Abstract
The class A β-lactamase GES-22 has been identified in Acinetobacter baumannii isolates in Turkey, and subsequently shown to differ from GES-11 by a single substitution (M169L). Because M169 is part of the omega loop, a structure that is known to have major effects on substrate selectivity in class A β-lactamases, we expressed, purified and kinetically characterized this novel variant. Our results show that compared to GES-116XHis, GES-226XHis displays more efficient hydrolysis of penicillins, and aztreonam, but a loss of efficiency against ceftazidime. Additionally, the M169L substitution confers on GES-22 more efficient hydrolysis of the mechanistic inhibitors clavulanic acid and sulbactam. These effects are highly similar to other mutations at the homologous position in other class A β-lactamases, suggesting that this methionine plays a key structural role in aligning active site residues and in substrate selectivity across the class.
Keywords: GES-22, M169L, Acinetobacter baumannii
Introduction
β-lactamases are bacterial enzymes that hydrolyze β-lactam antibiotics, rendering these compounds ineffective.1 These enzymes are grouped into four classes A, B, C and D, with class A, C and D enzymes making use of a catalytic serine to hydrolyze the β-lactam ring, and class B enzymes using a metal cofactor (Zn). Penicillins and cephalosporins have been used to treat bacterial infections, but their efficacy has been greatly diminished by resistance mechanisms. When these antibiotics began to be inactivated by class A β-lactamases, several generations of cephalosporins were developed for use in clinical settings. Inappropriate use of these drugs resulted in the extended spectrum β-lactamases (ESBL).2 ESBLs confer resistance to penicillins, first-, second-, and third-generation cephalosporins, and aztreonam (but not the cephamycins or carbapenems) and they are inhibited by β-lactamase inhibitors. ESBL type β-lactamases are found most commonly in class A, though there are a growing number of examples in class C and D).3 Most class A ESBLs are found in the TEM, SHV and CTX-M families,1 though variants in PER, VEB, TLA-1, GES/IBC, SFO-1, BES-1 have also been reported).4
A. baumannii is a Gram-negative, opportunistic pathogen that causes a range of infections, including bacteraemia, pneumonia, meningitis, urinary tract infections and wound infections.5 Extended-spectrum β-lactamases (ESBLs) from the Ambler class A group including VEB-1, PER-1, PER-2, TEM-92, TEM-116, CTXM-2, CTX-M-43, GES- 11, -12, -14, -22 and 24 have been found in A. baumannii.6
The origin of GES type β-lactamases remains unknown, but they generally are found in class 1 integron gene cassettes on both chromosomes and plasmids.7 The first GES β-lactamase, GES-1, was described in France in 2000.8 This enzyme confers resistance to penicillins, narrow- and expanded-spectrum cephalosporins, and ceftazidime9 and 26 variants have been reported to date (http://lahey.org/studies/other.asp). GES-2 showed better hydrolytic efficiency against imipenem than GES-1 and its activity was less inhibited by clavulanic acid, tazobactam and imipenem than GES-1. There is a single amino acid change between GES-1 and GES-2 (G170N) at position 170 in the omega loop of Ambler class A enzymes.8 GES-3 has two amino acid substitutions compared to GES-1 (E104K, M62T).10 Substitution at the G243 residue in GES-9 and GES-11 confers increased activity against aztreonam and ceftazidime.9 GES-4, GES-5, GES-6, and GES-14 possess a substitution at the G170 residue (N or S) that leads to carbapenemase activity.11 GES-12 differs from GES-11 by a single amino acid (T237A), which causes 2-fold higher efficiency against aztreonam and ceftazidime. GES-14, with two amino acid substitutions (G243A and G170S), can hydrolyze both oxyimino-cephalosporins and carbapenems.9
GES-type β-lactamases have been found in Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Aeromonas media, Aeromonas veronii, Klebsiella oxytoca, Acinetobacter baumannii, Serratia marcescens and Enterobacter cloacae. More recently, GES-22 was found in an Acinetobacter baumannii isolate from Turkey.12 GES-22 differs from GES-11 by one amino acid substitution M169L, the only known variant at this position. GES-22 and its parent GES-11 have been reported in previous studies from Turkey.5, 13 In another study from Kuwait, GES-type ESBLs were found in Acinetobacter baumannii.14 Together, these studies show that the Middle East and Turkey could be a reservoir for Acinetobacter baumannii producing GES-type ESBLs.5 Sequencing of the integron carrying blaGES-11 and blaGES-22 in isolates from Turkey show that they possess the same genetic structure as GES-11 from France. This suggests that GES-22 evolved from GES-11 under conditions of antibiotic stress with one amino acid change.12
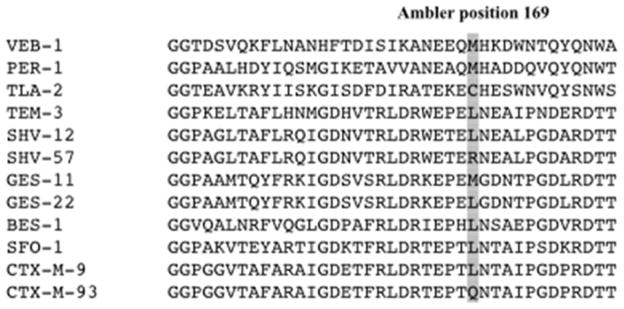
Position 169 is located in the omega loop of class A β-lactamases including members of the GES-type β-lactamases (Figure 1). In a previous study of the SHV β-lactamase subfamily, it was shown that the substitution R169L in SHV-57 induced a conformational change in N170. This mutation that causes resistance to ceftazidime, but not to cefazolin is inhibited with clavulanic acid.15 Also, increased ceftazidime hydrolysis caused by mutations at position 169 have been described in other studies. 16–18 Position 169 is most typically occupied by methionine or leucine in class A β-lactamases, though some exceptions exist (TLA-2, SHV-57, CTX-M-93) (Figure 2). The neighboring residue at position 170 is important in imipenem hydrolysis in GES-type β-lactamases.11, 19 The residue at position 170 affects the conformation of the active site of the GES-type β-lactamases through interactions with the E166 side chain. The presence of a hydrogenbonding interaction between S170 and E166 has been found to be vitally important for carbapenemase activity.19 Given the importance of M169 and other proximal residues, we wished to determine whether position 169 in GES-22 altered the activity of this β-lactamase against pencillins, cephalosporins and carbapenems. Towards this aim, we purified the enzyme and used steadystate kinetic analysis to measure its activity against a wide variety of these substrates.
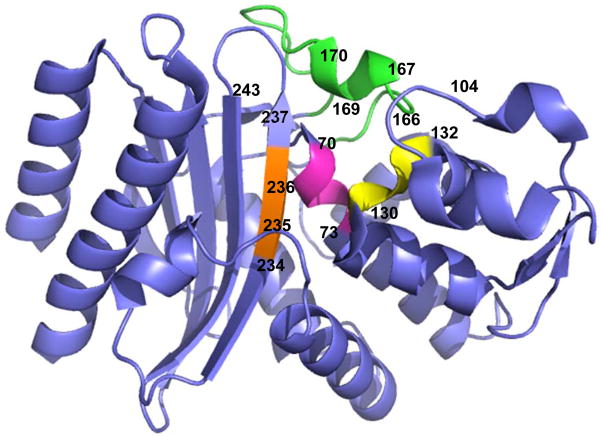
Figure 1. Overall structure of GES-1.
The conserved element 1 (S70-K73) is in magenta, conserved element number 2 (S130-N132) is in yellow, conserved element number 3 (K234-G236) is in orange and the omega loop (residues E166, P167, M169 and G170) is in green. G243, T237, E104 and G170 are important residues in substrate selectivity of GES-type β-lactamases. (PDB: 3V3R)
Figure 2. Class A β-lactamase sequence alignment.
Various class A β-lactamases were aligned using ClustalW, and the portion of the proteins surrounding M169 is shown.
Materials and Methods
Plasmids
The genes coding GES-11 and GES-22 in Acinetobacter baumannii isolates were amplified by using PCR.5,20 These coding regions were cloned into the pET28a expression vector using EcoRI and XhoI restriction sites. The signal peptides of GES-22 and GES-11 were determined using SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/). The DNA sequences of GES-11 and GES-22 without the signal sequence (ie. the first 18 residues) were amplified using iProof™ High-Fidelity DNA Polymerase (Bio-Rad, USA) and primers with restriction sites for EcoRI and XhoI (GES_EcoRI_F: 5′-GAATTCTCGGAAAAATTAACC TTCAAGACC-3′ and GES _XhoI_R: 5′-CTCGAGCTATTTGTCCGTGCTCAGGATGA-3′). After restriction, the PCR amplicons were introduced into pET28a with T4 DNA ligase. Sequencing of the coding regions was carried out by Macrogen Inc., Seoul, Korea. Sequencing results were analyzed using the alignment search tool BLAST (http://www.ncbi.nlm.nih.gov/BLAST) and the multiple sequence alignment program CLUSTALW (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Expression and Purification of GES-22 and GES-11
pET28a-GES-22 and pET28a-GES-11 were transformed into E. coli BL21 (DE3) for overexpression. E. coli cells harboring pET28a-GES-22 and pET28a-GES-11 vector were grown to an optical density at 600 nm of approximately 0.6 in LB medium containing kanamycin (25 μg/mL) at 37 °C, and over-expression was induced by the addition of 0.1 mM isopropyl β-D-thiogalactopyranoside (IPTG) overnight at 18 °C. The cells were harvested by centrifugation and lysed by sonication. After sonication, the lysate was centrifuged at 15000 rpm in a Sorvall SS-34 rotor for 30 min at 4°C. The clarified supernatants were loaded onto a HisPur™ Cobalt Resin (Thermo Scientific, USA) equilibrated with 50 mM Tris-HCl, 0.5 M NaCl, 10 mM imidazole, pH 7.4. The column was then washed extensively with column equilibration buffer, and then washed with 50 mM Tris-HCl, 0.5 M NaCl, 25 mM imidazole, pH 7.4. The purified β-lactamase protein was eluted with 50 mM Tris-HCl, 0.5 M NaCl, 250 mM imidazole, pH 7.4. The GES-22 and GES-11 containing fractions were pooled and dialyzed against 50 mM Tris-HCl, 0.2 M NaCl, pH 7.4 overnight. The protein concentrations were determined by measuring absorbance at 280 nm, and its purity was shown to be >95% by SDS-PAGE. Protein samples were stored at −80°C.
Determinations of Kinetic Parameters
The kinetic parameters of the GES-22 and GES-11 were determined with several β-lactamase substrates by UV spectroscopy (Cary 60, Agilent, USA). Hydrolysis of substrates was carried out in 100 mM sodium phosphate buffer pH 7.0 at room temperature. Initial velocities were obtained from the change in absorbance per second and converted into velocity units of μM/s using the Beer-Lambert Law equation, as described previously.21 Changes in absorbance as a function of time were converted to velocity (μM/s) using the following Δε values (M−1 cm−1): ampicillin, −900 (λ = 235 nm); penicillin G, −560 (λ = 240 nm); imipenem, −9000 (λ = 300 nm); cefotaxime, −7500 (λ = 260 nm); ceftriaxone, −9400 (λ = 260 nm); ceftazidime, −8660 (λ = 260 nm); cefoxitin, −7700 (λ = 260 nm); aztreonam, −700 (λ = 320 nm); nitrocefin, +15000 (λ = 482 nm); clavulanic acid, −1630 (λ = 235 nm); sulbactam, +1784 (λ = 236 nm). All experiments were carried out in triplicate. Microsoft Excel+SDAS was used to fit the data to the Michaelis–Menten equation and to determine Km and kcat values. For Km values that were too low or too high to be measured by UV spectroscopy, Ks values were determined by competition with a nitrocefin reporter substrate and the Cheng-Prusoff equation.22 For very high Km values, kcat/Km values were determined directly under conditions in which [S] ≪ Km, and kcat was determined indirectly from kcat/Km and Km
Results
In order to examine the effects of the M169L substitution, we expressed and purified both GES-11 M169 and GES-22 M169L. Constructs for each gene were set up to produce a 6X histidine tag followed by mature proteins (ie. with no export sequences) starting with position 19 (SEKL…). Expression and purification of GES-11 and GES-22 using a cobalt-affinity column yielded 7–11 mg of protein per liter of culture with a purity estimated to be > 95% by SDS-PAGE. We have not determined the effect of the 6X-histidine tag on the kinetics, so we limit our analysis to comparisons between the similarly-tagged GES-11 and GES-22.
Purified GES-11 and GES-22 were used to determine kinetic parameters for a variety of β-lactam substrates via UV-visible spectroscopy (Table 1). The substitution at position 169 in GES- 22 led to an overall increase in the efficiency of hydrolysis for two substrates as reflected by in- creases in kcat/Km ratios of 43–240%. Most notably, the kcat for aztreonam increased from 24 s−1 to 120 s−1, while its Km increased from 1500 μM to 3200 μM. Gains in turnover (kcat) between GES-11 and GES-22 were observed for the two penicillin substrates tested, penicillin G (3.7 s−1 to 34 s−1) and ampicillin (21 s−1 to 71 s−1) (Table 1). As with aztreonam, the gains in kcat for the two penicillins were offset by higher Km values, thereby moderating the overall gain of efficiency observed in the presence of the M169L substitution. A different trend was observed for the oxyimino-cephalosporin ceftazidime, for which the M169L substitution led to a lower kcat (40 s−1 to 17 s−1) and no significant change in Km (1400 μM to 1350 μM).
Table 1.
[Kinetic data for GES-116XHis and GES-226XHis for normal β-lactam substrates]
| Km or KS (μM) | kcat (s−1) | kcat/Km (μM−1 s−1) | |
|---|---|---|---|
|
|
|||
| GES-116XHis | |||
| Ampicillin | 51±3 | 21±0.3 | 0.41±0.02 |
| Penicillin G | <15 | 3.7±0.2 | >0.25 |
| Ceftazidime | 1400±250a | 40±7 | 0.029±0.001 |
| Cefotaxime | 2800±500a | 340±60 | 0.12±0.01 |
| Cefoxitin | 20±3 | 0.033±0.002 | 0.0017±0.0003 |
| Ceftriaxone | 1800±300a | 260±40 | 0.14±0.01 |
| Aztreonam | 1500±200a | 24±4 | 0.016±0.001 |
| ımipenem | 0.13±0.01a | 0.0092±0.0004 | 0.070±0.006 |
| Nitrocefin | 170±20 | 120±5 | 0.68±0.10 |
| GES-226XHis | |||
| Ampicillin | 121±10 | 71±2 | 0.59±0.05 |
| Penicillin G | 52±8 | 34±1 | 0.65±0.10 |
| Ceftazidime | 1350±210a | 17±3 | 0.013±0.001 |
| Cefotaxime | 2050±190a | 320±30 | 0.16±0.01 |
| Cefoxitin | 19±6 | 0.014±0.001 | 0.00072±0.00022 |
| Ceftriaxone | 1550±220a | 340±50 | 0.22±0.01 |
| Aztreonam | 3200±280a | 120±10 | 0.039±0.001 |
| ımipenem | 0.11±0.02a | 0.013±0.001 | 0.11±0.02 |
| Nitrocefin | 270±20 | 320±10 | 1.2±0.1 |
KS values determined by competition kinetics with ampicillin and nitrocefin as reporter substrat
Next, GES-11 and GES-22 were tested for their ability to hydrolyze the mechanistic inhibitors sulbactam, clavulanic acid and tazobactam. We observed a 10-fold decrease in Km for clavulanic acid when the M169L substitution was added to GES-11 to make GES-22 (41 μM to 4.8 μM) (Table 2). This apparent increase in binding affinity was offset by a modest decrease in kcat, but the overall kcat/Km ratio still increased ~3-fold (0.011 μM−1·s−1 to 0.032 μM−1·s−1). The change in kinetic parameters observed between GES-11 and GES-22 for sulbactam were reflected in a modest increase in both kcat and Km and an approximately 50% reduction in kcat/Km. No turnover of tazobactam was observed by either GES-11 or GES-22, and its binding affinity (KS determined by competition assay) was unaffected by the mutation.
Table 2.
[Kinetic data for GES-116XHis and GES-226XHis for inhibitors]
| KS (μM) | Km (μM) | kcat (s−1) | kcat/Km (μM−1 s−1) | |
|---|---|---|---|---|
|
|
||||
| GES-116XHis | ||||
| Tazobactam | 1.6±0.3 | - | - | - |
| Clavulanate | 9.3±1.4 | 41±4 | 0.42±0.01 | 0.011±0.0001 |
| Sulbactam | 6.1±1.6 | 23±7 | 0.33±0.01 | 0.014±0.004 |
| GES-226XHis | ||||
| Tazobactam | 1.2±0.14 | - | - | - |
| Clavulanate | 0.73±0.08 | 4.8±1.4 | 0.15±0.01 | 0.032±0.0095 |
| Sulbactam | 10±2 | 110±61 | 0.83±0.10 | 0.0078±0.0045 |
Representatives of two other substrate classes tested, cefoxitin and imipenem, showed minor changes in parameters between GES-11 and GES-22, but the activities were so close to the limit of detection for these substrates that the trends are not deemed reliable.
Discussion
Our analysis of the effect of the M169L substitution on the hydrolytic activity of GES-type β-lactamases towards a wide variety of β-lactam substrates reveals interesting trends. The presence of the leucine at this position leads to an apparent increase in catalytic turnover, but a loss of affinity, for penicillin G and aztreonam. Conversely, the same substitution leads to a decrease in the rate of catalytic turnover towards ceftazidime. While this pattern may appear somewhat scattered at first, it is highly notable that very similar trends have been observed when the homologous positions in other class A β-lactamases have been substituted. The presence of a leucine at this position in CTX-M-27 (compared to a glutamine in CTX-M-93) is also associated with higher activity against aztreonam and several penicillins, but lower activity against ceftazidime.16 SHV-1, which also has a leucine at positon 169, displays a similar pattern of activity: lower activity against ceftazidime, but higher activity against penicillins when compared to SHV-57 (with an arginine at position 169).15 A C169L substitution in TLA-2 shows increased activity against penicillins.23 The presence of a leucine in OXY-2-2 lowers ceftazidime resistance but increases resistance against penicillins and aztreonam compared to OXY-2-15, which contains a deletion of two residues (168 and 169).24 In another striking similarity, the presence of a leucine in SHV-1, TLA-2 C169L and OXY-2-2 consistently leads to higher susceptibility to clavulanic acid compared to their counterparts that have a substitution or deletion at that position. Our results, along with these other studies, thus confirm that the identity of the residue at this key omega loop position displays distinct effects on ceftazidime compared to other cephalosporins across the class A β-lactamase family. Additionally, common effects can be observed in the modulation of activity toward penicillins, clavulanic acid and sulbactam.
The location of M169 in the structure of GES-11 makes it unsurprising that substitutions there affect cephalosporin binding and turnover. M169 is part of the omega loop, which is known to affect substrate selection in Classes A, C and D β-lactamases.25 Alignment of GES-11 with the class A β-lactamase Toho-1 bound to cefotaxime shows that the bulky thiazolidine ring of that drug would be expected to make very close contact with the β-carbon (3.3 Å) and the carbonyl oxygen (3.0 Å) of P167, a residue that sits on the opposite side of a short α helix from M169 (Figure 3).26 Additionally, M169 is at the center of a hydrophobic core, making van der Waals contacts with the side-chain of F72, M68, the γ-carbon of E166 and the β-carbon of D176 (Figure 4). There are several plausible mechanisms by which the substitution of a leucine for a methionine at this position might affect substrate binding and/or catalytic turnover. First, the close proximity of position 169 to the general base E166 means that a substitution of the former residue could lead to subtle changes in the orientation of the carboxylate of the latter, modulating its ability to activate the deacylating water. If the substitution increased the general base activity of E166, for instance, it could be responsible the ~ 5-fold increase in kcat observed for the substrate aztreonam or ~9-fold increase for penicillin G. Second, the addition of a γ-branched carbon on leucine might disrupt the tight packing observed in the hydrophobic core. Alternatively, the substitution of leucine for methionine shortens the side-chain, and may lead to similar structural rearrangements. Even small changes in packing could be transmitted through the α-helix to residues that would be expected to directly contact the substrate (e.g. P167). If such structural rearrangements led to less room for substrate binding, we might expect the substitution to lead to higher Km values (for instance, the 2–3 fold increases we observe for penicillin substrates, and aztreonam).
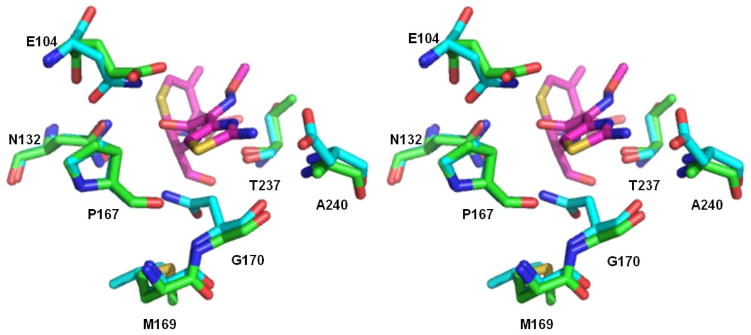
Figure 3. Superposition of GES-11 (PDB: 3V3R; green) with the class A β-lactamase Toho-1 E166A (PDB 1IYO; cyan) in complex with cefotaxime (magenta).
Residues P167, N170, S237, D240, and R274 create a binding site for the bulky thiazolidine side chain of cefotaxime in Toho-1 E166A structure complex.
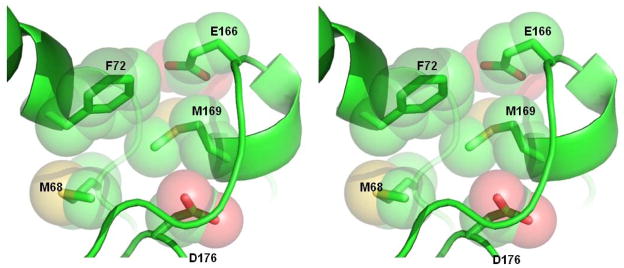
Figure 4. The hydrophobic environment around M169.
The side-chain of M169 makes contacts with F72, M68, the γ-carbon of E166 and the β-carbon of D176. This group of side-chains forms a hydrophobic core directly underneath the omega loop.
The structure of GES-1 with imipenem (PDB: 4GOG) bound to it shows that the hydroxyethyl moiety of the inhibitor makes very close contacts to the α-carbon (4.3 Å) and main-chain nitrogen (4.7 Å) of G170.19, 28 The carbapenem hydroxethyl moiety has been shown to influence the activation of water for deacylation of these drugs in more than one class of β-lactamase29, so therefore it is not surprising that substitutions at the 170 position lead to gains in hydrolytic activity towards carbapenems.8, 9, 11 It is interesting that the M169L substitution at a position so close to G170 leads to an increase in binding and hydrolysis of many β-lactams, but not carbapenems. This suggests that the acquisition of gain-of-function activity against carbapenems occurs by a different mechanism than that observed for the other classes of substrates.
Lastly, we note the somewhat puzzling observation that gains in hydrolytic activity for aztreonam appears to have arisen from a gene present in Acinetobacter baumannii, for which this drug is not typically used for treatment. This is a phenomenon however, that has been observed in several instances for both class C and class D β-lactamases in A. baumannii. 21, 30 One possible explanation is that these drugs are used for the treatment of patients infected by A. baumannii and a different species for which these drugs are used (e.g. Pseudomonas aeruginosa). Another possibility is that A. baumannii infections arise during the course of prophylactic treatment with these antibiotics, thus resulting in exposure to them. In either case, it must be assumed that the A. baumannii strains initially retain some susceptibility to these drugs but can selectively evolve enzymes that can effectively bind to these drugs and thereby confer resistance.
Acknowledgments
This study was supported by grants from Recep Tayyip Erdogan University (BAP-2013.102.03.12), The Scientific and Technical Research Council of Turkey (TUBıTAK-113Z054), National Institutes of Health grant 1R15AI082416 (D.A.L.) and a scholarship to A. Saral from Scientific and Technological Research Council of Turkey (TUBıTAK) (2214-A).
References
- 1.Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Shoichet B, Bonnet R. Structure, function, and inhibition along the reaction coordinate of CTX-M β-lactamases. J Am Chem Soc. 2005;127:5423–5434. doi: 10.1021/ja042850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cicek AC, et al. OXA- and GES-type β-lactamases predominate in extensively drug-resistant Acinetobacter baumannii isolates from a Turkish University Hospital. Clin Microbiol Infect. 2014;20:410–405. doi: 10.1111/1469-0691.12338. [DOI] [PubMed] [Google Scholar]
- 6.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotsakis SD, Miriagou V, Tzelepi E, Tzouvelekis LS. Comparative biochemical and computational study of the role of naturally occurring mutations at Ambler positions 104 and 170 in GES β-lactamases. Antimicrob Agents Chemother. 2010;54:4864–4871. doi: 10.1128/AAC.00771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirel L, et al. GES-2, a class A β-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob Agents Chemother. 2001;45:2598–2603. doi: 10.1128/AAC.45.9.2598-2603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delbrück H, et al. Kinetic and crystallographic studies of extended-spectrum GES-11, GES-12, and GES-14 β-lactamases. Antimicrob Agents Chemother. 2012;56:5618–5625. doi: 10.1128/AAC.01272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachino J, et al. Nosocomial spread of ceftazidime-resistant Klebsiella pneumoniae strains producing a novel class a β-lactamase, GES-3, in a neonatal intensive care unit in Japan. Antimicrob Agents Chemother. 2004;48:1960–1967. doi: 10.1128/AAC.48.6.1960-1967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bebrone C, et al. GES-18, a new carbapenem-hydrolyzing GES-Type β-lactamase from Pseudomonas aeruginosa that contains Ile80 and Ser170 residues. Antimicrob Agents Chemother. 2013;57:396–401. doi: 10.1128/AAC.01784-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castanheira M, et al. Evaluation of clonality and carbapenem resistance mechanisms among Acinetobacter baumannii-Acinetobacter calcoaceticus complex and Enterobacteriaceae isolates collected in European and Mediterranean countries and detection of two novel β-lactamases, GES-22 and VIM-35. Antimicrob Agents Chemother. 2014;58:7358–7366. doi: 10.1128/AAC.03930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeka AN, et al. GES-type and OXA-23 carbapenemase-producing Acinetobacter baumannii in Turkey. J Antimicrob Chemother. 2014;69:1145–1146. doi: 10.1093/jac/dkt465. [DOI] [PubMed] [Google Scholar]
- 14.Bonnin RA, et al. Wide dissemination of GES-type carbapenemases in Acinetobacter baumannii isolates in Kuwait. Antimicrob Agents Chemother. 2013;57:183–188. doi: 10.1128/AAC.01384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, et al. Novel SHV-derived extended-spectrum β-lactamase, SHV-57, that confers resistance to ceftazidime but not cefazolin. Antimicrob Agents Chemother. 2005;49:600–605. doi: 10.1128/AAC.49.2.600-605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djamdjian L, et al. CTX-M-93, a CTX-M variant lacking penicillin hydrolytic activity. Antimicrob Agents Chemother. 2011;55:1861–1866. doi: 10.1128/AAC.01656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vakulenko S, Golemi D. Mutant TEM β-lactamase producing resistance to ceftazidime, ampicillins, and β-lactamase inhibitors. Antimicrob Agents Chemother. 2002;46:646–653. doi: 10.1128/AAC.46.3.646-653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmas J, Robin F, Carvalho F, Mongaret C, Bonnet R. Prediction of the evolution of ceftazidime resistance in extended-spectrum β-lactamase CTX-M-9. Antimicrob Agents Chemother. 2006;50:731–738. doi: 10.1128/AAC.50.2.731-738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CA, et al. Structural basis for progression toward the carbapenemase activity in the GES family of β-lactamases. J Am Chem Soc. 2012;134:19512–19515. doi: 10.1021/ja308197j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moubareck C, Brémont S, Conroy MC, Courvalin P, Lambert T. GES-11, a novel integron-associated GES variant in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:3579–3581. doi: 10.1128/AAC.00072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaitany KC, et al. Structures of the class D Carbapenemases OXA-23 and OXA-146: mechanistic basis of activity against carbapenems, extended-spectrum cephalosporins, and aztreonam. Antimicrob Agents Chemother. 2013;57:4848–4855. doi: 10.1128/AAC.00762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 23.Naas T, Iorga B, Djamdjian L, Maros M, Nordmann P. European Society of Clinical Microbiology and Infectious Diseases. Berlin, Germany: 2013. Insights in penicillin-hydrolysis activity of class A extended-spectrum β-lactamases (ESBL) TLA-2. [Google Scholar]
- 24.Nijhuis RH, et al. OXY-2-15, a novel variant showing increased ceftazidime hydrolytic activity. J Antimicrob Chemother. 2015;70:1429–1433. doi: 10.1093/jac/dkv002. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JM, et al. Structural basis of activity against aztreonam and extended spectrum cephalosporins for two carbapenem-hydrolyzing class D β-lactamases from Acinetobacter baumannii. Biochemistry. 2015;54:1976–1987. doi: 10.1021/bi501547k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura T, et al. Acyl-intermediate structures of the extended-spectrum class A β-lactamase, Toho-1, in complex with cefotaxime, cephalothin, and benzylpenicillin. J Biol Chem. 2002;277:46601–46608. doi: 10.1074/jbc.M207884200. [DOI] [PubMed] [Google Scholar]
- 27.Schrödinger. The PyMOL Molecular Graphics System, Version 1.3. 2014. [Google Scholar]
- 28.Docquier JD, et al. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem Biol. 2009;16:540–547. doi: 10.1016/j.chembiol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Taibi P, Mobashery S. Mechanism of turnover of imipenem by the TEM β-lactamase revisited. J Am Chem Soc. 2002;117:7600–7605. [Google Scholar]
- 30.Poirel L, et al. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 2011;55:2546–2551. doi: 10.1128/AAC.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]