Abstract
Linoleic acid (LA, 18:2n-6) is the most abundant polyunsaturated fatty acid in the North American diet and is a precursor to circulating bioactive fatty acid metabolites implicated in brain disorders. This exploratory study tested the effects of increasing dietary LA on plasma and cerebral cortex metabolites derived from LA, it’s elongation-desaturation products dihomo-gamma linolenic (DGLA, 20:3n-6) acid and arachidonic acid (AA, 20:4n-6), as well as omega-3 alpha-linolenic (α-LNA, 18:3n-3), eicosapentaenoic (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3). Plasma and cortex were obtained from rats fed a 0.4%, 5.2% or 10.5% energy LA diet for 15 weeks and subjected to liquid chromatography tandem mass spectrometry analysis. Total oxylipin concentrations, representing the esterified and unesterified pool, and unesterified oxylipins derived from LA and AA were significantly increased and EPA metabolites decreased in plasma at 5.2% or 10.5% energy LA compared to 0.4% energy LA. Unesterified plasma DHA metabolites also decreased at 10.5% energy LA. Total oxylipins did not significantly change in cortex, whereas unesterified LA and AA metabolites increased and unesterified EPA metabolites decreased at 5.2 or 10.5% LA. DGLA and α-LNA metabolites did not significantly change in plasma or cortex. Dietary LA lowering represents a feasible approach for targeting plasma and brain LA, AA, EPA or DHA-derived metabolite concentrations.
Keywords: omega-6 linoleic acid (LA), high LA, oxylipin, metabolites, mediators, plasma, brain
1. Introduction
US intake of omega-6 linoleic acid (LA, 18:2n-6) has increased over the past century from a historic norm of 2% energy to 7% energy, owing to the growth of corn and soybean agriculture [1]. High intakes of LA could potentially be harmful to the brain because it can be converted non-enzymatically or enzymatically via 5 or 12/15lipoxygenase (LOX), cyclooxygenase (COX)-1 or 2, cytochrome P450 (CYP)-2C, 2E, 4A or 4F or soluble expoxide hydrolase (sEH) enzymes [2–4] into oxidized linoleic acid metabolites (OXLAMs) which are mostly pro-inflammatory [5–7]. OXLAMs have been linked to brain disorders including Alzheimer’s disease [8], chronic migraine [9] and Parkinson’s disease [10, 11]. It is not known how increased dietary LA regulates brain OXLAM composition, which would be important for understanding the role of OXLAMs in brain disorders.
COX, LOX, CYP and sEH enzymes also catalyze the formation of oxidized fatty acids known as ‘oxylipins’, from other fatty acid substrates including LA’s elongation/desaturation product dihomo-gamma-linolenic acid (DGLA, 20:3n-6) and arachidonic acid (AA, 20:4n-6), as well as omega-3 α-linolenic acid (α-LNA) and its elongation/desaturation products eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) [12, 13]. DGLA, AA, EPA and DHA are synthesized from their dietary-essential LA or α-LNA precursors in the liver and transported through the circulation to the brain and other tissues [14–17]. In brain, COX, LOX and CYP enzymes convert LA, DGLA, AA, α-LNA, EPA or DHA into hydroxylated, epoxidized, prostacyclin or ketone metabolites that mediate the inflammatory response or resolution of inflammation (Figure 1) [18–21]. sEH catalyzes the conversion of epoxide oxylipins into dihydroxy and possibly trihydroxy fatty acid metabolites (Figure 1) [22].
Figure 1.
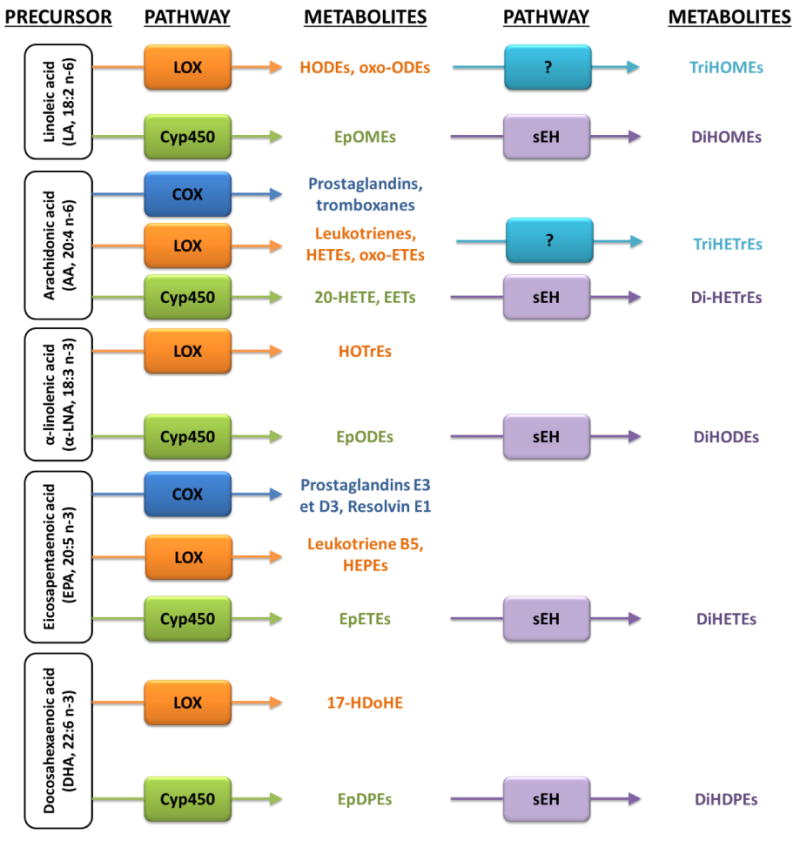
Oxylipin formation pathways from fatty acids. Linoleic acid (LA, 18:2n-6), dihomo-gamma linolenic acid (DGLA, 20:3n-6), arachidonic acid (AA, 20:4n-6), α-linolenic acid (α-LNA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) are precursors for cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P45 (CYP) enzymes, which convert them into mono-hydroxylated, epoxidized or ketone products. Soluble epoxide hydrolase (sEH) catalyzes the formation of dihydroxylated metabolites from epoxidized metabolites. sEH may catalyze tri-hydroxylated metabolite formation, but this remains to be tested.
Kim et al. reported that compared to an n-6 polyunsaturated fatty acid (PUFA) adequate diet containing 27.6% LA, n-6 PUFA deficiency achieved by lowering LA to 3% of total fatty acids for 15 weeks, decreased brain AA-releasing group IVA calcium-dependent phospholipase A2 (cPLA2) mRNA, protein and activity, and COX-2 protein, and increased group VIA DHA-releasing calcium independent phospholipase A2 (iPLA2) mRNA, protein and activity, and 15-LOX mRNA and protein [23]. N-6 PUFA deficiency also increased brain DHA incorporation rate and turnover within membrane phospholipids [24], and reduced the rate of loss of AA from brain membrane phospholipids [25].
The changes in COX-2 and 15-LOX following n-6 PUFA deficiency suggest possible changes in their oxidized fatty acid metabolites. Supporting this suggestion, is evidence of increased hydroxyeicosapentaenoic acid (HEPE) metabolites of EPA and reduced hydroxyeicosatetraenoic acid (HETE) and prostaglandin (PG) F2α metabolites of AA following 15 weeks of n-6 PUFA deficiency (low LA) in rats [25]. Brain DHA-metabolite concentrations did not change, whereas LA, DGLA and α-LNA metabolites were not measured [25].
In view of the evidence showing that dietary LA levels regulate brain AA and EPA-derived mediator concentrations and COX and LOX protein expression [23, 25], the present study tested the hypothesis that graded increments in dietary LA would alter brain oxylipin concentrations. Plasma oxylipins were also measured to determine the extent to which circulating oxylipins reflect brain oxylipin concentrations.
Rats were fed a 0.4%, 5.2% or 10.5% energy LA diet for 15 weeks, and their plasma cerebral cortex oxylipin concentrations quantified. Plasma and cortex fatty acid concentrations, which were measured in the same batch of animals and recently reported [26], are depicted in Supplementary Figure 1. The 0.4% and 5.2% energy diets are equivalent to the 3% and 27% (of total fatty acids) LA diets used in previous studies [23–25, 27]. The 10.5% energy LA diet containing 52% LA of total fatty acids was added to explore the effect of increased LA consumption on plasma and brain oxylipin metabolism at clinically relevant levels, which range from approximately 2 to 20% energy [1]. In this manuscript, we will refer to the 0.4%, 5.2% and 10.5% energy diets as the low, medium and high LA diets respectively.
Although AA, EPA and DHA-derived metabolites are often referred to as mediators because of their established roles in mediating the brain inflammatory or resolution cascades (reviewed in [28]), very little is known about what LA, DGLA or α-LNA mediate in the brain. Thus, the term metabolites or oxylipins will be used in lieu of mediators when referring to COX, LOX, CYP or sEH fatty acid products.
A total of 84 metabolites (Supplementary Table 1) derived from LA, DGLA, AA, α-LNA, EPA or DHA were quantified with liquid chromatography tandem mass spectrometry (LC-MS/MS) using an established lipidomics platform [29]. Total oxylipins, reflecting the sum of esterified and unesterified species, and unesterified (free) oxylipins were measured [30, 31], because the unesterified pool represents the bioactive form of oxylipins [32], whereas the total pool containing esterified oxylipins represents the amount potentially available for release [33].
We found that increasing dietary LA increased plasma total or unesterified LA and AA-derived metabolites, and decreased EPA and DHA metabolites. Brain (cortex) total oxylipins did not change, whereas unesterified LA and AA metabolites increased and EPA metabolites decreased following increased dietary LA intake.
2. Materials and Methods
2.1 Animals
Ethical approval for the animal protocol was obtained from the Animal Care and Use Committee of the Eunice Kennedy Schriver National Institute of Child Health and Human Development. The protocol followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23). Male Fischer-344 (CDF) rat pups (18–21 days old) and their surrogate mothers were purchased from Charles River Laboratories (Portage, MI, USA). The pups were weaned upon arrival from their surrogate mothers and randomized to a low (n=8), moderate (n=8), or high LA diet (n=8) for 15-weeks. Animals were housed in an animal facility with regulated temperature, humidity and a 12 hour light/dark cycle. The three study diets were prepared by Dyets Inc. (Bethlehem, PA, USA) based on the AIN-93G formulation, and were isocaloric [27, 34]. The diets contained 60% carbohydrate, 20% protein, 10% fat and 10% vitamin, mineral and other additives as shown in Supplementary Table 2. The fat source was balanced to provide 0.4%, 5.2% and 10.5% energy LA. The fatty acid composition of the diets was measured by gas-chromatography (see below) and is presented in Table 1. The energy contribution of each fatty acid is shown in Supplementary Table 3. Rats had free access to food and water throughout the study; their food was replaced every 3 or 4 days.
Table 1.
Dietary fatty composition, expressed as percent of total fatty acids.
| 0.4% Group | 5.2% Group | 10.5% Group | |
|---|---|---|---|
| 10:0 | 33.4 ± 1.3 | 20.6 ± 1.7 | 7.1 ± 0.1 |
| 12:0 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.03 |
| 14:0 | 20.5 ± 0.2 | 11.0 ± 0.1 | 4.1 ± 0.01 |
| 16:0 | 13.3 ± 0.3 | 9.9 ± 0.2 | 8.2 ± 0.02 |
| 16:1n-7 | 0.1 ± 0.002 | 0.1 ± 0.002 | 0.1 ± 0.001 |
| 18:0 | 15.1 ± 0.4 | 14.5 ± 0.7 | 9.9 ± 0.2 |
| 18:1n-9 | 7.8 ± 0.1 | 8.7 ± 0.2 | 13.7 ± 0.03 |
| 18:2n-6 | 3.1 ± 0.05 | 29.7 ± 0.6 | 51.9 ± 0.1 |
| 18:3n-3 | 6.1 ± 0.1 | 5.1 ± 0.1 | 4.6 ± 0.01 |
Data are mean ± SD of n = 3 per diet.
Dietary fatty acids were extracted by the Folch method [35] and analyzed by gas chromatography [36]. Food pellets were crushed with pestle and mortar, weighed and extracted in 30 ml of chloroform/methanol (2:1 v/v) and 7.5 ml of 0.1% potassium chloride. The chloroform layer containing total lipids was separated, and the sample re-extracted with 20 ml of chloroform. The chloroform extract was dried and reconstituted in 5 ml chloroform/methanol (2:1 v/v). A portion total lipid extract (50 μl) was methylated for 3 hours at 70 °C with 1% H2SO4 in methanol after adding 25μl of 8.11 nmol/μl 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine as an internal standard. The fatty acid methyl esters were extracted in 3 ml heptane, further neutralized with 1.5 ml water to remove residual H2SO4 and reconstituted in 100 μl isooctane. Fatty acid methyl esters were analyzed by gas-chromatography as detailed elsewhere [36].
2.2 Rat tissue collection
The rats were killed by CO2 asphyxiation and decapitated. Trunkal blood was collected into tubes containing ethylenediaminetetraacetic acid (EDTA). Blood was centrifuged for 1 minute at 13,000 rpm and plasma collected and stored at −80°C. Brain was excised, separated into cerebrum, cerebellum and brainstem and frozen on dry-ice chilled isobutene. Samples were stored at −80°C. Only cerebral cortex tissue was available for analysis because brainstem and cerebellum were used for fatty acid composition measurements, which were recently reported [26].
2.3 Oxylipin analysis
A total of 84 oxylipins listed and abbreviated in Supplementary Table 1 were quantified using LC-MS/MS by running calibration curves for each oxylipin using authentic standards obtained from Cayman Chemicals (Ann Arbor, Michigan) or synthetic standards produced by Dr. Hammock’s laboratory. Both total (unesterified and esterified) and unesterified oxylipins were measured. The oxylipin extraction and analysis was done in a blinded manner.
2.3.1 Plasma oxylipin extraction
Fatty acid metabolites were analyzed as free oxylipins and after base hydrolysis to determine the sum of both free and esterified oxylipins as previously described [31]. Oxylipins were extracted from plasma using 60 mg Waters Oasis HLB 3cc cartridges (Catalogue #: WAT094226; Milford, MA). The columns were cleaned with one wash of ethyl acetate and two washes of methanol, and conditioned with two washes of buffer containing 0.1% acetic acid and 5% methanol in Millipore water. Each column was spiked with 10 μl antioxidant solution and 10μl surrogate standard mix containing 10 nmol of d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-6-keto-PGF1a, d4-9-HODE, d4-LTB4, d4-PGE2, d4-TXB2, d6-20-HETE and d8-5-HETE. The antioxidant solution contained three antioxidants mixed at a 1:1:1 ratio (v/v/v) consisting of 0.6 mg/ml Ethylenediaminetetraacetic acid (EDTA) in water, 0.6 mg/ml butylated hydroxytoluene in methanol and 0.6 mg/ml Triphenylphosphine in water/methanol (1:1 v/v). The antioxidant solution was passed through a Millipore filter to remove solid particles. The final concentration of each antioxidant was 0.2 mg/ml.
Fifty to one hundred μl of ice-thawed plasma was added directly to the column, which was then topped to volume with buffer containing 0.1% acetic acid and 5% MeOH in Millipore water. The solid phase extraction chamber plug, to which the column was connected, was opened to allow the solution to pass through. In doing so, the oxylipins and surrogate oxylipin standards adsorb to the column filter and other polar compounds (sugars, proteins, etc.) elute into the extraction chamber. The column was washed with the buffer twice and then dried under vacuum suction (~20psi) for 20 minutes. Oxylipins were eluted into a 2 ml centrifuge tube containing 6μl of 30% glycerol (in methanol) by washing the column with 0.5 ml methanol and 1.5 ml ethyl acetate. The collected methanol/ethyl acetate extract containing oxylipins was dried in a vacuum centrifuge and reconstituted in 50 μl of 200 nM 1-cyclohexyl ureido, 3-dodecanoic acid in methanol solution as a recovery standard.
To measure total oxylipins (esterified and free), 10 μl of antioxidant mix and 10 μl surrogate standard were mixed with 45–100 μl of the remaining plasma, which was then hydrolyzed in 100 μl of 0.5 M sodium carbonate solution (26.5 mg per ml of 1:1 v/v methanol/water) at 60 °C for 30 min under constant shaking. The samples were neutralized with 25 μl acetic acid and 1575 μl water was added. The pH was confirmed to be neutral after adding the acetic acid by spiking a litmus paper with a few microliters from one of the samples. The hydrolyzed oxylipins were poured into pre-cleaned and pre-conditioned solid phase extraction columns and washed with buffer and extraction solvents as described above.
2.3.2 Cerebral cortex oxylipin extraction
Ice-cold methanol (400 μl) containing 0.1% acetic acid and 0.1% butylated hydroxytoluene (BHT) was added to approximately 0.35 g of frozen cortex, following the addition of 10 μl antioxidant mix and 20 μl surrogate standard solution containing 200 nmol of d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-6-keto-PGF1a, d4-9-HODE, d4-LTB4, d4-PGE2, d4-TXB2, d6-20-HETE and d8-5-HETE in methanol. The samples were cooled in a −80°C freezer for 30 minutes and then homogenized for 5 minutes at 30 vibrations per second using a bead homogenizer. The homogenized samples were stored overnight in a −80°C freezer and then centrifuged at high speed in a 5145R Eppendorf microcentrifuge (Hauppauge, NY) for 10 minutes. Approximately half of the supernatant was subjected to direct solid phase extraction to extract free oxylipins. The remaining supernatant was hydrolyzed in equal volumes of 0.5 M sodium carbonate solution (26.5 mg per ml of 1:1 v/v methanol/water) at 60 °C for 30 min under constant shaking. Following neutralization with 25 μl acetic acid and addition of 1575 μl water, the hydrolyzed oxylipins were subjected to solid phase extraction.
All samples stored in −80°C until LC-MS/MS analysis. Samples were analyzed within a week following extraction.
2.3.3 LC-MS/MS analysis
Oxylipins were analyzed as previously described [29, 31, 37] on an Agilent 1200 SL (Agilent Corporation, Palo Alto, CA) UPLC system connected to a 4000 QTrap tandem mass spectrometer (Applied Biosystems Instrument Corporation, Foster City, CA) equipped with an electrospray source (Turbo V). The UPLC was equipped with an Agilent 2.1 × 150 mm Eclipse Plus C18 column with a 1.8 μm particle size.
The autosampler was kept at 4 °C, and the column at 50 °C. The mobile phase A contained water with 0.1% glacial acetic acid and mobile phase B consisted of acetonitrile/methanol (84:15) with 0.1% glacial acetic acid. Gradient elution was performed at a flow rate of 0.25 ml/min. The total run time was 21.5 minutes. Solvent B was held at 35% for 0.25 minutes, then increased to 45% between 0.25–1 min, 55% from 1 – 3 min, 66% from 3 – 8.5 min, 72% from 8.5 to 12.5 min, 82% from 12.5–15 min and 95% from 15–16.5 min, maintained at 95% to 18 min, and finally lowered to 35% from 18 to 18.1 min and held at 35% between 18.1 and 21 min. This gradient enables the separation of oxylipins according to polarity, with the most polar oxylipins eluting first (prostaglandins and leukotrienes) followed by less polar hydroxy and epoxy fatty acids. The instrument was operated in negative electrospray ionization mode and using optimized multiple reaction monitor (MRM) conditions of the parent and fragmentation product ion [37]. Analyst software 1.4.2 was used to quantify the peaks according to the standard curves used to establish the response factor for each oxylipin after correcting for the surrogate standard recovery.
Oxylipins that were base-hydrolyzed and used d4-PGE2 as a surrogate standard were not quantified due to degradation of the d4-PGE2 during the hydrolysis step [31]. Oxylipins that were three or more times lower than the minimum standard concentration used for the standard curve were not analyzed, because they likely represent baseline noise.
2.3.4 Statistical analysis
The oxylipin measurements were completed for all 24 plasma samples (8 per group), but only 19 out of 24 cortex samples were analyzed because of sample loss during homogenization (n=3) or samples not injected into the LC-MS/MS due to syringe failure (n=2), an issue that was realized during the semi-automated data analysis. These samples were not re-injected due to possible storage effects on oxylipin levels. Thus, the sample size for the cortex was 6, 6, and 7 for the 0.4%, 5.2% and 10.5% energy dietary LA groups, respectively.
Dietary data are presented as mean ± standard deviation (SD). Oxylipin data are presented as medians and interquartile ranges. Oxylipin values that were below the detection or quantitation limit were imputed as one-half the minimum value of the other samples. Oxylipin data were analyzed using Kruskal Wallis one-way analysis of variance (ANOVA). In an exploratory manner, we conducted Dunn’s post-hoc test to determine differences amongst the groups. Oxylipin values that were below the detection or quantitation limit were imputed as one-half the limit of quantitation. Oxylipin data are presented as medians and interquartile ranges. The reference (‘control’) group is the low (0.4%) LA diet. Statistical significance was accepted at p <= 0.05.
3. Results
3.1 Dietary fatty acid composition
Dietary LA was altered as a controlled variable by replacing coconut or olive oil with high-LA safflower oil as shown in Supplementary Table 2. The fatty acid composition of the diets is in Table 1. As shown, the low, medium and high LA diets contained 3.1%, 29.7% and 51.9% LA of total fatty acids, respectively. This provided 0.4%, 5.2% and 10.5% energy LA as shown in Supplementary Table 3. The fatty acid composition of the low and medium LA diets is consistent with previous reports that used the same diets [23, 24, 27].
3.2 Plasma oxylipin concentrations
Plasma concentrations of several total or unesterified oxylipins derived from LA, AA, EPA and DHA were significantly affected by dietary LA.
Total di-hydroxylated (9–10 DiHOME, 12,13 DiHOME) metabolites of LA were 3 to 8 times higher in the 5.2% and 10.5% LA groups compared to 0.4% controls. Similar changes in di-hydroxylated LA metabolites were seen within the unesterified pool, in which the epoxidized metabolites, 9(10)-EpOME and 12(13)-EpOME, were increased by 6–12 fold in the 5.2 and 10.5% LA diets relative to 0.4% LA. There were no significant differences between the 5.2% and 10.5% LA diets.
Total AA-derived 15-oxo-ETE (a ketone metabolite) the dihydroxylated metabolite, 8,9-diHETrE were significantly higher by 3–4 fold in the 5.2% LA group compared to 0.4% LA controls. 5,6-DiHETrE, 14,15-DiHETrE and TBX2 were significantly increased by 4 to 6 fold in the 5.2% and 10.5% LA groups compared to 0.4% controls. Similar 4 to 6 fold increments in 15-oxo-ETE, 14,15-DiHETrE, 11,12-DiHETrE and TBX2 were detected within the unesterified oxylipin pool. Unesterified mono-hydroxylated 12-HETE and epoxidized 11(12)-EpETrE and 14(15)-EpETrE AA metabolites also significantly increased by 4 to 10 fold in the 5.2% and 10.5% LA groups compared to 0.4% LA controls. Conversely, total LXA4 was reduced by 88–95% with the 5.2% and 10.5% LA diets compared to 0.4% LA controls.
Total and unesterified mono-hydroxy (HEPEs), epoxy (EpETEs) and di-hydroxy (diHETEs) EPA metabolites were significantly decreased by the 5.2% and 10.5% LA diets compared to 0.4% LA, with the exception of total 8,15-diHETE, which was significantly higher in the 5.2% LA compared to 0.4% LA controls. Changes ranged from 2 to 89 fold within the total oxylipin pool and from 2 to 9 within the unesterified pool.
No change in total DHA-metabolites was detected. Unesterified dihydroxylated DHA-metabolites (10,11-DiHDPE, 13,14-DiHDPE, 16,17-DiHDPE and 19,20-DiHDPE), however, decreased significantly by 2–4 folds in the 10.5% group compared to 0.4% LA.
Total and unesterified metabolites of α-LNA and DGLA did not differ significantly between the groups.
3.2 Cerebral cortex oxylipin concentrations
Total and unesterified hydroxy (9- and 13-HODE), epoxy (9(10)- and 12(13)-EpOME), and dihydroxy (9,10- and 12,13-DiHOME) LA-metabolites were significantly increased in the 10.5% LA groups compared to 0.4% LA. The increase in LA metabolites ranged from approximately 2 to 3 fold, except for total 9(10)-EpOME, which increased by 92 fold. Unesterified 9-oxo-ODE and tri-hydroxy (9,10,13- and 9,12,13-TriHOME) LA-metabolites were significantly increased in the 10.5% group relative to 0.4% LA controls by 2 to 3 fold.
Except for an 1.8-fold higher concentration in 8,9-DiHETrE in the 10.5% LA group compared to 0.4% LA, no change in total AA-derived metabolites was observed. AA-derived unesterified 11,12-DiHETrE, 14,15-DiHETrE and PGD2 were significantly higher by 1.5–1.6 fold in the 10.5% LA groups compared to 0.4% LA. No significant changes in other unesterified AA metabolites were detected.
EPA-derived total and unesterified hydroxy (5 and 15-HEPE), epoxy (8(9)-, 14(15)- and 17(18)-EpETE) and di-hydroxy (17,18-DiHETE) metabolites were significantly reduced in the 5.2% and 10.5% LA groups relative to 0.4% LA by 3 to 19 fold. Unesterified PGE3 was also significantly reduced by 86% in the 5.2% and 10.5% LA compared to the 0.4% LA group.
Total and unesterified α-LNA, DGLA and DHA-derived metabolites did not significantly change in cerebral cortex.
3.3 Summary of oxylipin changes
Table 4 summarizes significant changes in plasma and cortex total and unesterified oxylipin concentrations. As indicated, changes in total oxylipins reflected changes in the unesterified pool in both plasma and cortex.
Table 4.
Plasma and cortex oxylipins that changed significantly in the total or unesterified pools
| TOTAL | UNESTERIFIED | |||
|---|---|---|---|---|
|
| ||||
| Plasma | Cortex | Plasma | Cortex | |
| n-6 Derivatives LA | ||||
| 9-HODE | ↔ | ↑(10.5%) | ↔ | ↑(10.5%) |
| 13-HODE | ↔ | ↑(10.5%) | ↔ | ↑(10.5%) |
| Total HODE | ↔ | ↑(10.5%) | ↔ | ↑(10.5%) |
| 9-oxo-ODE | ↔ | ↔ | ↔ | ↑(10.5%) |
| 9(10)-EpOME | ↔ | ↑(10.5%) | ↑↑ | ↑(10.5%) |
| 12(13)-EpOME | ↔ | ↔ | ↑↑ | ↑(10.5%) |
| Total EpOME | ↔ | ↑(10.5%) | ↑↑ | ↑(10.5%) |
| 9,10-DiHOME | ↑↑ | ↑(10.5%) | ↑(10.5%) | ↑(10.5%) |
| 12,13-DiHOME | ↑↑ | ↑(10.5%) | ↑↑ | ↑(10.5%) |
| Total DiHOME | ↑↑ | ↑(10.5%) | ↑(10.5%) | ↑(10.5%) |
| 9,10,13-TriHOME | ND | ND | ↔ | ↑(10.5%) |
| 9,12,13-TriHOME | ND | ND | ↔ | ↑(10.5%) |
| Total TriHOME | ND | ND | ↔ | ↑(10.5%) |
| AA | ||||
| 5-HETE | ↔ | ↔ | ↔ | ↔ |
| 12-HETE | ↔ | ↔ | ↑↑ | ↔ |
| 15-oxo-ETE | ↑(5.2%) | ↔ | ↑↑ | ↔ |
| 11(12)-EpETrE | ↔ | ↔ | ↑↑ | ↔ |
| 14(15)-EpETrE | ↔ | ↔ | ↑↑ | ↔ |
| 5,6-DiHETrE | ↑↑ | ↔ | ↔ | ↔ |
| 8,9-DiHETrE | ↑(5.2%) | ↑(10.5%) | ND | ↔ |
| 11,12-DiHETrE | ↔ | ↔ | ↑↑ | ↑(10.5%) |
| 14,15-DiHETrE | ↑↑ | ↔ | ↑↑ | ↑(10.5%) |
| PGD2 | ND | ND | ND | ↑(10.5%) |
| TXB2 | ↑↑ | ↔ | ↑↑ | ↔ |
| LXA4 | ↓↓ | ND | ↔ | ND |
| n-3 Derivatives EPA | ||||
| 5-HEPE | ↓↓ | ↓↓ | ↓(10.5%) | ↓↓ |
| 12-HEPE | ↓↓ | ↔ | ND | ↔ |
| 15-HEPE | ↔ | ↓↓ | ↓(10.5%) | ↓(10.5%) |
| 8(9)-EpETE | ↓(10.5%) | ↓↓ | ND | ↓↓ |
| 11(12)-EpETE | ↓↓ | ↔ | ↓↓ | ↔ |
| 14(15)-EpETE | ↓↓ | ↓↓ | ↔ | ↓↓ |
| 17(18)-EpETE | ↓↓ | ↓↓ | ND | ↓↓ |
| Total EpETE | ↓↓ | ↓↓ | ↓↓ | ↓↓ |
| 8,15-DiHETE | ↑(5.2%) | ↔ | ND | ↔ |
| 17,18-DiHETE | ↓(10.5%) | ↓↓ | ↓↓ | ↓↓ |
| PGE3 | ND | ND | ↔ | ↓↓ |
| DHA | ||||
| 10,11-DiHDPE | ND | ↔ | ↓(10.5%) | ↔ |
| 13,14-DiHDPE | ND | ↔ | ↓ (10.5%) | ↔ |
| 16,17-DiHDPE | ↔ | ↔ | ↓(10.5%) | ↔ |
| 19,20-DiHDPE | ↔ | ↔ | ↓(10.5%) | ↔ |
One arrow means that the 5.2% and/or 10.5% LA group differed significantly from 0.4% LA controls. Parenthesis indicate the specific group (5.2% or 10.5%) that differed from the 0.4% LA group. Two arrows mean that both the 5.2% and 10.5% LA groups differed significantly from 0.4% LA. A left-right arrow (↔) means that there were no significant differences between any groups. ND means that the metabolites were not detected.
Changes in plasma did not entirely reflect changes in cortex. In total oxylipin pool, incremental amounts of dietary LA intakes affected 16 and 12 metabolites in plasma and cortex, respectively, but overlapping changes were observed in only 7 metabolites – 9,10- and 12,13-DiHOME, 5-HEPE, 8(9)-, 14(15)- and 17(18)-EpETE and 17,18-DiHETE. Unesterified oxylipin concentrations changed in 19 metabolites in both plasma and cortex, but overlapping changes were detected in only 9 metabolites – 9(10)- and 12(13)-EpOME, 9,10- and 12,13-DiHOME, 11,12- and 14,15-DiHETrE, 5- and 15-HEPE and 17,18-DiHETE.
4. Discussion
This exploratory study showed significant changes in plasma and cortex oxylipin concentrations following increased dietary LA intake. In plasma, LA and AA-derived metabolites increased and EPA and DHA-derived metabolites decreased within total or unesterified oxylipins following increased dietary LA (Table 2). Cortex total oxylipins did not change, whereas unesterified LA and AA metabolites increased and EPA (but not DHA) metabolites decreased following graded increments in dietary LA (Table 3). Changes in plasma did not reflect changes in cortex with the exception of 7 or 9 oxylipins (Table 4).
Table 2.
Plasma oxylipin concentrations (pmol/ml plasma)
| TOTAL | UNESTERIFIED | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Compound | 0.4% Group Median (IQR) | 5.2% Group Median (IQR) | 10.5% Group Median (IQR) | 0.4% Group Median (IQR) | 5.2% Group Median (IQR) | 10.5% Group Median (IQR) |
| n-6 Derivatives LA | ||||||
| 9-HODE | 54.1 (35.9, 121.5) | 46.5 (25.5, 115.9) | 81.0 (54.7, 102.9) | 43.2 (27.9, 74.8) | 28.0 (20.0, 45.3) | 37.6 (32.2, 58.8) |
| 13-HODE | 86.0 (55.3, 168.6) | 68.1 (40.1, 180.6) | 106.8 (80.5, 131.9) | 76.1 (51.4, 119.8) | 49.5 (39.1, 77.5) | 74.6 (60.0, 106.5) |
| Total HODE | 147.5 (91.2, 282.7) | 114.6 (65.5, 296.6) | 188.4 (133.8, 234.1) | 119.3 (79.3, 194.5) | 76.3 (60.2, 122.8) | 114.7 (92.5, 162.5) |
| 9-oxo-ODE | 251.9 (108.7, 353.1) | 265.4 (204.1, 329.4) | 498.5 (123.1, 770.0) | 128.3 (80.7, 222.5) | 200.5 (109.0, 357.0) | 265.6 (151.3, 362.3) |
| 13-oxo-ODE | 19.8 (11.8, 34.7) | 27.9 (18.6, 46.5) | 58.9 (6.2, 102.5) | 14.2 (6.8, 23.2) | 19.1 (9.8, 60.8) | 13.4 (9.3, 19.2) |
| 9(10)-EpOME | 120.1 (59.5, 391.7) | 208.4 (114.1, 310.7) | 196.2 (125.4, 499.5) | 14.2 (9.2, 25.2)a | 93.5 (49.5, 185.0)b | 175.0 (68.0, 520.0)b |
| 12(13)-EpOME | 600.5 (153.2, 1,255.0) | 1,037.5 (364.7, 2,060.0) | 583.8 (382.8, 2,765.3) | 14.2 (12.9, 29.9)a | 82.0 (39.6, 182.8)b | 169.0 (73.0, 529.0)b |
| Total EpOME | 848.6 (212.7, 1,518.6) | 1,321.1 (478.8, 2,295.4) | 740.3 (547.8, 3,264.8) | 27.9 (22.5, 55.1)a | 175.4 (88.2, 367.8)b | 346.3 (141.0, 1,059.3)b |
| 9,10-DiHOME | 4.1 (3.4, 4.8)a | 16.6 (11.5, 21.0)b | 32.3 (21.5, 45.6)b | 2.8 (2.2, 4.5)a | 8.1 (7.1, 8.7)ab | 16.1 (14.6, 24.1)b |
| 12,13-DiHOME | 5.7 (4.8, 6.2)a | 13.3 (6.9, 15.4)b | 18.3 (12.0, 26.5)b | 3.5 (2.1, 3.9)a | 5.9 (5.5, 8.2)b | 8.7 (7.9, 13.1)b |
| Total DiHOME | 9.2 (8.8, 10.2)a | 31.3 (18.3, 35.3)b | 52.7 (33.4, 69.7)b | 5.8 (4.8, 8.3)a | 13.9 (13.1, 16.4)ab | 24.0 (22.3, 37.2)b |
| 9,10,13-TriHOME | 2.0 (1.2, 2.5) | 2.1 (1.0, 3.3) | 4.0 (1.8, 6.3) | |||
| 9,12,13-TriHOME | 7.8 (6.4, 13.8) | 10.3 (3.8, 14.9) | 14.1 (7.6, 25.4) | |||
| Total TriHOME | 9.5 (7.8, 16.7) | 12.2 (5.1, 18.2) | 17.9 (9.3, 31.7) | |||
| EKODE | 13.0 (9.1, 16.4) | 16.5 (10.6, 24.2) | 21.6 (14.0, 27.1) | |||
| DGLA | ||||||
| 15(S)-HETrE | 3.4 (1.4, 8.0) | 2.3 (0.6, 4.0) | 3.1 (0.5, 7.6) | 3.9 (2.8, 4.6) | 4.6 (0.8, 7.7) | 2.1 (1.5, 4.1) |
| AA | ||||||
| 5-HETE | 23.5 (18.1, 35.9) | 80.8 (68.5, 108.4) | 138.0 (43.8, 212.6) | 2.9 (2.3, 3.5) | 5.8 (4.6, 8.3) | 5.2 (4.4, 10.3) |
| 8-HETE | 26.5 (16.1, 33.5) | 28.0 (16.7, 34.3) | 33.6 (13.8, 57.9) | 2.2 (0.4, 8.0) | 6.1 (1.7, 13.6) | 3.2 (1.1, 6.7) |
| 12-HETE | 13.5 (9.8, 24.5) | 42.2 (30.8, 56.6) | 64.1 (15.5, 123.5) | 8.0 (3.7, 14.9)a | 59.3 (24.1, 77.3)b | 29.2 (20.1, 57.3)b |
| 15-HETE | 22.0 (13.4, 33.8) | 54.9 (33.2, 71.9) | 79.0 (18.4, 138.3) | 5.8 (2.1, 8.0) | 11.7 (5.8, 20.5) | 8.5 (6.1, 11.9) |
| 20-HETE | 20.3 (6.5, 32.9) | 14.1 (12.3, 21.1) | 16.5 (4.0, 43.7) | 9.4 (6.6, 15.6) | 5.1 (2.0, 17.4) | 5.6 (3.4, 10.3) |
| 5-oxo-ETE | 15.9 (3.4, 25.2) | 27.5 (22.7, 37.8) | 41.9 (2.3, 65.9) | 9.3 (6.1, 15.9) | 10.4 (6.2, 16.5) | 4.5 (2.3, 17.6) |
| 15-oxo-ETE | 9.9 (5.5, 11.7)a | 36.2 (24.6, 62.9)b | 35.6 (8.4, 91.1)ab | 2.1 (1.1, 3.1)a | 11.6 (4.8, 14.8)b | 6.4 (4.5, 12.0)b |
| 5(6)-EpETrE | 19.7 (11.2, 92.1) | 83.4 (32.0, 197.9) | 85.3 (66.8, 307.4) | |||
| 8(9)-EpETrE | 0.5 (0.3, 1.2) | 0.8 (0.4, 1.3) | 0.7 (0.5, 1.0) | |||
| 11(12)-EpETrE | 277.1 (109.6, 514.7) | 951.9 (409.7, 2,399.3) | 596.3 (332.0, 2,079.0) | 3.3 (1.9, 9.9)a | 20.2 (12.6, 73.8)b | 31.6 (14.2, 84.7)b |
| 14(15)-EpETrE | 400.9 (165.8, 1,157.2) | 1,821.9 (812.5, 5,082.1) | 1,193.8 (620.0, 4,524.4) | 2.9 (0.9, 4.1)a | 14.5 (7.3, 34.7)b | 17.3 (10.8, 52.6)b |
| 5,6-DiHETrE | 2.4 (2.2, 2.9)a | 10.0 (6.5, 15.6)b | 13.1 (9.4, 16.2)b | 0.1 (0.0, 0.3) | 0.2 (0.2, 0.5) | 0.4 (0.1, 0.5) |
| 8,9-DiHETrE | 0.7 (0.3, 1.1)a | 2.3 (1.4, 2.8)b | 1.1 (0.8, 1.9)ab | |||
| 11,12-DiHETrE | 0.6 (0.5, 1.0) | 2.1 (0.9, 3.5) | 1.6 (1.1, 2.3) | 0.4 (0.1, 0.6)a | 1.1 (0.8, 1.3)b | 1.1 (0.7, 2.1)b |
| 14,15-DiHETrE | 0.5 (0.2, 0.8)a | 2.8 (2.0, 4.2)b | 2.8 (2.2, 4.7)b | 0.3 (0.2, 1.0)a | 1.8 (1.3, 2.4)b | 1.9 (1.4, 2.1)b |
| TXB2 | 0.5 (0.4, 0.8)a | 1.8 (1.3, 3.2)b | 1.9 (1.2, 2.1)b | 0.4 (0.3, 0.8)a | 1.6 (1.3, 2.0)b | 1.6 (1.1, 3.2)b |
| LTB3 | 3.1 (1.2, 4.1) | 1.7 (0.6, 3.0) | 2.4 (0.4, 4.9) | |||
| LTB4 | 1.3 (0.1, 4.1) | 0.1 (0.0, 1.1) | 0.2 (0.1, 1.2) | |||
| 6-trans-LTB4 | 1.6 (0.0, 5.4) | 0.7 (0.0, 3.4) | 1.6 (0.3, 2.6) | |||
| 20-COOH-LTB4 | 14.2 (7.3, 25.8) | 14.7 (10.3, 23.1) | 12.5 (9.0, 35.6) | 3.2 (1.3, 5.0) | 2.0 (1.2, 3.4) | 3.9 (1.6, 6.0) |
| 20-OH-LTB4 | 1.6 (1.4, 1.8) | 0.7 (0.0, 1.7) | 1.5 (0.2, 3.2) | |||
| LXA4 | 5.8 (2.1, 6.7)a | 0.7 (0.5, 1.8)b | 0.3 (0.1, 1.8)b | 0.3 (0.1, 0.5) | 0.1 (0.0, 0.3) | 0.3 (0.2, 0.5) |
| 11,12,15-TriHETrE | 0.3 (0.2, 0.4) | 0.4 (0.3, 0.5) | 0.5 (0.4, 0.8) | |||
| n-3 Derivatives α-LNA | ||||||
| 9-HOTrE | 2.3 (0.2, 6.3) | 1.8 (0.3, 2.8) | 2.2 (1.4, 2.7) | 7.3 (1.6, 11.0) | 2.7 (1.9, 5.4) | 3.0 (2.4, 6.4) |
| 9(10)-EpODE | 26.2 (18.7, 103.1) | 12.4 (6.9, 36.9) | 12.5 (8.8, 31.3) | 15.1 (9.3, 29.1) | 13.4 (9.1, 42.3) | 30.1 (8.6, 74.2) |
| 12(13)-EpODE | 55.4 (19.5, 116.6) | 30.1 (11.4, 45.3) | 11.7 (6.0, 53.8) | 5.8 (3.6, 16.0) | 4.3 (2.7, 19.1) | 10.0 (3.5, 30.6) |
| 15(16)-EpODE | 466.4 (145.3, 790.0) | 217.2 (78.2, 328.6) | 99.7 (63.3, 375.7) | 34.8 (14.1, 57.1) | 24.9 (19.8, 67.2) | 45.2 (18.1, 110.4) |
| 15,16-DiHODE | 3.8 (3.1, 4.8) | 4.2 (3.5, 5.9) | 4.6 (3.9, 6.3) | 3.8 (3.2, 5.8) | 3.2 (2.6, 3.7) | 3.6 (2.9, 4.8) |
| EPA | ||||||
| 5-HEPE | 62.4 (31.7, 72.8)a | 10.4 (4.4, 14.9)b | 6.3 (2.8, 16.2)b | 9.5 (4.7, 14.4)a | 1.4 (0.9, 4.2)ab | 1.6 (0.3, 1.7)b |
| 12-HEPE | 2,284.6 (683.6, 3,233.9)a | 145.4 (80.6, 338.1)b | 25.8 (0.1, 66.3)b | |||
| 15-HEPE | 6.3 (4.9, 13.4) | 3.9 (1.6, 6.6) | 2.7 (0.1, 5.7) | 9.9 (6.6, 15.2)a | 4.7 (1.3, 11.9)ab | 3.5 (2.2, 4.2)b |
| 8(9)-EpETE | 41.9 (19.3, 140.5)a | 2.9 (1.0, 11.1)ab | 0.0 (0.0, 1.4)b | |||
| 11(12)-EpETE | 171.4 (57.1, 267.0)a | 8.9 (5.2, 21.9)b | 4.0 (1.4, 5.1)b | 6.4 (4.8, 10.5)a | 1.0 (0.3, 2.7)b | 1.5 (0.8, 3.2)b |
| 14(15)-EpETE | 293.9 (131.8, 559.8)a | 23.0 (6.4, 53.9)b | 3.9 (3.1, 21.7)b | 3.9 (3.7, 6.0) | 3.0 (1.3, 5.2) | 1.1 (0.4, 3.4) |
| 17(18)-EpETE | 382.5 (220.8, 803.9)a | 29.5 (10.9, 93.1)b | 10.9 (7.4, 19.1)b | |||
| Total EpETE | 951.2 (431.2, 1,712.8)a | 62.9 (26.0, 190.0)b | 18.2 (13.1, 46.5)b | 9.9 (8.6, 15.4)a | 4.6 (2.4, 7.3)b | 2.2 (1.6, 9.3)b |
| 5,15-DiHETE | 0.8 (0.5, 1.6) | 0.9 (0.3, 1.7) | 1.7 (0.9, 2.0) | |||
| 8,15-DiHETE | 0.7 (0.3, 1.1)a | 2.3 (1.4, 2.8)b | 1.1 (0.8, 1.9)ab | |||
| 17,18-DiHETE | 1.7 (1.0, 3.6)a | 0.7 (0.3, 1.0)ab | 0.5 (0.3, 0.8)b | 3.7 (3.1, 4.2)a | 0.8 (0.3, 1.2)b | 0.4 (0.2, 0.8)b |
| PGD3 | 0.7 (0.4, 1.0) | 0.4 (0.3, 0.6) | 0.4 (0.4, 0.6) | |||
| PGE3 | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.1 (0.0, 0.1) | |||
| DHA | ||||||
| 7(8)-EpDPE | 1346.7 (627.4, 4,955.1) | 489.8 (386.9, 941.5) | 515.8 (199.5, 1,132.4) | 96.2 (46.4, 187.1) | 139.8 (47.5, 301.8) | 108.8 (30.2, 260.8) |
| 10(11)-EpDPE | 155.1 (37.6, 345.1) | 75.7 (29.3, 112.0) | 31.3 (19.8, 114.2) | 8.3 (4.4, 11.1) | 6.0 (3.8, 17.3) | 3.2 (2.8, 9.7) |
| 13(14)-EpDPE | 218.8 (78.7, 417.7) | 103.6 (29.8, 245.8) | 42.6 (19.8, 189.3) | 2.8 (1.4, 4.1) | 4.5 (1.0, 9.7) | 1.0 (0.3, 4.4) |
| 16(17)-EpDPE | 236.8 (75.9, 524.5) | 133.8 (46.9, 332.6) | 48.7 (31.8, 234.1) | |||
| 19(20)-EpDPE | 504.3 (164.2, 1,047.8) | 231.9 (95.6, 722.6) | 133.4 (67.0, 448.5) | 3.5 (2.8, 4.6) | 6.6 (1.5, 21.0) | 3.0 (0.9, 10.1) |
| Total EpDPE | 2,851.7 (1,074.4, 6,809.5) | 1,241.8 (588.6, 2,201.9) | 696.8 (401.6, 2,118.5) | 105.6 (55.4, 204.6) | 157.4 (61.3, 339.4) | 119.0 (34.6, 281.4) |
| 17-HDoHE | 14.5 (8.7, 35.6) | 3.1 (1.9, 13.2) | 6.0 (1.3, 17.1) | 5.1 (4.2, 8.4) | 4.7 (0.6, 7.1) | 2.8 (1.3, 5.6) |
| 10,11-DiHDPE | 0.7 (0.4, 0.9)a | 0.2 (0.1, 0.4)ab | 0.2 (0.1, 0.3)b | |||
| 13,14-DiHDPE | 0.4 (0.3, 0.4)a | 0.2 (0.2, 0.4)ab | 0.1 (0.1, 0.3)b | |||
| 16,17-DiHDPE | 1.0 (0.6, 1.6) | 0.9 (0.6, 1.7) | 1.0 (0.5, 1.5) | 1.3 (0.7, 2.2)a | 0.5 (0.3, 1.0)ab | 0.4 (0.3, 0.7)b |
| 19,20-DiHDPE | 6.8 (4.4, 9.6) | 2.5 (1.3, 4.2) | 2.6 (1.3, 4.5) | 3.3 (2.3, 6.5)a | 2.1 (1.5, 3.7)ab | 1.6 (1.2, 2.0)b |
Data are presented as median and interquartile ranges (IQR) of n=8 per group. The lower and upper boundaries of the IQR represent 25th and 75th percentile values, respectively. Data were analyzed by Kruskal Wallis one-way analysis of variance (ANOVA) followed by Dunn’s post-hoc test. Different alphabetical superscripts mean that the groups differed significantly (P<0.05) from each other.
Table 3.
Cerebral cortex oxylipin concentrations (pmol/g wet weight)
| TOTAL | UNESTERIFIED | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Compound | 0.4% Group Median (IQR) | 5.2% Group Median (IQR) | 10.5% Group Median (IQR) | 0.4% Group Median (IQR) | 5.2% Group Median (IQR) | 10.5% Group Median (IQR) |
| n-6 Derivative s La | ||||||
| 9-HODE | 15.5 (13.8, 20.5)a | 32.4 (27.4, 39.7)ab | 43.3 (40.2, 48.1)b | 12.7 (12.5, 18.9)a | 29.0 (26.8, 32.0)ab | 30.7 (27.5, 40.3)b |
| 13-HODE | 21.8 (19.6, 25.4)a | 43.6 (34.9, 50.5)ab | 59.0 (57.4, 65.9)b | 17.4 (15.2, 23.7)a | 40.8 (34.5, 44.1)ab | 48.6 (40.5, 54.0)b |
| Total HODE | 37.3 (33.4, 45.9)a | 76.0 (62.3, 90.2)ab | 101.5 (99.2, 114.0)b | 30.1 (27.7, 42.6)a | 69.8 (61.3, 76.3)ab | 79.2 (67.6, 94.4)b |
| 9-oxo-ODE | 10.4 (7.7, 15.0) | 19.9 (13.6, 20.9) | 18.4 (13.1, 22.5) | 12.3 (10.1, 16.2)a | 15.9 (11.7, 19.7)ab | 21.0 (18.2, 29.7)b |
| 13-oxo-ODE | 7.9 (6.7, 8.9) | 8.3 (5.5, 9.4) | 6.9 (5.0, 12.2) | 9.8 (7.4, 12.5) | 8.0 (6.1, 10.3) | 9.7 (6.2, 13.2) |
| 9(10)-EpOME | 13.3 (9.5, 307.0)a | 24.3 (11.7, 576.6)ab | 1,226.1 (586.7, 1,837.4)b | 11.2 (6.3, 12.8)a | 19.4 (13.3, 27.2)ab | 25.3 (18.7, 29.8)b |
| 12(13)-EpOME | 12.2 (9.1, 16.1) | 16.7 (10.6, 17.0) | 27.2 (18.8, 40.9) | 12.5 (7.0, 15.2)a | 24.0 (16.6, 32.2)ab | 27.3 (22.5, 43.2)b |
| Total EpOME | 32.2 (22.9, 316.2)a | 57.9 (28.4, 593.6)ab | 1,252.5 (605.5, 1,860.3)b | 23.7 (13.3, 28.0)a | 43.5 (29.8, 59.3)ab | 52.6 (41.2, 73.0)b |
| 9,10-DiHOME | 2.0 (1.2, 2.5)a | 3.0 (2.9, 3.6)ab | 4.7 (4.2, 5.0)b | 1.5 (1.1, 2.3)a | 2.9 (2.4, 3.4)ab | 4.3 (3.7, 4.7)b |
| 12,13-DiHOME | 2.3 (1.9, 3.2)a | 3.5 (3.4, 3.6)ab | 4.4 (3.4, 5.2)b | 1.7 (1.7, 3.2)a | 3.1 (2.7, 3.7)ab | 4.0 (3.3, 4.7)b |
| Total DiHOME | 4.4 (3.1, 5.7)a | 6.5 (6.4, 7.0)ab | 9.3 (7.5, 9.9)b | 3.2 (2.8, 5.5)a | 6.0 (5.0, 7.1)ab | 8.3 (7.2, 9.3)b |
| 9,10,13-TriHOME | 0.3 (0.2, 0.4)a | 0.8 (0.5, 1.0)ab | 1.0 (0.7, 1.1)b | |||
| 9,12,13-TriHOME | 3.0 (2.0, 3.9)a | 6.6 (6.0, 7.3)ab | 7.1 (4.8, 8.7)b | |||
| Total TriHOME | 3.3 (2.2, 4.4)a | 7.4 (6.7, 8.2)ab | 8.1 (5.5, 9.6)b | |||
| EKODE | 0.4 (0.2, 0.6) | 0.5 (0.5, 0.5) | 0.5 (0.4, 0.6) | |||
| DGLA | ||||||
| PGD1 | 2.2 (2.0, 3.2) | 2.5 (2.4, 2.7) | 2.7 (2.6, 2.8) | |||
| 15(S)-HETrE | 16.5 (7.9, 19.7) | 12.1 (10.2, 13.7) | 9.5 (8.5, 14.4) | 12.7 (7.4, 23.5) | 11.6 (8.4, 12.8) | 11.3 (8.8, 14.5) |
| AA | ||||||
| 5-HETE | 50.4 (34.9, 61.1) | 54.9 (53.8, 78.1) | 58.1 (44.1, 72.6) | 41.0 (27.1, 46.3) | 55.6 (37.6, 61.9) | 53.0 (43.9, 54.8) |
| 8-HETE | 36.8 (21.6, 48.7) | 35.2 (32.0, 46.1) | 37.7 (33.1, 58.7) | 40.0 (25.8, 46.7) | 44.4 (31.8, 50.9) | 47.4 (41.6, 54.2) |
| 9-HETE | 166.3 (119.8, 219.9) | 206.2 (198.9, 240.4) | 212.3 (180.2, 325.1) | 167.0 (118.0, 236.6) | 229.6 (173.5, 297.2) | 244.4 (211.5, 297.2) |
| 12-HETE | 387.0 (157.3, 592.8) | 360.5 (315.0, 686.9) | 461.0 (359.9, 620.9) | 392.3 (220.7, 737.7) | 534.9 (375.6, 693.6) | 693.4 (420.9, 780.0) |
| 15-HETE | 187.3 (130.8, 259.3) | 251.0 (238.5, 296.6) | 217.4 (208.0, 374.0) | 217.9 (135.3, 313.8) | 323.8 (239.8, 329.1) | 308.8 (254.6, 381.7) |
| 20-HETE | 12.3 (9.6, 15.8) | 10.1 (8.0, 18.8) | 10.3 (8.9, 14.5) | 14.9 (11.2, 16.5) | 14.1 (10.5, 15.0) | 12.3 (11.3, 19.4) |
| 5-oxo-ETE | 8.6 (5.8, 16.3) | 9.0 (8.6, 12.8) | 9.6 (8.0, 18.1) | 26.1 (14.5, 30.6) | 26.8 (20.8, 27.2) | 30.2 (21.4, 31.3) |
| 12-oxo-ETE | 88.7 (71.6, 147.3) | 145.7 (54.0, 147.7) | 79.6 (70.3, 144.7) | 258.0 (180.7, 281.3) | 325.9 (304.3, 359.3) | 276.9 (249.3, 401.1) |
| 15-oxo-ETE | 13.3 (10.8, 19.3) | 18.6 (9.5, 20.5) | 15.5 (14.3, 28.7) | 28.8 (18.2, 44.7) | 40.1 (24.0, 41.9) | 41.5 (34.5, 56.0) |
| 5(6)-EpETrE | 3.2 (2.1, 43.8) | 4.1 (4.1, 27.3) | 1.9 (0.3, 142.5) | 114.7 (54.2, 172.7) | 141.1 (94.3, 191.9) | 188.6 (126.6, 223.5) |
| 11(12)-EpETrE | 0.2 (0.2, 125.6) | 4.7 (0.2, 106.2) | 0.2 (0.1, 7.6) | 386.9 (171.7, 510.4) | 472.5 (237.4, 649.8) | 596.0 (391.9, 874.2) |
| 14(15)-EpETrE | 1.1 (0.4, 66.7) | 3.3 (0.9, 79.8) | 1.5 (0.6, 2.8) | 360.6 (140.8, 530.6) | 348.3 (173.1, 656.6) | 562.0 (389.0, 1,043.7) |
| 5,6-DiHETrE | 2.0 (1.5, 2.7) | 2.2 (1.8, 2.3) | 3.6 (3.0, 4.1) | 0.4 (0.3, 0.6) | 0.6 (0.5, 0.8) | 0.8 (0.6, 1.0) |
| 8,9-DiHETrE | 1.0 (0.8, 1.2)a | 1.3 (1.2, 1.6)ab | 1.8 (1.7, 2.0)b | 0.9 (0.6, 1.1) | 1.5 (1.1, 1.6) | 1.8 (1.2, 1.9) |
| 11,12-DiHETrE | 1.3 (0.9, 2.0) | 1.6 (1.4, 2.0) | 2.0 (2.0, 2.1) | 1.3 (1.0, 1.5)a | 1.8 (1.6, 2.0)ab | 2.1 (1.8, 2.4)b |
| 14,15-DiHETrE | 2.4 (2.1, 3.5) | 2.8 (2.6, 3.7) | 3.6 (3.5, 3.8) | 2.1 (1.7, 2.8)a | 3.3 (2.6, 3.5)ab | 3.5 (3.3, 3.9)b |
| PGD2 | 94.2 (88.3, 118.8)a | 132.1 (128.6, 135.5)ab | 139.3 (137.0, 150.7)b | |||
| PGE2 | 8.4 (7.7, 14.2) | 13.0 (12.1, 15.7) | 13.3 (12.9, 14.3) | |||
| PGF2a | 46.5 (33.9, 65.2) | 66.9 (63.6, 92.5) | 74.9 (72.2, 84.5) | |||
| PGJ2 | 3.1 (2.5, 8.3) | 5.4 (3.9, 6.6) | 7.1 (5.9, 7.8) | |||
| TXB2 | 46.6 (40.0, 55.8) | 63.6 (56.1, 67.4) | 72.3 (59.8, 83.5) | 56.8 (54.7, 65.2) | 69.2 (66.1, 85.4) | 78.4 (71.4, 85.8) |
| 6-trans-LTB4 | 0.5 (0.1, 0.9) | 0.0 (0.0, 1.3) | 0.6 (0.1, 1.1) | |||
| 6-keto-PGF1a | 840.2 (87.3, 2,478.4) | 424.8 (83.8, 3,798.8) | 1,200.8 (432.5, 1,518.1) | 36.7 (33.5, 55.5) | 61.0 (52.7, 69.1) | 54.7 (49.6, 73.2) |
| n-3 Derivative s α-LNA | ||||||
| 9-HOTrE | 0.6 (0.3, 0.8) | 1.0 (0.8, 1.0) | 0.8 (0.8, 1.2) | 0.6 (0.5, 1.0) | 0.9 (0.7, 1.0) | 1.1 (0.7, 1.4) |
| 13-HOTrE | 1.3 (0.9, 1.6) | 1.6 (1.1, 1.6) | 1.7 (1.1, 1.9) | 1.5 (0.9, 1.9) | 1.3 (1.2, 1.7) | 1.8 (1.4, 2.2) |
| 9(10)-EpODE | 0.9 (0.7, 1.1) | 0.8 (0.6, 1.5) | 1.6 (1.1, 2.2) | 1.4 (0.7, 1.6) | 1.5 (0.9, 2.3) | 1.8 (1.4, 2.9) |
| 12(13)-EpODE | 0.7 (0.2, 0.9) | 0.2 (0.1, 0.4) | 0.8 (0.5, 1.0) | 0.6 (0.5, 1.4) | 0.9 (0.2, 1.7) | 0.9 (0.7, 1.5) |
| 15(16)-EpODE | 3.6 (2.1, 4.2) | 2.4 (2.4, 3.3) | 3.9 (3.1, 5.1) | 3.7 (2.5, 5.1) | 4.2 (2.2, 8.0) | 4.0 (2.9, 9.5) |
| 15,16-DiHODE | 0.6 (0.3, 1.0) | 0.7 (0.5, 0.7) | 1.0 (0.8, 1.1) | 0.4 (0.4, 0.9) | 0.7 (0.5, 0.9) | 0.9 (0.8, 1.2) |
| EPA | ||||||
| 5-HEPE | 2.8 (2.6, 3.7)a | 1.0 (0.8, 1.2)b | 0.7 (0.4, 1.0)b | 4.2 (2.5, 5.6)a | 1.0 (0.8, 1.1)b | 1.0 (0.4, 1.0)b |
| 12-HEPE | 98.6 (46.4, 127.9) | 75.0 (52.0, 86.1) | 106.9 (56.3, 232.6) | 134.6 (43.1, 235.4) | 72.2 (51.0, 286.1) | 241.8 (127.1, 398.8) |
| 15-HEPE | 12.5 (8.1, 16.8)a | 3.0 (1.5, 3.0)b | 1.2 (1.1, 3.0)b | 15.1 (8.5, 20.3)a | 2.1 (1.6, 4.3)ab | 1.6 (1.1, 6.3)b |
| 8(9)-EpETE | 3.8 (2.0, 7.0)a | 0.3 (0.1, 0.9)b | 0.6 (0.1, 1.1)b | 5.6 (2.3, 6.8)a | 0.3 (0.1, 0.9)b | 0.6 (0.3, 0.9)b |
| 11(12)-EpETE | 6.3 (3.9, 6.6) | 1.9 (1.4, 2.4) | 3.4 (1.8, 6.5) | 8.7 (3.4, 12.8) | 2.6 (1.5, 4.7) | 6.5 (2.8, 7.3) |
| 14(15)-EpETE | 5.2 (4.5, 6.4)a | 0.3 (0.1, 0.4)b | 0.3 (0.2, 0.3)b | 7.5 (2.8, 12.3)a | 0.8 (0.2, 1.6)b | 0.9 (0.5, 1.3)b |
| 17(18)-EpETE | 7.1 (5.5, 12.8)a | 0.9 (0.8, 0.9)b | 0.6 (0.1, 1.0)b | 11.1 (4.0, 16.0)a | 0.9 (0.5, 1.7)b | 1.1 (0.9, 1.7)b |
| Total EpETE | 22.6 (15.8, 32.3)a | 4.1 (2.8, 4.9)b | 6.1 (2.3, 8.6)b | 33.5 (11.2, 46.7)a | 6.4 (2.4, 8.7)b | 9.5 (5.2, 11.0)b |
| 5,15-DiHETE | 2.1 (1.6, 2.4) | 1.7 (1.6, 2.9) | 2.6 (1.8, 3.3) | 1.4 (1.1, 1.6) | 1.1 (0.9, 1.5) | 1.4 (1.1, 1.8) |
| 8,15-DiHETE | 5.7 (4.8, 6.2) | 6.7 (6.7, 7.0) | 7.4 (5.2, 8.3) | 3.5 (3.1, 4.1) | 4.3 (3.9, 5.6) | 4.6 (3.6, 6.8) |
| 17,18-DiHETE | 2.0 (1.6, 2.0)a | 0.4 (0.3, 0.5)b | 0.6 (0.5, 0.6)b | 1.3 (1.1, 1.9)a | 0.5 (0.4, 0.6)b | 0.5 (0.4, 0.6)b |
| PGE3 | 0.7 (0.6, 0.9)a | 0.1 (0.1, 0.2)b | 0.1 (0.1, 0.2)b | |||
| DHA | ||||||
| 7(8)-EpDPE | 608.6 (530.4, 673.3) | 576.0 (514.5, 744.7) | 753.8 (545.1, 973.3) | 706.5 (386.9, 986.8) | 737.3 (297.9, 1,003.4) | 778.0 (467.3, 931.8) |
| 10(11)-EpDPE | 41.5 (39.7, 47.6) | 53.1 (33.9, 55.9) | 64.8 (35.0, 75.6) | 49.8 (26.7, 74.3) | 52.5 (20.8, 76.1) | 61.3 (37.7, 73.5) |
| 13(14)-EpDPE | 31.4 (30.2, 40.9) | 31.8 (27.5, 43.1) | 45.0 (32.5, 59.9) | 35.8 (18.3, 52.7) | 34.6 (14.8, 53.9) | 41.8 (24.9, 63.7) |
| 16(17)-EpDPE | 40.1 (28.7, 41.3) | 33.8 (29.9, 48.6) | 50.4 (33.5, 74.1) | 37.4 (19.5, 59.1) | 31.2 (15.2, 52.2) | 47.0 (28.9, 71.3) |
| 19(20)-EpDPE | 69.5 (62.4, 80.6) | 71.3 (57.4, 103.6) | 103.4 (65.5, 133.8) | 63.5 (33.0, 94.8) | 61.2 (22.4, 87.5) | 72.2 (50.5, 115.4) |
| Total EpDPE | 801.8 (712.0, 841.5) | 766.0 (663.3, 996.1) | 1,019.1 (711.6, 1,331.6) | 893.1 (484.4, 1,267.8) | 964.5 (371.2, 1,273.1) | 1,058.9 (609.3, 1,257.2) |
| 17-HDoHE | 23.4 (14.9, 41.3) | 19.7 (19.4, 21.0) | 23.2 (21.1, 24.4) | 20.3 (11.8, 39.6) | 15.2 (12.6, 17.8) | 14.4 (12.3, 22.3) |
| 4,5-DiHDPE | 3.7 (2.8, 6.3)ab | 2.7 (2.6, 2.8)a | 4.9 (3.7, 5.9)b | 0.7 (0.3, 0.8) | 0.4 (0.3, 0.4) | 0.6 (0.2, 0.6) |
| 7,8-DiHDPE | 0.3 (0.1, 0.4) | 0.3 (0.2, 0.3) | 0.3 (0.3, 0.4) | 0.4 (0.3, 0.5) | 0.3 (0.2, 0.4) | 0.4 (0.3, 0.5) |
| 10,11-DiHDPE | 0.4 (0.3, 0.5) | 0.3 (0.3, 0.5) | 0.5 (0.4, 0.6) | 0.4 (0.3, 0.6) | 0.4 (0.4, 0.5) | 0.5 (0.4, 0.6) |
| 13,14-DiHDPE | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.6) | 0.7 (0.6, 0.8) | 0.5 (0.3, 0.7) | 0.5 (0.5, 0.5) | 0.6 (0.5, 0.7) |
| 16,17-DiHDPE | 1.2 (1.1, 1.5) | 1.2 (1.2, 1.2) | 1.3 (1.3, 1.7) | 1.1 (0.8, 1.6) | 1.3 (0.9, 1.3) | 1.3 (1.0, 1.6) |
| 19,20-DiHDPE | 5.9 (5.0, 8.2) | 6.0 (5.5, 6.1) | 6.7 (6.2, 8.0) | 6.1 (4.3, 7.4) | 5.5 (4.8, 5.8) | 5.9 (5.3, 6.3) |
Data are presented as median and interquartile ranges (IQR) of n=6–7 per group. The lower and upper boundaries of the IQR represent 25th and 75th percentile values, respectively. The 0.4% and 5.2% LA groups contained six samples each and the 10.5% LA group contained seven samples. Data were analyzed by Kruskal Wallis one-way analysis of variance (ANOVA) followed by Dunn’s post-hoc test. Different alphabetical superscripts mean that the groups differed significantly (P<0.05) from each other.
The discrepancy between changes in plasma and cortex total and unesterified oxylipins suggests that plasma oxylipins are not representative biomarkers of brain oxylipin levels. Causes of this discrepancy could be due to their differing compartmental half-lives or degradation of plasma oxylipins upon entering the brain. The extent of oxylipin entry into the brain is not known. Akanuma et al. reported that the elimination of labeled PGE2 through the blood brain barrier is reduced following intracerebrovascular injection of bacterial lipopolysaccharide, suggesting mechanisms that regulate PGE2 (and possibly other oxylipin) partitioning in and out of brain [38].
The increase in plasma and cortex LA and AA-derived oxylipin concentrations was statistically similar between the 5.2% and 10.5% LA diets relative to 0.4% LA, with a few notable exceptions (Table 4). Mono-hydroxylated, mono-oxygenated (ketones) and epoxidized LA or AA metabolites did not increase beyond 5.2% LA compared to 0.4% LA, whereas many of the epoxide di or tri-hydroxylated sEH degradation products (Figure 1) were significantly higher at 10.5% LA compared to 5.2% or 0.4% LA (Table 4). This suggests that the degradation of CYP-derived epoxidized metabolites by plasma and cortex sEH was enhanced when LA intake increased beyond 5.2% energy. Future studies should confirm this hypothesis by measuring sEH enzyme activity following graded increments in dietary LA. It would be valuable to also identify the enzyme that converts hydroxy, epoxy or ketone metabolites into trihydroxylated products (Figure 1).
The concomitant increase in cortex AA-metabolites and decrease in EPA-metabolites is consistent with a study by Lin et al. which reported higher brain AA-derived HETEs and PGF2α, and lower EPA-derived HEPEs in rats on a 24% LA diet (similar to our 5.2% LA group) compared to rats on a 2% LA diet (similar to our 0.4% LA group) [25]. The reciprocal changes in AA and EPA metabolites following graded LA increments could potentially be explained by two processes – 1) precursor fatty acid availability and 2) COX, LOX, sEH or CYP selectivity towards fatty acids. Brain AA concentration exceeds EPA concentration by > 600-fold [25], yet CYP 4F2, CYP 2E1 and CYP 2J2 preferentially metabolize EPA over AA [13]. Thus, under moderate or high dietary LA conditions, the reduction in cortex EPA concentration (Supplementary Figure 1-B) is substrate limiting to COX, LOX, sEH or CYP enzymes. When LA is lowered, however, cortex EPA concentration increases by approximately 10-fold (Supplementary Figure 1-B) and becomes available for CYP-mediated epoxylation. A similar process likely governs plasma AA and EPA-metabolite levels, but at different substrate thresholds, since plasma AA and EPA concentrations are within the same order of magnitude (Supplementary Figure 1-A). Substrate preference for COX, LOX and sEH enzymes remains to be confirmed.
The increase in plasma LA-derived oxylipins and decrease in DHA-derived oxylipin concentrations are likely related to changes in their precursor fatty acid pool, which increased and decreased respectively with increased dietary LA intake (Supplementary Figure 1-A). LA lowering was reported to decrease plasma LA-derived metabolites (OXLAMs) in humans [39], consistent with the findings of this study. In mice and humans, fish oil supplementation raised circulating DHA concentrations in association with increased plasma DHA metabolites [13, 31].
Consistent with the findings of Lin et al., [25], brain DHA-metabolites were not altered by dietary LA (Table 2), despite the reported increase in brain DHA incorporation rate and turnover (acylation-reacylation rate) within membrane phospholipids [23]. The increase in DHA turnover reflects an increase in the rate of phospholipid remodelling and does not necessarily imply an increase in the rate of formation of DHA-metabolites. It is possible that the rate of formation of DHA-metabolites increased without causing significant accumulation if they are rapidly metabolized. This interpretation would be consistent with the Lin et al study which showed increased rate of loss of DHA from brain following LA lowering [23]. Alternative pathways of loss may also include β-oxidation products or recycling into sterols in response to increased LA incorporation by the brain [40, 41].
In plasma, the concentration of total oxylipins exceeded unesterified oxylipins by 5–10 fold, whereas in cortex, total and unsterified oxylipins were similarly concentrated. This suggests that the majority of oxylipins in plasma are esterified to triacylglyerol, phospholipid, cholesteryl ester or other lipids, whereas in cortex, most oxylipins are unesterified. In plasma, oxylipins are released from the esterified fraction via lipoprotein lipase [33]. The abundance of unesterified oxylipins in cortex is consistent with the notion that they are actively produced when needed via COX, LOX, CYP or sEH enzymes, rather than stored in membrane phospholipids [22]. This is in agreement with the changes seen in this study, which occurred in the cortex unesterified fraction but not the total oxylipin pool.
Changes in oxylipins in response to dietary LA are likely underestimated in this study because the rats were not subjected to head-focused microwave fixation, which stops brain lipid metabolism [42–45]. Brain unesterified fatty acids and their eicosanoid and docosanoid metabolites were reported to increase following decapitation or ischemia compared to microwave fixation [43, 44]. Thus, baseline oxylipin values in this study reflect an ischemic state, which may have masked dietary effects or underestimated the amount of oxylipins esterified to phospholipids. Lin et al. measured brain unesterified AA, EPA and DHA metabolite concentrations in microwave-fixated rat brain following 15 weeks of a low (2% of fatty acid, 0.4% energy) or moderate (24% of fatty acids, 5.2% energy) LA diet [25]. Of the measured AA and EPA metabolites that overlapped with this study, they observed higher AA-derived HETE concentrations in the moderate LA group compared to the low LA group, whereas this study did not find significant changes in HETEs [25]. In both studies, however, AA-derived PGE2 was unaltered and EPA-derived HEPE metabolites increased in the moderate LA group compared to low LA controls [25].
The findings of this study are strengthened by the blinded measurements, which eliminate analytical bias associated with sample handling or LC-MS/MS peak integrations [46, 47], and by measuring oxylipins in both total and unesterified fractions. Limitations include the possibility of statistical false positives and false negatives associated with the small sample size (6–8 per group). Many metabolites were measured on each rat and several statistical tests were performed to identify differences in the diet groups, thus inflating type I errors. Power calculations were not performed prior to initiating the study because it was exploratory, and we did not know the magnitude of variance and change to expect.
In summary, graded increments in dietary LA increased plasma and cortex LA and AA metabolites, and decreased EPA or DHA metabolites. Changes in plasma did not entirely reflect in cortex, thus pointing out the limitation of using plasma measurements as relevant biomarkers of brain oxylipin concentrations. This could be overcome by using positron emitting tomography to image fatty acid incorporation into the brain, as a way to quantify the amount lost due to metabolism (into oxylipins and other signaling molecules) [48, 49]. The changes in brain oxylipins suggest that dietary LA lowering could be used to target disturbed LA, AA or EPA metabolism in brain disorders [9, 49–51].
Supplementary Material
Highlights.
Increased LA intake raised omega-6 and reduced omega-3 derived oxylipins in rat plasma and cortex.
Acknowledgments
We thank Mark S. Horowitz for statistical programming expertise and Dr. Helene Blanchard and Lisa Chang for their help with the brain dissection and plasma collection. This study was funded by the UC Davis College of Agriculture and Environmental Sciences start-up funds to A.Y.T and the National Institute on Aging (NIA) and Alcohol Abuse and Alcoholism (NIAAA) Intramural Research Programs. Partial support was provided by NIEHS R01 ES002710 and Superfund Research Program P42 ES04699.
Abbreviations
- AA
arachidonic acid
- ANOVA
one-way analysis of variance
- BHT
butylated hydroxytoluene
- cPLA2
calcium-dependent phospholipase A2
- COX
cyclooxygenase
- CYP
cytochrome P450
- DGLA
dihomo-gamma-linoleic acid
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EDTA
ethylenediaminetetraacetic acid
- EPA
eicosapentaenoic acid
- GC
gas-chromatography
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- IQR
interquartile ranges
- iPLA2
calcium-independent phospholipase A2
- LA
linoleic acid
- α-LNA
α–linolenic acid
- LOX
lipoxygenase
- MRM
multiple reaction monitoring
- OXLAM
oxidized linoleic acid metabolite
- PUFA
polyunsaturated fatty acid
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- sEH
soluble expoxide hydrolase
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None to declare
References
- 1.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinaud O, Delaforge M, Boucher JL, Rocchiccioli F, Mansuy D. Oxidative metabolism of linoleic acid by human leukocytes. Biochem Biophys Res Commun. 1989;161:883–891. doi: 10.1016/0006-291x(89)92682-x. [DOI] [PubMed] [Google Scholar]
- 3.Engels F, Willems H, Nijkamp FP. Cyclooxygenase-catalyzed formation of 9-hydroxylinoleic acid by guinea pig alveolar macrophages under non-stimulated conditions. FEBS Lett. 1986;209:249–253. doi: 10.1016/0014-5793(86)81121-8. [DOI] [PubMed] [Google Scholar]
- 4.Moghaddam M, Motoba K, Borhan B, Pinot F, Hammock BD. Novel metabolic pathways for linoleic and arachidonic acid metabolism. Biochim Biophys Acta. 1996;1290:327–339. doi: 10.1016/0304-4165(96)00037-2. [DOI] [PubMed] [Google Scholar]
- 5.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida Y, Yoshikawa A, Kinumi T, Ogawa Y, Saito Y, Ohara K, Yamamoto H, Imai Y, Niki E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiology of aging. 2009;30:174–185. doi: 10.1016/j.neurobiolaging.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, Ringel A, Hibbeln JR, Feldstein AE, Mori TA, Barden A, Lynch C, Coble R, Mas E, Palsson O, Barrow DA, Mann JD. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyurina YY, Polimova AM, Maciel E, Tyurin VA, Kapralova VI, Winnica DE, Vikulina AS, Domingues MR, McCoy J, Sanders LH, Bayir H, Greenamyre JT, Kagan VE. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: Implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic Res. 2015;49:681–691. doi: 10.3109/10715762.2015.1005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J, Beard JD, Umbach DM, Park Y, Huang X, Blair A, Kamel F, Chen H. Dietary fat intake and risk for Parkinson’s disease. Mov Disord. 2014;29:1623–1630. doi: 10.1002/mds.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aukema HM, Winter T, Ravandi A, Dalvi S, Miller DW, Hatch GM. Generation of Bioactive Oxylipins from Exogenously Added Arachidonic, Eicosapentaenoic and Docosahexaenoic Acid. Primary Human Brain Microvessel Endothelial Cells, Lipids. 2015 doi: 10.1007/s11745-015-4074-0. [DOI] [PubMed] [Google Scholar]
- 13.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassam AG, Sinclair AJ, Crawford MA. The incorporation of orally fed radioactive gamma-linolenic acid and linoleic acid into the liver and brain lipids of suckling rats. Lipids. 1975;10:417–420. [PubMed] [Google Scholar]
- 15.Sinclair AJ, Crawford MA. The incorporation of linolenic aid and docosahexaenoic acid into liver and brain lipids of developing rats. FEBS Lett. 1972;26:127–129. doi: 10.1016/0014-5793(72)80557-x. [DOI] [PubMed] [Google Scholar]
- 16.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trepanier MO, Lim J, Lai TK, Cho HJ, Domenichiello AF, Chen CT, Taha AY, Bazinet RP, Burnham WM. Intraperitoneal administration of docosahexaenoic acid for 14days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model. Epilepsy & behavior: E&B. 2014;33:138–143. doi: 10.1016/j.yebeh.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP. Unesterified docosahexaenoic acid is protective in neuroinflammation. Journal of neurochemistry. 2013;127:378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, Zhang C, Jun B, Obenaus A, Fredman G, Zhu M, Winkler JW, Petasis NA, Serhan CN, Belayev L. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Experimental neurology. 2012;236:122–130. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard HC, Taha AY, Rapoport SI, Yuan ZX. Low-dose aspirin (acetylsalicylate) prevents increases in brain PGE2, 15-epi-lipoxin A4 and 8-isoprostane concentrations in 9 month-old HIV-1 transgenic rats, a model for HIV-1 associated neurocognitive disorders. Prostaglandins, leukotrienes, and essential fatty acids. 2015;96:25–30. doi: 10.1016/j.plefa.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr. 2015;6:513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HW, Rao JS, Rapoport SI, Igarashi M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim Biophys Acta. 2011;1811:111–117. doi: 10.1016/j.bbalip.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igarashi M, Kim HW, Chang L, Ma K, Rapoport SI. Dietary n-6 polyunsaturated fatty acid deprivation increases docosahexaenoic acid metabolism in rat brain. Journal of neurochemistry. 2012;120:985–997. doi: 10.1111/j.1471-4159.2011.07597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin LE, Chen CT, Hildebrand KD, Liu Z, Hopperton KE, Bazinet RP. Chronic dietary n-6 PUFA deprivation leads to conservation of arachidonic acid and more rapid loss of DHA in rat brain phospholipids. J Lipid Res. 2015;56:390–402. doi: 10.1194/jlr.M055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsden CE, Ringel A, Majchrzak-Hong SF, Yang J, Blanchard H, Zamora D, Loewke JD, Rapoport SI, Hibbeln JR, Davis JM, Hammock BD, Taha AY. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: Implications for idiopathic pain syndromes? Molecular Pain. 2016 doi: 10.1177/1744806916636386. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nature reviews Neuroscience. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 29.Zivkovic AM, Yang J, Georgi K, Hegedus C, Nording ML, O’Sullivan A, German JB, Hogg RJ, Weiss RH, Bay C, Hammock BD. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics: Official journal of the Metabolomic Society. 2012;8:1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuchardt JP, Schmidt S, Kressel G, Dong H, Willenberg I, Hammock BD, Hahn A, Schebb NH. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins, leukotrienes, and essential fatty acids. 2013;89:19–29. doi: 10.1016/j.plefa.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins & other lipid mediators. 2014;113–115:21–29. doi: 10.1016/j.prostaglandins.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaik JS, Ahmad M, Li W, Rose ME, Foley LM, Hitchens TK, Graham SH, Hwang SH, Hammock BD, Poloyac SM. Soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is neuroprotective in rat model of ischemic stroke. Am J Physiol Heart Circ Physiol. 2013;305:H1605–1613. doi: 10.1152/ajpheart.00471.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins, leukotrienes, and essential fatty acids. 2008;79:215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. The Journal of nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 35.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 36.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akanuma S, Uchida Y, Ohtsuki S, Tachikawa M, Terasaki T, Hosoya K. Attenuation of prostaglandin E2 elimination across the mouse blood-brain barrier in lipopolysaccharide-induced inflammation and additive inhibitory effect of cefmetazole. Fluids and barriers of the CNS. 2011;8:24. doi: 10.1186/2045-8118-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins, leukotrienes, and essential fatty acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunnane SC, Anderson MJ. The majority of dietary linoleate in growing rats is beta-oxidized or stored in visceral fat. The Journal of nutrition. 1997;127:146–152. doi: 10.1093/jn/127.1.146. [DOI] [PubMed] [Google Scholar]
- 41.Cunnane SC, Trotti D, Ryan MA. Specific linoleate deficiency in the rat does not prevent substantial carbon recycling from [(14)C]linoleate into sterols. J Lipid Res. 2000;41:1808–1811. [PubMed] [Google Scholar]
- 42.Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochemical research. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- 43.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochemical research. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- 45.Brose SA, Golovko SA, Golovko MY. Brain 2-Arachidonoylglycerol Levels Are Dramatically and Rapidly Increased Under Acute Ischemia-Injury Which Is Prevented by Microwave Irradiation. Lipids. 2016;51:487–495. doi: 10.1007/s11745-016-4144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke; a journal of cerebral circulation. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macleod MR, Fisher M, O’Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, Donnan GA, Howells DW. Good laboratory practice: preventing introduction of bias at the bench. Stroke; a journal of cerebral circulation. 2009;40:e50–52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- 48.Thambisetty M, Gallardo KA, Liow JS, Beason-Held LL, Umhau JC, Bhattacharjee AK, Der M, Herscovitch P, Rapoport JL, Rapoport SI. The utility of (11)C-arachidonate PET to study in vivo dopaminergic neurotransmission in humans. J Cereb Blood Flow Metab. 2012;32:676–684. doi: 10.1038/jcbfm.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, Hallett M, Herscovitch P, Eckelman WC, Carson RE, Rapoport SI. Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008;49:1414–1421. doi: 10.2967/jnumed.107.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsden CE, Faurot KR, Zamora D, Palsson OS, MacIntosh BA, Gaylord S, Taha AY, Rapoport SI, Hibbeln JR, Davis JM, Mann JD. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain. 2015;156:587–596. doi: 10.1097/01.j.pain.0000460348.84965.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alashmali SM, Hopperton KE, Bazinet RP. Lowering dietary n-6 polyunsaturated fatty acids: interaction with brain arachidonic and docosahexaenoic acids. Curr Opin Lipidol. 2016;27:54–66. doi: 10.1097/MOL.0000000000000255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


