems by Life Technologies™, Austin, TX) using a standard three-temperature protocol with denaturing at 94°C, annealing at the primer specific temperature and extension at 72°C. The primer sequences, annealing temperatures and sizes of amplicons are listed in the supplementary data (Supplementary Table 1). The purified PCR products were Sanger sequenced by MacrogenUSA (Rockville, MD). The obtained sequences were analyzed following alignment with reference sequences NM_000143.3 and NP_000134.2 (www.ncbi.nml.nih.gov).
RESULTS
The observed losses of fumarase (FH) immunostaining in uterine and selected other smooth muscle tumors are summarized in Table 1. The frequency of FH-deficiency was highest in atypical uterine leiomyomas (37.3%), while it was similarly low, 1-2% in conventional non-atypical and cellular spindle cell leiomyomas. None of the leiomyosarcomas showed FH-deficiency. Images of FH loss in uterine smooth muscle tumors are shown in Fig. 1.
Table 1.
Occurrence of fumarase deficiency in uterine and non-uterine smooth muscle tumors.
| Fumarase loss/all cases | % of cases with fumarase loss | |
|---|---|---|
| Unselected leiomyoma, non-atypical* | 17/1058 | 1.6 |
| Cellular leiomyoma without atypia | 1/57 | 1.8 |
| Leiomyoma with epithelioid component | 1/61 | 1.6 |
| Atypical leiomyoma | 68/182 | 37.3 |
| Leiomyosarcoma | 0/226 | 0 |
| Total for uterine smooth muscle tumors | 86/1583 | |
| Non-uterine smooth muscle tumors | ||
| Retroperitoneal leiomyoma, ER-positive | 1/40 | 2.5% |
| Angioleiomyoma | 0/33 | 0 |
| Glomus tumor | 0/23 | 0 |
| Leiomyosarcoma, trunk and extremities | 0/30 | 0 |
| Leiomyosarcoma, retroperitoneum | 0/31 | 0 |
| Total for non-uterine smooth muscle tumors | 157 |
Includes one cellular leiomyoma also shown on line 2 for cellular leiomyomas.
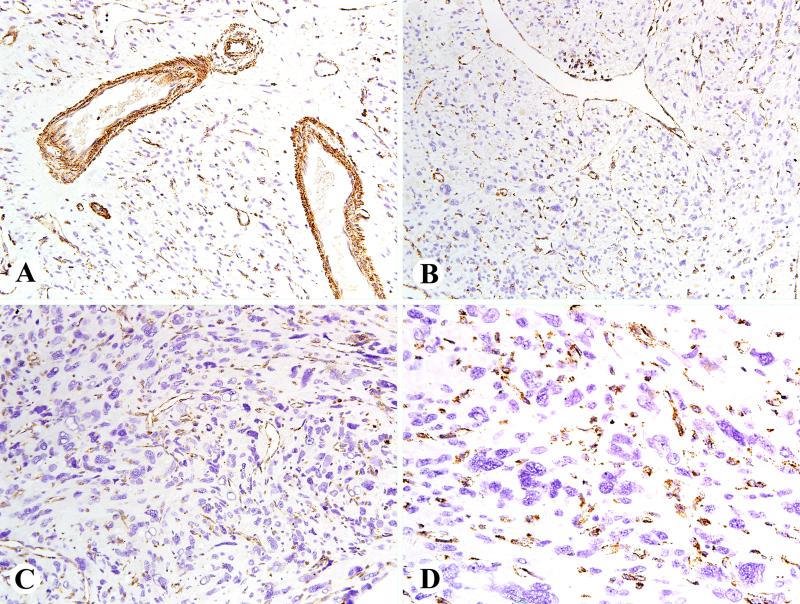
Fig. 1.
Examples of FH immunostains in FH deficient uterine leiomyomas. A. This tumor with mildly atypical features shows retained staining in capillary endothelia and vascular smooth muscle cells. B. This example shows retained FH in a staghorn vessels and clusters of lymphocytes in the middle near top. C. An atypical leiomyoma with pleomorphism shows a prominent microvascular capillary pattern highlighted by FH immunostain. D. Higher magnification of a pleomorphic leiomyoma with prominent FH-positive capillaries.
The only FH-deficient extrauterine smooth muscle tumor was a retroperitoneal ER-positive, non-atypical leiomyoma in a woman. FH loss was not detected in any soft tissue angioleiomyomas, glomus tumors, or extrauterine leiomyosarcomas.
Clinical features of FH-deficient uterine smooth muscle tumors
These tumors occurred in 86 women with age range of 20-79 years (median age, 38 years). In few cases, history was notable beyond common leiomyoma symptoms by rapidly growing myomas (two patients), and Meig's syndrome with 3 liters of ascites, and early hysterectomy in sister and mother at ages of 21 and 23 years, in one patients each. None of the patients had a history or family history of kidney cancer.
Hysterectomy was performed in 30 cases, myomectomies in 23 cases, while there was incomplete information in the remaining cases. Multiple leiomyomas were seen in 43 patients, while there was a solitary leiomyoma removed in 23 cases, and no data on multiplicity in 21 cases. Many myomectomy specimens were morcellated not allowing to specify tumor size or multiplicity.
Follow-up data were available for 24 patients with FH-deficient atypical leiomyomas. Nineteen patients were alive 19-35 years after the procedure (median, 27 years) with no further information, and 5 patients were dead at 9-30 years after surgery at ages of 56-97 years (median, 72 years) with no further information on tumor status or cause of death.
Gross pathology of FH-deficient uterine smooth muscle tumors
Commonly observed gross features included homogeneous, white tumor tissue with a soft and amorphous texture without nodularity or whirling patterns typically seen in uterine leiomyomas, giving resemblance to a fibroma-fibrothecoma. The tumor size varied from < 2 cm to 17 cm. Among cases with specified myoma size, there were 2 tumors < 2 cm, 23 tumors 2-5 cm, 40 tumors 5-10 cm, and 11 tumors > 10 cm.
Histopathology of FH-deficient non-atypical uterine leiomyomas (n = 17)
Microscopic features in cases ascertained from randomly selected non-atypical leiomyomas included common lack of bundling of tumor cells by collagenous bands and absent or poorly developed fascicular arrangements. Characteristic features were higher cellularity than that typically seen in uterine leiomyomas, and distinctive chain-like arrangements of tumor cells with oval nuclei set in a diffuse collagenous matrix (Fig. 2A, 2B). Most cases contained at least some cells with mildly enlarged eosinophilic nucleoli (13/17), eosinophilic cytoplasmic inclusions (13/17), and staghorn vessels (17/17). One tumor had an appearance of cellular spindle cell leiomyoma containing vague nuclear palisading (Fig 2C, 2D). Two cases each contained microcystic degeneration and infiltrative borders. Only mild if any nuclear atypia occurred so that none of these tumors were considered atypical leiomyomas. Mitotic activity was rare (0-1/10 HPFs) with no atypical mitotic figures present.
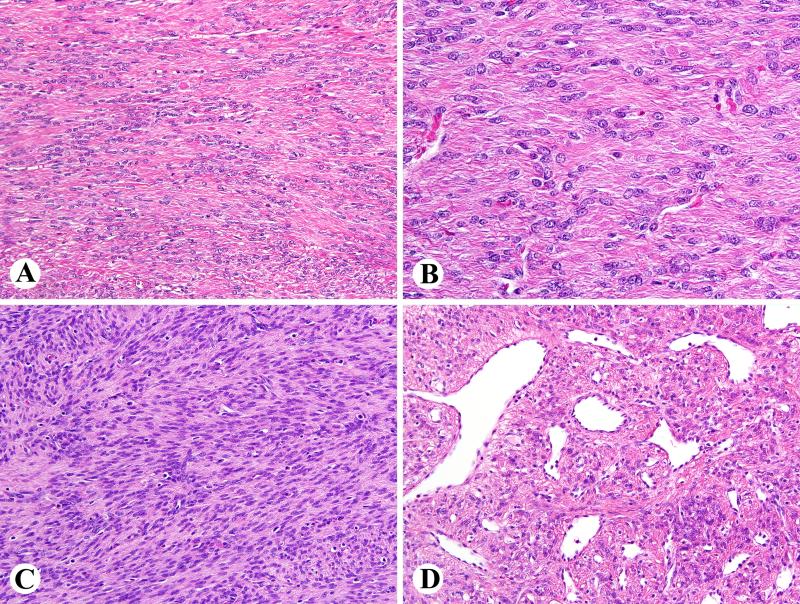
Fig. 2.
Examples of FH-deficient leiomyomas with little or no atypia. A. Ribbon-like arrangement of tumor cells is a common distinctive feature. B. Higher magnification also reveals eosinophilic cytoplasmic globules. C. A rare example of FH deficient leiomyoma shows cellular leiomyoma features with vague nuclear palisading. D. Prominent staghorn vessels are typically seen.
Histopathology of FH-deficient atypical uterine leiomyomas (n = 68)
Nuclear atypia was moderate in 62 cases, and severe with pleomorphism in 6 cases. At least some nuclei contained eosinophilic nucleoli in most cases, sometimes with perinucleolar clearing (65/68). Multinucleation was common, often with wreath-like nuclear arrangements and nuclear pseudoinclusions (Fig. 3 A-D). Mitotic activity varied 0-7 per 10 HPFs (median, 1/10 HPFs). Only 4 cases had mitotic rate > 5/10 HPFs (3 cases with 6 and 1 cases with 7 mitoses) with atypical mitotic figures seen in 23 cases.
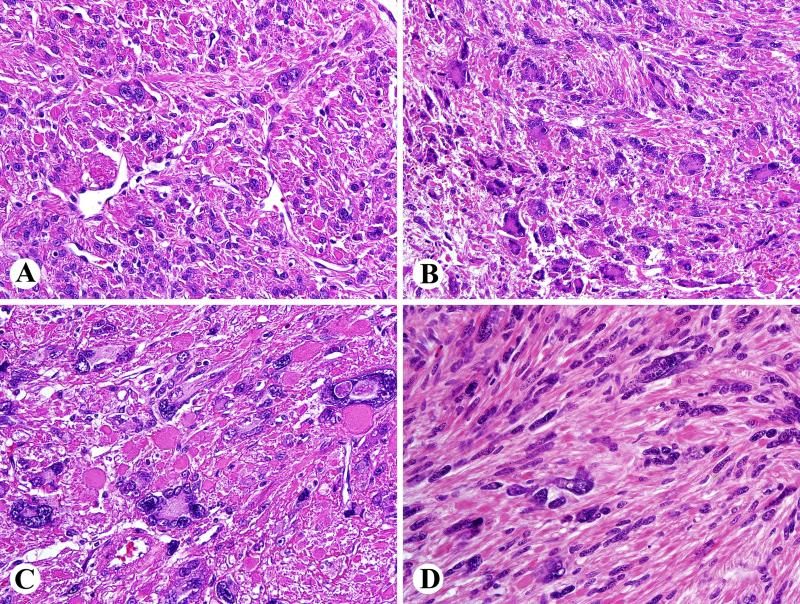
Fig. 3.
Histology of atypical FH-deficient leiomyomas. A. Focal atypia. B. Prominent nuclear pleomorphism and multinucleated cells with wreath-like nuclear arrangement. C. Some nuclei also contain pseudoinclusions and eosinophilic cytoplasmic globules are prominent. D. An example with a focal fascicular pattern and scattered pleomorphic nuclei.
FH-deficient atypical leiomyomas often had a diffuse non-fascicular architecture (35/68) and even more often had a poorly defined fascicular pattern. They had a less prominent tendency for chain-like nuclear arrangements typically seen in the non-atypical FH-deficient leiomyomas. Eosinophilic globular cytoplasmic inclusions (62/68) and staghorn vessels (56/68) were usually present, and sometimes there was an infiltrative border whenever this determination could be made (12/44).
Histopathology of non FH-deficient atypical leiomyomas (n = 114)
In comparison, non FH-deficient atypical leiomyomas less commonly lacked fascicular pattern (12/112) or contained eosinophilic cytoplasmic inclusions (21/110). Like the FH-deficient atypical leiomyomas, they also often although less consistently had staghorn vessels (45/113), prominent eosinophilic nucleoli (76/110) including macronuceoli and perinucleolar halo-like clearing in some cases. Border was sometimes infiltrative (26/81); not all parameters could be evaluated in all cases.
Other fumarase deficient smooth muscle tumors (n = 2)
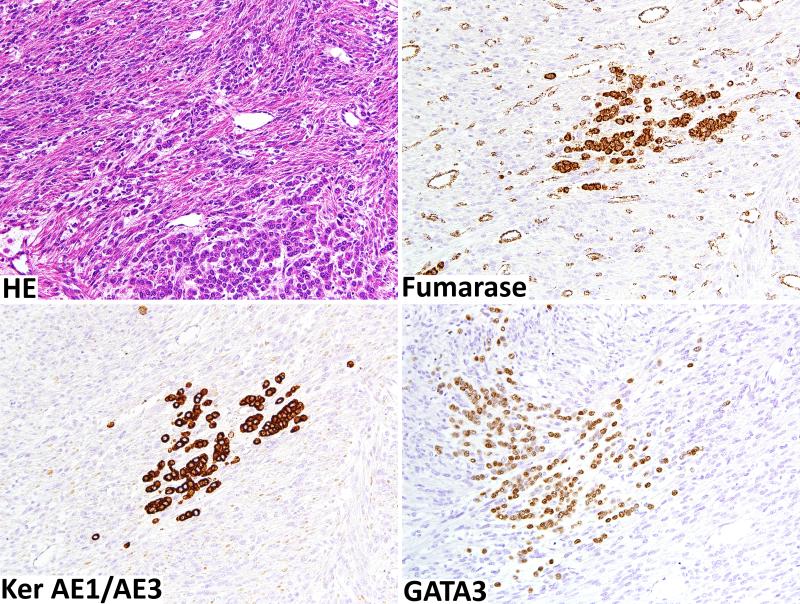
The only FH-deficient case in the epithelioid leiomyoma category was notable for an atypical keratin-positive epithelioid component comprising approximately 10% of the sample (Fig. 3). FH deficiency was seen in leiomyoma cells but not in the epithelioid cells. The epithelioid cells were positive for keratins AE1/AE3, GATA3, and focally for gross cystic disease fluid protein and were interpreted most likely as a metastatic carcinoma of the breast involving the leiomyoma.
The only fumarase-deficient retroperitoneal leiomyoma was histologically similar to non-atypical fumarase-deficient leiomyomas showing poorly defined fascicular morphology with nuclear palisading and chain-like nuclear arrangements.
Immunohistochemistry of FH-deficient uterine leiomyomas
Immunohistochemically FH-deficient leiomyomas were positive for smooth muscle actin, heavy caldesmon and desmin, often showing cytoplasmic inclusions highlighted with the latter immunostain. Estrogen receptor-positivity was typically seen in most cases, with occasional cases showing focally restricted pattern. All cases were negative for CD10. In comparison with non FH-deficient conventional leiomyomas, the patterns of immunostains were similar, with the exception of FH-loss.
Molecular genetic findings of FH–deficient uterine leiomyomas
Sixteen cases with available DNA were screened for FH alterations using MLPA and Sanger sequencing. All together 8 (50%) tumors were shown to have FH genetic alteration. There were 5 whole gene deletions identified by MLPA and 3 frameshift mutations predicted to result in STOP codon formation detected by Sanger sequencing. The latter were in exon 4, c.567delA (p.E168Dfs*34) and exon 7, c.944_997delAA (p.K311Ifs*1) and c.1004_1005insC (p.A314Afs*5). Exon 4 mutation was previously listed by Database of Single Nucleotide Polymorphisms at National Center for Biotechnology Information as rs. 776190273. Successful Sanger sequencing experiments are detailed in Table 2 in the supplementary data. Also, representative examples of MLPA and Sanger sequencing are provided in Figure 1 and 2 in the supplementary data.
DISCUSSION
Germline mutations in the fumarase/fumarate hydratase (FH)-gene characterize hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome, which predisposes carriers to cutaneous and uterine smooth muscle tumors and kidney cancer. The syndrome-associated tumors are FH-deficient resulting from combination of germline and somatic loss-of-function mutations. These mutations vary from single nucleotide substitutions to whole gene deletions. The most commonly reported are mutations followed by frameshift, splice site mutations, and gene deletions. 13,15,16 As a consequence, fumarate and succinate accumulate in the tumor cells, and this metabolic aberration is believed to be an oncogenic factor, for example by stabilizing hypoxia inducible factors causing their overexpression. 1-4
Earlier studies evaluated the loss of FH indirectly by studying accumulation of succinylated proteins (succinylcysteine adducts). 9-11 Recently, antibodies to FH have become available opening a practical way to detect this abnormality. 7.8,12 This is analogous to detection of succinate dehydrogenase deficiency in certain tumors, especially subsets of paragangliomas, gastrointestinal stromal tumors, and renal cancers. 18
In this study, we evaluated 1583 uterine and 157 non-uterine smooth muscle tumors for FH expression using a specific antibody in order to identify FH-deficient tumors and subsequently study histopathology, FH gene mutations, and clinical correlation in such cases. We found FH-deficiency with a high frequency in atypical leiomyomas (37%), and infrequent occurrence (0-2%) in non-atypical leiomyomas and leiomyosarcomas. One retroperitoneal ER-positive leiomyoma in a woman (2.5% of all retroperitoneal leiomyomas) was also FH-deficient. As these extrauterine tumors are in many ways comparable with uterine leiomyomas, occurrence of FH deficiency also in this group is not very surprising.
In this study, 8 of 16 immunohistochemically FH-deficient tumors showed FH gene alterations. In 5 cases, deletion of the whole gene was detected by MLPA, and in 3 cases, frame shift mutations were identified by Sanger sequencing. The latter occurred in exon 4 and 7, typical mutation “hot spots” in FH-deficient tumors. 13,15,16 Our frequency of FH mutations (50%) is similar to the one found in a recent study of immunohistochemically screened FH-deficient leiomyomas (6/10). This study also used massive parallel sequencing (MPS) to supplement Sanger sequencing data but noted discordant results: additional mutations found but considered not definitively confirmed in the absence of ability to reproduce data by Sanger sequencing. 8 However, another study found all 5 FH-deficient leiomyomas to carry FH mutations. 7 Complexity of FH mutations is a major challenge in comprehensive detection requiring use of multiple techniques and access to high quality DNA not always possible in archival specimens.
Although FH-deficiency has been traditionally associated with the HLRCC-syndrome, none of our patients had evidence for renal cancer, and only one patient had history of familial early onset uterine leiomyomas suggestive for HLRCC. Similarly, a recent study identified 6 FH-mutants among 10 FH-deficient uterine leiomyomas with none of the patients having a history of HLRCC syndrome. In addition, MPS data and Sanger sequencing of normal tissue suggested somatic nature of mutations in that study. 8 An earlier study searching for somatic FH mutations in uterine leiomyomas of non-syndromic leiomyoma patients without immunohisto-chemical, but instead allelic loss screening, found such mutations in 2 of 166 tumors of 51 patients, an 1.2% frequency by leiomyoma count and 3.9% frequency by patient count. 13 The frequency obtained in the latter study cannot be compared with blind immunohistochemical screening studies. Another investigation failed to identify FH mutations in 7 sporadic leiomyomas with allele imbalance at 1q42-q43. 17 The mutation screening strategy employed in the latter study could have contributed to the negative results.
Based on our study and previously reported studies, 8,13,17 FH-deficient sporadic leiomyomas may carry mutations similar to the ones documented in HLRCC related tumors, and these sporadic cases might contribute to the majority of FH-deficient leiomyomas found by screening studies, such as ours. Prospective clinical and combined germline and somatic genetic studies are needed to definitively determine how often FH-deficient leiomyomas are related to the HLRCC syndrome. Apparent common somatic nature of FH-deficiency in uterine leiomyomas would lessen the likelihood of association with HLRCC syndrome and diminish the impetus for compulsory germline studies and genetic counseling, unless there are clinical features support this, such as familial occurrence of early onset leiomyomas.
One could also consider epigenetic silencing as a potential mechanism for FH-inactivation as known for SDH-deficiency in 40% of SDH-deficient GISTs. 19 However, one study found no epigenetic silencing for FH gene in FH-deficient tumors. 20
Atypical FH-deficient uterine leiomyomas had a set of histologic features including nuclear atypia, at least focally prominent eosinophilic nucleoli, staghorn vessels, prominent cytoplasmic eosinophilic inclusions, and common lack of fascicular morphology. Some of these features have been reported in previous studies on HLRCC syndrome-associated or in some cases, FH-deficient sporadic uterine leiomyomas. 5-7 However, when comparing with non FH-deficient leiomyomas we observed similar features in non-FH-deficient atypical leiomyomas so that none of these features seem to be specific. Especially, prominent eosinophilic nucleoli, sometimes with perinucleolar halo-like clearing occurred in both FH-deficient and non-deficient leiomyomas, so that this feature is not specific for FH-deficiency. Furthermore, perinucleolar clearing is difficult to define and its presence may depend on histological processing.
Illustrations of series of atypical uterine leiomyomas also highlight features similar to those seen in FH-deficient uterine leiomyomas also indicating that FH-deficient tumors form a large subset of those atypical leiomyomas. 21,22 A recent study divided atypical uterine leiomyomas in types 1 and 2 suggesting that type 1 (seen in 53% of cases) corresponds to FH-deficient tumors because of morphologic similarity to previously reported atypical FH-deficient leiomyomas. However, FH expression was not studied in this investigation. 23
The non-atypical FH-deficient uterine leiomyomas also had a characteristic histology lacking bundling of smooth muscle cells around collagenous bands and a fascicular architecture. Additional distinct features are chain-like arrangement of nuclei (and tumors cells), and staghorn vessels. Based on our observations, FH-deficient tumors may also be tentatively identified by gross features as these tumors are often soft and lack whirling appearance typical of common uterine leiomyomas forming amorphous masses resembling fibroma-fibrothecoma tumors.
The biologic potential of FH-deficient atypical uterine leiomyomas appears low based on our direct and indirect evidence. There was no significant mortality in the patient cohort with follow-up, and most deaths were at old age and thus likely unrelated to the previous smooth muscle tumor. Also, there were no FH-deficient cases among the leiomyosarcomas neither defined by mitotic rate > 10/10 HPFs nor necrosis and lower mitotic rates indicating that FH-deficiency is rarely if ever associated with leiomyosarcoma. Therefore, FH deficiency could be a useful feature supporting a low biologic potential of an atypical uterine smooth muscle tumor. Histologic features such as atypia, atypical mitoses, and infiltrative border do not seem to have malignant implications in the context of FH-deficient uterine smooth muscle tumors.
In conclusion, we examined 1583 uterine smooth muscle tumors immunohistochemically for FH loss finding 1.6-1.8% occurrence in unselected non-atypical leiomyomas and 37% frequency in atypical leiomyomas. FH-deficient tumors were often associated with FH-mutations. Both follow-up data and inability to find FH deficient tumors among leiomyosarcomas suggest low biologic potential for FH-deficient uterine smooth muscle tumors. Although many FH-mutations in these leiomyomas are likely somatic, additional clinical and genetic studies are needed to determine how often FH-deficient leiomyomas are related to FH-germline mutations and the HLRCC syndrome.
Supplementary Material
Fig. 4.
An unusual FH deficient leiomyoma with epithelioid infiltrate that probably represent breast cancer metastatic to leiomyoma. Tumor shows loss of FH, but FH expression is retained in the carcinoma cells and tumor capillaries. Carcinoma component is positive for keratins and GATA3 suggesting the possibility of breast origin.
REFERENCES
- 1.Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014 Jun 20;7:253–60. doi: 10.2147/IJNRD.S42097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam NA, Rowan AJ, Wortham NC, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–52. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 4.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–92. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg K, Tickoo SK, Soslow RA, et al. Morphologic features of uterine leiomyomas associated with hereditary leiomyomatosis and renal cell carcinoma syndrome: a case report. Am J Surg Pathol. 2011;35:1235–7. doi: 10.1097/PAS.0b013e318223ca01. [DOI] [PubMed] [Google Scholar]
- 6.Sanz-Ortega J, Vocke C, Stratton P, et al. Morphologic and molecular characteristics of uterine leiomyomas in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Am J Surg Pathol. 2013;37:74–80. doi: 10.1097/PAS.0b013e31825ec16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph NM, Solomon DA, Frizzell N, et al. Morphology and Immunohistochemistry for 2SC and FH Aid in Detection of Fumarate Hydratase Gene Aberrations in Uterine Leiomyomas From Young Patients. Am J Surg Pathol. 2015;39:1529–39. doi: 10.1097/PAS.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 8.Harrison WJ, Andrici J, Maclean F, et al. Fumarate Hydratase-deficient Uterine Leiomyomas Occur in Both the Syndromic and Sporadic Settings. Am J Surg Pathol. 2016;40:599–607. doi: 10.1097/PAS.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardella C, El-Bahrawy M, Frizzell N, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. doi: 10.1002/path.2932. [DOI] [PubMed] [Google Scholar]
- 10.Reyes C, Karamurzin Y, Frizzell N, et al. Uterine smooth muscle tumors with features suggesting fumarate hydratase aberration: detailed morphologic analysis and correlation with S-(2-succino)-cysteine immunohistochemistry. Mod Pathol. 2014;27:1020–7. doi: 10.1038/modpathol.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YB, Brannon AR, Toubaji A, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. 2014;38:627–37. doi: 10.1097/PAS.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llamas-Velasco M1, Requena L, Kutzner H, et al. Fumarate hydratase immunohistochemical staining may help to identify patients with multiple cutaneous and uterine leiomyomatosis (MCUL) and hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome. J Cutan Pathol. 2014;41:859–865. doi: 10.1111/cup.12396. [DOI] [PubMed] [Google Scholar]
- 13.Lehtonen R, Kiuru M, Vanharanta S, et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164:17–22. doi: 10.1016/S0002-9440(10)63091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker KT, Bevan S, Wang R, et al. Low frequency of somatic mutations in the FH/multiple cutaneous leiomyomatosis gene in sporadic leiomyosarcomas and uterine leiomyomas. Br J Cancer. 2002;87:446–8. doi: 10.1038/sj.bjc.6600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 16.Gill AJ. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology. 2012;44:285–92. doi: 10.1097/PAT.0b013e3283539932. [DOI] [PubMed] [Google Scholar]
- 17.Alam NA, Olpin S, Leigh IM. Fumarate hydratase mutations and predisposition to cutaneous leiomyomas, uterine leiomyomas and renal cancer. Br J Dermatol. 2005 Jul;153(1):11–7. doi: 10.1111/j.1365-2133.2005.06678.x. [DOI] [PubMed] [Google Scholar]
- 18.Kiuru M, Lehtonen R, Arola J, et al. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62:4554–7. [PubMed] [Google Scholar]
- 19.Killian JK, Miettinen M, Walker RL, et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med. 2014;6(268):268ra177. doi: 10.1126/scitranslmed.3009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker KT, Spendlove HE, Banu NS, et al. No evidence for epigenetic inactivation of fumarate hydratase in leiomyomas and leiomyosarcomas. Cancer Lett. 2006;235:136–40. doi: 10.1016/j.canlet.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Ly A, Mills AM, McKenney JK, et al. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37:643–9. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 22.Croce S, Young RH, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014;38:1330–9. doi: 10.1097/PAS.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 23.Ubago JM, Zhang Q, Kim JJ, et al. Two Subtypes of Atypical Leiomyoma: Clinical, Histologic, and Molecular Analysis. Am J Surg Pathol. 2016 Mar 24; doi: 10.1097/PAS.0000000000000646. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.