Abstract
Objective
Although many treatment modalities have been introduced for trigeminal neuralgia (TN), the long-term clinical results remain unsatisfactory. It has been particularly challenging to determine an appropriate treatment strategy for patients who have responded poorly to initial therapies. We analyzed the surgical outcomes in TN patients who failed prior treatments.
Methods
We performed a retrospective analysis of 37 patients with recurrent or persistent TN symptoms who underwent surgery at our hospital between January 2010 and December 2014. Patients with follow-up data of at least one year were included. The prior treatment modalities of the 37 patients included microvascular decompression (MVD), gamma knife radiosurgery (GKRS), and percutaneous procedures such as radiofrequency rhizotomy (RFR), balloon compression, and glycerol rhizotomy (GR). The mean follow-up period was 69.9 months (range : 16–173). The mean interval between the prior treatment and second surgery was 26 months (range : 7–123). We evaluated the surgical outcomes using the Barrow Neurological Institute (BNI) pain intensity scale.
Results
Among the 37 recurrent or persistent TN patients, 22 underwent MVD with partial sensory rhizotomy (PSR), 8 received MVD alone, and 7 had PSR alone. Monitoring of the surgical treatment outcomes via the BNI pain intensity scale revealed 8 (21.6%) patients with a score of I, 13 (35.1%) scoring II, 13 (35.1%) scoring III, and 3 (8.2%) scoring IV at the end of the follow-up period. Overall, 91.8% of patients had good surgical outcomes. With regard to postoperative complications, 1 patient had transient cerebrospinal fluid rhinorrhea (2.7%), another had a subdural hematoma (2.7%), and facial sensory changes were noted in 8 (21.1%) patients after surgery.
Conclusion
Surgical interventions, such as MVD and PSR, are safe and very effective treatment modalities in TN patients who failed initial or prior treatments. We presume that the combination of MVD with PSR enabled us to obtain good short- and long-term surgical outcomes. Therefore, aggressive surgical treatment should be considered in patients with recurrent TN despite failure of various treatment modalities.
Keywords: Trigeminal neuralgia, Microvascular decompression, Rhizotomy
INTRODUCTION
Trigeminal neuralgia (TN) is characterized by sudden attacks of severe facial pain41,42). Various surgical procedures are recommended for patients who are refractory to medical treatment. These procedures include microvascular decompression (MVD) and percutaneous procedures such as glycerol rhizotomy (GR), balloon microcompression of the trigeminal ganglion (BC), radio frequency rhizotomy (RFR), and stereotactic radiosurgery (SRS)34,40,43). One study analyzed the surgical outcomes for each of these treatment modalities. In the short term, good outcomes (i.e., BNI scores of I-III) were observed in 75% of MVD cases, 92% of SRS, 86% of GR, 86% of balloon compression, and 80% of RFR cases. Long-term outcomes of these same procedures were favorable in 70%, 70%, 46%, 43.5%, and 27.1% of cases, respectively. In other words, aside from MVD and SRS, none of the known treatment modalities showed good long-term clinical results12,18,28). Due the effects of neurovascular conflict, MVD has become the first choice for patients with TN refractory to medication treatment35,36,37).
Unfortunately, despite surgical intervention, some patients still have recurrent symptoms, with an annual recurrence risk of 1–4%2,9,14,30). Although neurodestructive procedures such as glycerol injection provide short-term relief, their long-term results are unsatisfactory; only 40–50% are successful17,38). Therefore, it is difficult to determine an appropriate treatment strategy for patients continuing to have facial pain after prior treatments. In addition, if ablative procedures are performed repeatedly, patients are at risk for facial numbness. Several authors have compiled there currence rates for each of the surgical and non-surgical procedures currently available (MVD, 30%; SRS, 43%, GR, 68–78%; BC, 45%; and RFR, 42%)4,7,22,26,32). Repeat surgical treatments (including MVD) have been reported to be reasonable options for recurrent TN, even after neurodestructive procedures; however, this approach has lower success rates and higher complication rates than primary surgery3,14,32). We reviewed our experiences in the surgical treatment of TN patients with persistent or recurrent pain despite prior treatment.
MATERIALS AND METHODS
Patient population
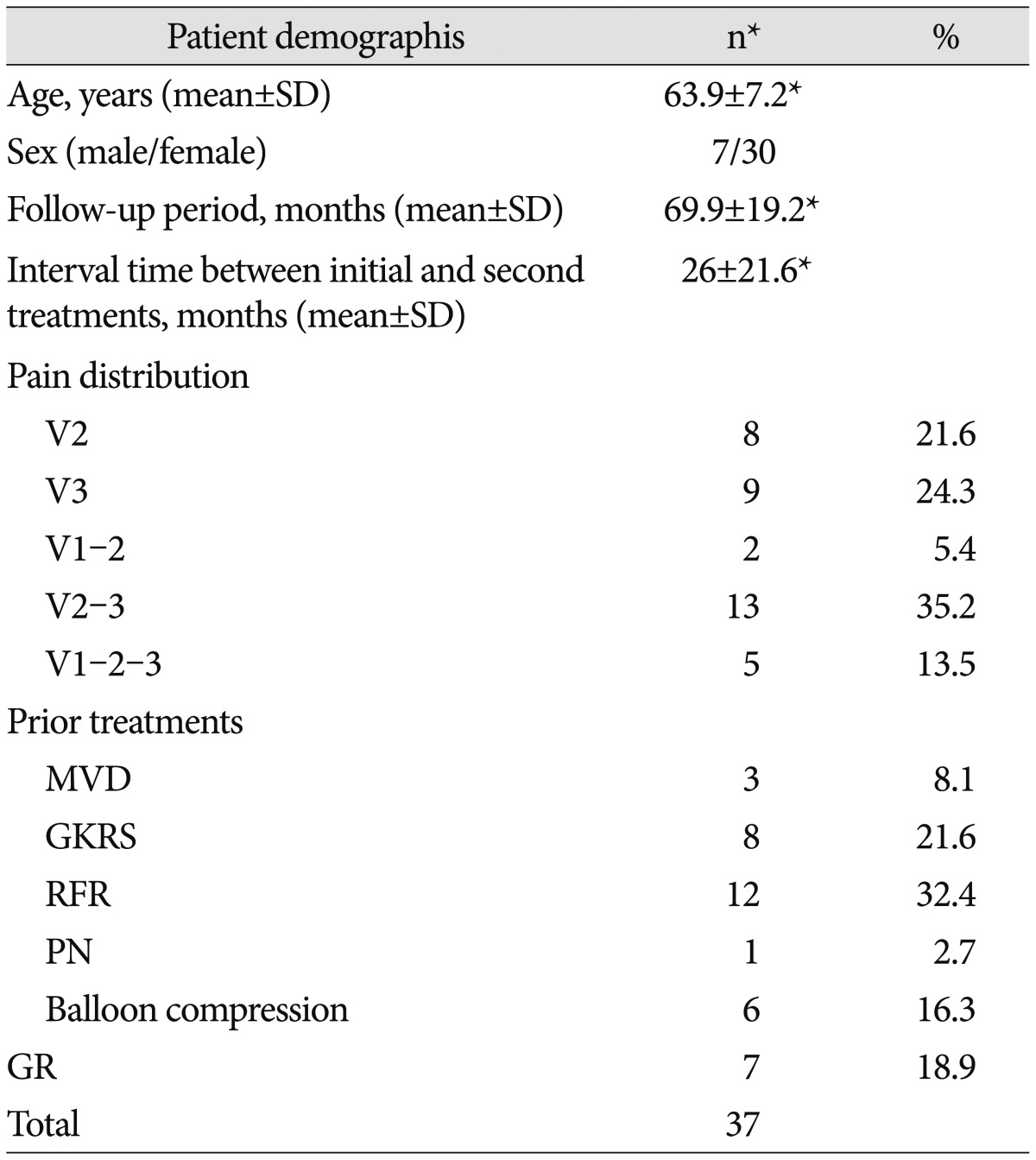
We retrospectively analyzed 37 patients with primary TN who underwent surgical treatment at our hospital between January 2010 and December 2014. Only patients with postoperative follow-up data for at least one year were included. Patients were selected if they had persistent facial pain or recurrent facial pain after prior treatment. If there was definitive evidence of neurovascular compression, surgery was performed. However, in cases where the offending vessel could not be clearly identified, the symptoms were believed to be the result of venous compression with an adequate duration of symptoms; when sufficient decompression could not be achieved, a partial sensory rhizotomy (PSR) was performed. Recurrent TN was defined as a resurgence of pain on the same side after a previously successful treatment. A successful previous treatment was defined as complete pain relief without the need for medication. A total of 37 patients underwent surgery as a re-treatment modality for persistent or recurrent TN. The group comprised 7 males and 30 females with a mean age of 63.9 years at the time of treatment (range : 40–80 years). These patients received their primary treatments at other centers, which were either unsuccessful at relieving their symptoms or were followed by recurrent symptoms. Prior to surgery, 26 patients underwent percutaneous procedures for TN : radiofrequency rhizotomy in 12 patients, GR in 8, and balloon compression in 6 patients. In addition, 8 patients were treated with gamma knife radiosurgery (GKRS) and 3 underwent MVD (Table 1). All of the patients were followed postoperatively for a minimum of 1 year.
Table 1. Patient demographics.
*Other value. MVD : microvascular decompression, PSR : partial sensory rhizotomy, PN : peripheral neurectomy, GKRS : gamma knife radiosurgery, RFR : radiofrequency rhizotomy, GR : glycerol rhizotomy, SD : standard deviation, n : number
Surgery
Patients were placed in a lateral position for surgery, as previously described25). All patients underwent intraoperative brain stem monitoring to measure their auditory evoked responses. In patients with a history of prior surgery, meticulous muscular dissections were performed to avoid dural injury due to adhesions. After the dura was opened, if an offending vessel could be clearly confirmed, MVD was performed. However, in certain cases (as listed below), PSR was performed by dividing the caudal two-thirds to one-half portion of the trigeminal nerve sensory root. This procedure was performed under the following circumstances : older age (>65 years); patients in poor medical condition; cases where no distinct compressive lesions could be identified; scenarios in which a venous offending vessel was found to be the culprit; and in cases of multiple prior percutaneous procedures. Additionally, if the offending vessels could be visualized intraoperatively without any radiological evidence of compression lesions, then both MVD and PSR were performed, even if only partial rhizotomy was planned preoperatively. Intraoperatively, arterial offending vessels were definitively identified in 20 patients, venous offending vessels were present in 9 patients, and no offenders were found in 5 patients. Teflon granuloma formation was evident in 1 patient, and the remaining 2 patients demonstrated inadequate decompression.
Pain assessment and analysis
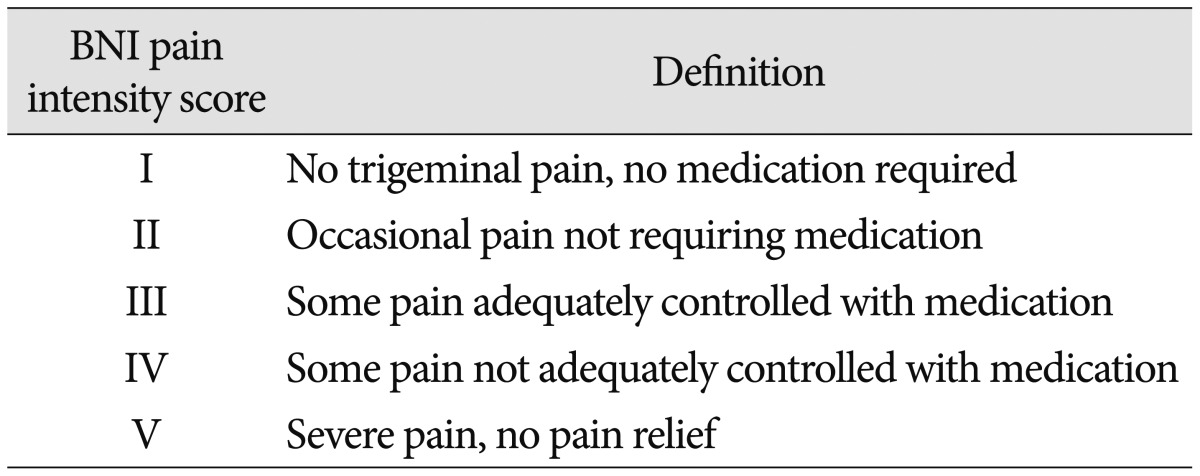
Pre- and post-operative pain measurements were obtained from the medical records or telephone inquiries based on the Barrow Neurological Institute (BNI) pain intensity scale (Table 2). The categories of pain relief evaluated by this scale included excellent (BNI pain score I-II), good (BNI pain score III), and poor (BNI pain score IV-V). These outcomes were assessed immediately after surgery and during the last follow-up visit in the outpatient department. In order to analyze the relationships between the patients' clinical features and long-term outcomes, we collected various descriptive statistical variables, including patient age, sex, interval time before re-treatment, pain distribution, prior treatment modalities, type of offending vessels, and whether rhizotomy was implemented. In order to investigate the factors effectively, the offending vessels were categorized as either arterial or venous vessels. Statistical analyses were performed using the chi-square test, Fisher's exact test, and Spearman correlation test. The Statistical Package for the Social Sciences (SPSS; ver. 21.0; Chicago, IL, USA) was used for all calculations. p-values <0.05 were considered to be statistically significant.
Table 2. BNI pain intensity score.
BNI : barrow neurological institute
RESULTS
Prior to surgery, all patients had facial pain BNI scores greater than IV despite initial TN treatment. A total of 22 patients underwent a combination of MVD with PSR. Postoperatively, 12 of these patients (54.6%) had improved facial pain, with a BNI score better than II. However, 5 patients (23%) complained of facial dysesthesia, 2 of whom had previously experienced facial sensory changes following the first treatment. One patient had cerebrospinal fluid leakage, and another patient had a subdural hematoma. Of the 8 patients who only underwent MVD, 6 (75%) experienced excellent pain relief, and none complained of facial dysesthesia. In contrast, of the 7 patients who were treated with PSR, 3 (42.9%) showed improvement with a BNI score of II, while 3 (42%) had facial dysesthesia. Of these three patients, one had facial sensory changes prior to re-treatment.
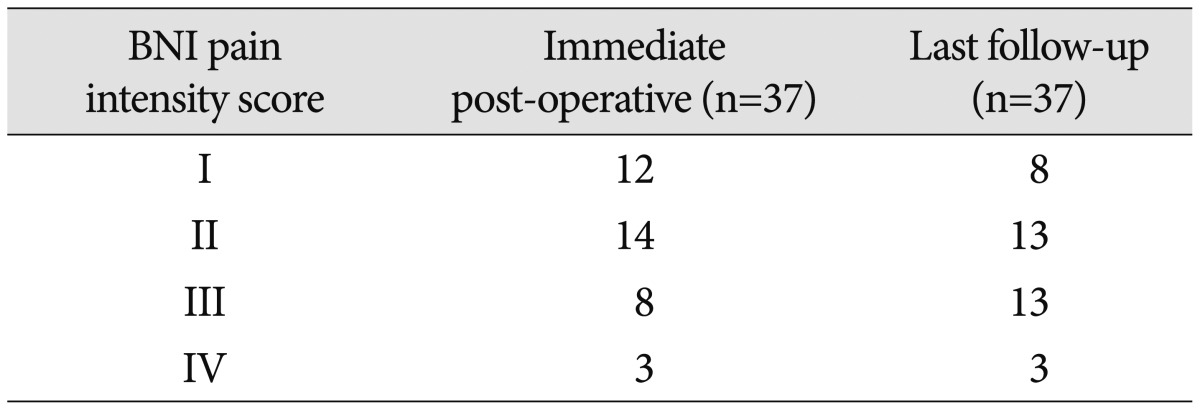
Immediately after repeat surgery, 12 patients had facial pain relief with a BNI score of I, 14 had a BNI score of II, and 8 patients increased to a BNI score of III. Three patients experienced persistent facial pain with a BNI score greater than IV. The mean follow-up period was 69.9 months (range : 16–173). The mean interval between the initial and second treatments was 26 months (range : 7–123). As of the end of the follow-up period, 21 (56.7%) patients had excellent pain relief (BNI score of I in 8 and II in 13 patients), 13 (35.1%) had good pain relief (BNI score of I-III in 34), and 3 (8.2%) had poor pain relief (Table 3). Overall, 56.7% of patients experienced excellent surgical outcomes.
Table 3. Postoperative BNI pain intensity scores.
BNI : barrow neurological institute
There were no intraoperative complications or increased mortality, although there were several postoperative complications. These included one case of cerebrospinal fluid leakage, one subdural hematoma, and facial dysesthesia in 8 (21.1%) patients. No postoperative hearing loss was noted.
There were no significant relationships between clinical factors and surgical outcomes. A Spearman's correlation revealed a p-value of 0.853 upon analysis of age and 0.541 with respect to the interval time. Sex, pain distribution, prior treatment modalities, types of offending vessels, and rhizotomy implementation were not significant factors, according to the results of the Fisher's exact test (p-value=0.771, 0.785, 0.984, 0.635, and 0.542, respectively).
DISCUSSION
Since the pioneering work of Jannetta in the 1970s, MVD has become one of the most common treatments for TN19). However, the recurrence of TN symptoms remains a challenge for neurosurgeons. The likelihood of recurrence is variable. Many authors have sought to explain the recurrence of TN after treatment. Kabatas et al.21) cited multiple factors, such as Teflon granuloma formation, excessive Teflon insertion, improper and inadequate operative techniques, Teflon dislocation, and venous compression after the MVD procedure, in the redevelopment of symptoms. In regard to the GR procedure, Blomstedt et al.8) reported that the needle should enter the trigeminal cistern via the foramen ovale. If the needle is improperly placed, it can enter the subtemporal region or pass through the foramen spinosum and cause venous or arterial bleeding, resulting in the recurrence of symptoms. Several factors may contribute to TN recurrence in patients after a balloon compression procedure. These include the formation of a cheek hematoma, inappropriate localization, or inadequate balloon inflation1,10). Gusmão et al.15) explained other situations in which TN symptoms may reoccur after a radiofrequency rhizotomy. For instance, if there was any difficulty in visualizing the foramen ovale, an inappropriate passage may be formed through the carotid canal, jugular foramen, or foramen Vesalius; these situations complicate the overall treatment process and may contribute to symptom recurrence. Controversy exists surrounding the most appropriate and tolerable cumulative dose that should be used during GKRS in the treatment of TN. Therefore, discrepancies in drug dosing may potentially contribute to the redevelopment of symptoms following GKRS13,24). Symptoms may also return after a peripheral neurectomy. Murali et al.27) hypothesized that this recurrence is due to the regeneration of peripheral nerves that occurs anywhere from18 months to 3 years postoperatively.
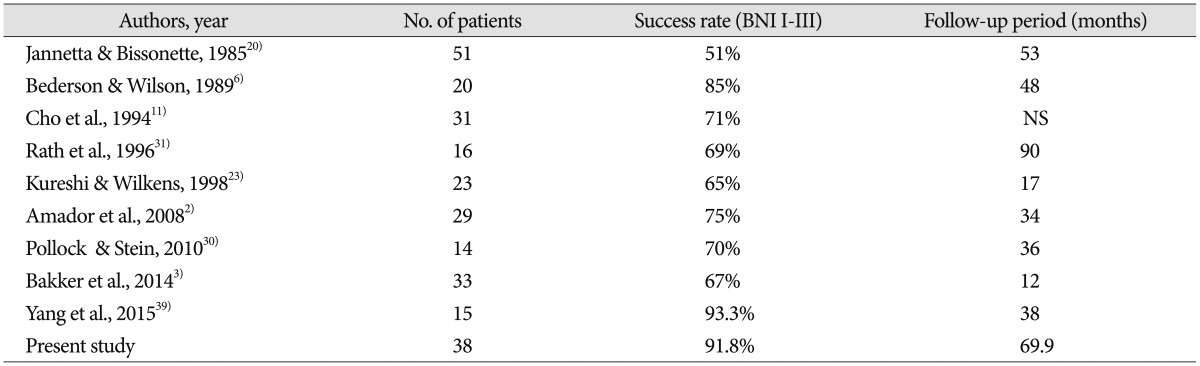
For several reasons, most neurosurgeons recommend that patients with persistent or recurrent TN undergo less invasive procedures to achieve pain relief. First, the success rate of repeat MVD is significantly lower than that of primary operations. According to previous studies, the success rate of secondary surgeries ranges from 51% to 93.3% (Table 4). Barker et al.5) reported complete pain relief in 42% of patients 10 years after undergoing a second operation, compared to 64% of patients after the initial MVD. In addition, when recurrent pain is treated with radiosurgery or GR, between 50% and 59% of patients continued to be pain-free without the use of medications 3 years later13,29). Secondly, the risk of facial numbness is greater with repeated operations. Barker et al.4) reported that 11 of 1204 patients (0.9%) had severe facial numbness after their initial MVD, in contrast to 11 (8%) of 132 patients after a second MVD.
Table 4. Summary of literature regarding the success rate of repeat surgical treatment.
No : number, NS : not stated, BNI : barrow neurological institute
Because decompression is a viable option in cases of neurovascular compression, several authors have suggested that re-exploration of the posterior fossa should be performed for recurrent or persistent TN, because appropriate decompression can be performed in cases of neurovascular compression. PSR can be employed in non-neurovascular cases or in those involving Teflon granulomas. In these instances, recurrence can be prevented. Although the success rate of re-operation was lower than that of the initial operation, performing a second surgery showed a higher long-term success rate than did other treatment modalities16,30). There was a higher risk of facial dysesthesia after a second surgery than after the first surgery. This was due to surgeons frequently performing a PSR at the time of repeat surgery in the absence of a compelling compressive lesion11,20,23). Therefore, the risk of trigeminal nerve injury was higher during the second MVD than during the first surgery, increasing the rate of facial dysesthesia. In the current study, eight patients (21%) complained of facial numbness after the second surgery. All of them underwent a PSR. With the exception of a single patient who had a postoperative BNI score of IV, the remaining 7 patients continue to be followed in our neurosurgical outpatient clinic and are satisfied with their postoperative results. Their symptoms have also been well controlled with the aid of medical treatment.
Although the success rate of re-operation falls between 51% and 93.3% (Table 4)2,3,6,11,20,23,30,31,39), the success rate of percutaneous procedures is up to 57%12). The performance of a PSR procedure with or without MVD can be considered as an option for repeat operative intervention. Despite the variety of treatments available in the management of recurrent TN, the patients in our study had failed these treatments at other centers and were receiving ineffective medical treatment for a prolonged period of time. Our center has an extensive history of treating TN patients, with a success rate matching that of multiple studies. Hence, these factors led us to perform repeat surgeries in these patients. In this study, we were able to obtain good surgical outcomes comparable to primary treatment due to the addition of PSR to conventional MVD procedures. Also, in the cases where definite MVD could not be achieved due to absence of neurovascular conflict, PSR along with additional techniques such as mechanical manipulation (squeezing of trigeminal nerve, thermal injury of trigeminal nerve) and teflon insertion in Meckel's cave (Rhee's method) were performed. We believe that our results are particularly noteworthy given that our analysis is based on long-term follow-up data (69.9 months). Although the rate of excellent outcomes was only 56.7%, the rate of good outcomes approached 91.9%. Together, these numbers illustrate excellent surgical results. Based on five years of data, we deduced that recurrence rates can be substantially reduced by complementing the conventional MVD procedure with PSR. Regardless, there is a need for further studies targeted at exploring the effects of PSR on the recurrence rate. The factors that have historically been associated with poor outcomes after an initial MVD included female sex, pain duration, atypical features, and venous compression4,33). In the current study, there was no statistical association between various factors and surgical outcomes. However, this discrepancy may indicate a difference in our study population, as Bakker et al.3) only investigated patients who underwent MVD alone.
This study has several limitations. First, it is a retrospective study. Second, being less than 10 years in duration, the follow-up period was relatively short. Third, the sample of patients included was fairly small. Therefore, further, larger studies conducted over longer than10 years are needed to substantiate our findings.
CONCLUSION
The present study confirms that revision surgery, an intervention that demonstrates a good success rate and a low complication rate, is a feasible therapeutic option for patients with recurrent TN. Although PSR caused mild to moderate facial dysesthesia, this symptom was tolerable in most patients. Therefore, MVD performed concurrently with PSR is a highly effective treatment strategy for patients with a history of failed pain management. For patients with recurrent TN despite various treatments, aggressive surgical treatment should be considered.
References
- 1.Abdennebi B, Guenane L. Technical considerations and outcome assessment in retrogasserian balloon compression for treatment of trigeminal neuralgia. Series of 901 patients. Surg Neurol Int. 2014;5:118. doi: 10.4103/2152-7806.137838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amador N, Pollock BE. Repeat posterior fossa exploration for patients with persistent or recurrent idiopathic trigeminal neuralgia. J Neurosurg. 2008;108:916–920. doi: 10.3171/JNS/2008/108/5/0916. [DOI] [PubMed] [Google Scholar]
- 3.Bakker NA, Van Dijk JM, Immenga S, Wagemakers M, Metzemaekers JD. Repeat microvascular decompression for recurrent idiopathic trigeminal neuralgia. J Neurosurg. 2014;121:936–939. doi: 10.3171/2014.7.JNS132667. [DOI] [PubMed] [Google Scholar]
- 4.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Jho HD. Trigeminal numbness and tic relief after microvascular decompression for typical trigeminal neuralgia. Neurosurgery. 1997;40:39–45. doi: 10.1097/00006123-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 6.Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71:359–367. doi: 10.3171/jns.1989.71.3.0359. [DOI] [PubMed] [Google Scholar]
- 7.Bender M, Pradilla G, Batra S, See A, Bhutiani N, James C, et al. Effectiveness of repeat glycerol rhizotomy in treating recurrent trigeminal neuralgia. Neurosurgery. 2012;70:1125–1133. doi: 10.1227/NEU.0b013e31823f5eb6. discussion 1133-1134. [DOI] [PubMed] [Google Scholar]
- 8.Blomstedt PC, Bergenheim AT. Technical difficulties and perioperative complications of retrogasserian glycerol rhizotomy for trigeminal neuralgia. Stereotact Funct Neurosurg. 2002;79:168–181. doi: 10.1159/000070830. [DOI] [PubMed] [Google Scholar]
- 9.Capelle HH, Brandis A, Tschan CA, Krauss JK. Treatment of recurrent trigeminal neuralgia due to Teflon granuloma. J Headache Pain. 2010;11:339–344. doi: 10.1007/s10194-010-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng JS, Lim DA, Chang EF, Barbaro NM. A review of percutaneous treatments for trigeminal neuralgia. Neurosurgery. 2014;10(Suppl 1):25–33. doi: 10.1227/NEU.00000000000001687. discussion 33. [DOI] [PubMed] [Google Scholar]
- 11.Cho DY, Chang CG, Wang YC, Wang FH, Shen CC, Yang DY. Repeat operations in failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1994;35:665–669. doi: 10.1227/00006123-199410000-00012. discussion 669-670. [DOI] [PubMed] [Google Scholar]
- 12.Czepko R, Kwinta B, Libionka W, Pietraszko W. [Direct and late outcome in trigeminal neuralgia treated by means of microvascular decompression in cerebellopontine angle] Przegl Lek. 2003;60:621–624. [PubMed] [Google Scholar]
- 13.Dvorak T, Finn A, Price LL, Mignano JE, Fitzek MM, Wu JK, et al. Retreatment of trigeminal neuralgia with Gamma Knife radiosurgery : is there an appropriate cumulative dose? Clinical article. J Neurosurg. 2009;111:359–364. doi: 10.3171/2008.11.JNS08770. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Zhao W. Microvascular decompression for recurrent trigeminal neuralgia. J Clin Neurosci. 2014;21:1549–1553. doi: 10.1016/j.jocn.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Gusmão S, Oliveira M, Tazinaffo U, Honey CR. Percutaneous trigeminal nerve radiofrequency rhizotomy guided by computerized tomography fluoroscopy. Technical note. J Neurosurg. 2003;99:785–786. doi: 10.3171/jns.2003.99.4.0785. [DOI] [PubMed] [Google Scholar]
- 16.Han I, Shin D, Chang J, Kim K, Chang J, Huh R, et al. Effect of various surgical modalities in recurrent or persistent trigeminal neuralgia. Stereotact Funct Neurosurg. 2010;88:156–162. doi: 10.1159/000303530. [DOI] [PubMed] [Google Scholar]
- 17.Harries AM, Mitchell RD. Percutaneous glycerol rhizotomy for trigeminal neuralgia : safety and efficacy of repeat procedures. Br J Neurosurg. 2011;25:268–272. doi: 10.3109/02688697.2011.558946. [DOI] [PubMed] [Google Scholar]
- 18.Henson CF, Goldman HW, Rosenwasser RH, Downes MB, Bednarz G, Pequignot EC, et al. Glycerol rhizotomy versus gamma knife radiosurgery for the treatment of trigeminal neuralgia : an analysis of patients treated at one institution. Int J Radiat Oncol Biol Phys. 2005;63:82–90. doi: 10.1016/j.ijrobp.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Jannetta PJ. Trigeminal neuralgia and hemifacial spasm--etiology and definitive treatment. Trans Am Neurol Assoc. 1975;100:89–91. [PubMed] [Google Scholar]
- 20.Jannetta PJ, Bissonette DJ. Management of the failed patient with trigeminal neuralgia. Clin Neurosurg. 1985;32:334–347. [PubMed] [Google Scholar]
- 21.Kabatas S, Albayrak SB, Cansever T, Hepgul KT. Microvascular decompression as a surgical management for trigeminal neuralgia : a critical review of the literature. Neurol India. 2009;57:134–138. doi: 10.4103/0028-3886.51279. [DOI] [PubMed] [Google Scholar]
- 22.Kondziolka D, Zorro O, Lobato-Polo J, Kano H, Flannery TJ, Flickinger JC, et al. Gamma Knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2010;112:758–765. doi: 10.3171/2009.7.JNS09694. [DOI] [PubMed] [Google Scholar]
- 23.Kureshi SA, Wilkins RH. Posterior fossa reexploration for persistent or recurrent trigeminal neuralgia or hemifacial spasm : surgical findings and therapeutic implications. Neurosurgery. 1998;43:1111–1117. doi: 10.1097/00006123-199811000-00061. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Choi HJ, Ko HC, Choi SK, Lim YJ. Long term outcomes of gamma knife radiosurgery for typical trigeminal neuralgia-minimum 5-year follow-up. J Korean Neurosurg Soc. 2012;51:276–280. doi: 10.3340/jkns.2012.51.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Park BJ, Shin HS, Park CK, Rhee BA, Lim YJ. Prognostic ability of intraoperative electromyographic monitoring during microvascular decompression for hemifacial spasm to predict lateral spread response outcome. J Neurosurg. 2016 Apr 22; doi: 10.3171/2016.1.JNS151782. [Epub] [DOI] [PubMed] [Google Scholar]
- 26.Meyer B, Lehmberg J. Treatment options for refractory trigeminal neuralgia. World Neurosurg. 2012;77:275–276. doi: 10.1016/j.wneu.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Murali R, Rovit RL. Are peripheral neurectomies of value in the treatment of trigeminal neuralgia? An analysis of new cases and cases involving previous radiofrequency gasserian thermocoagulation. J Neurosurg. 1996;85:435–437. doi: 10.3171/jns.1996.85.3.0435. [DOI] [PubMed] [Google Scholar]
- 28.Noorani I, Lodge A, Vajramani G, Sparrow O. Comparing percutaneous treatments of trigeminal neuralgia : 19 years of experience in a single centre. Stereotact Funct Neurosurg. 2016;94:75–85. doi: 10.1159/000445077. [DOI] [PubMed] [Google Scholar]
- 29.Pollock BE. Percutaneous retrogasserian glycerol rhizotomy for patients with idiopathic trigeminal neuralgia : a prospective analysis of factors related to pain relief. J Neurosurg. 2005;102:223–228. doi: 10.3171/jns.2005.102.2.0223. [DOI] [PubMed] [Google Scholar]
- 30.Pollock BE, Stein KJ. Surgical management of trigeminal neuralgia patients with recurrent or persistent pain despite three or more prior operations. World Neurosurg. 2010;73:523–528. doi: 10.1016/j.wneu.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Rath SA, Klein HJ, Richter HP. Findings and long-term results of subsequent operations after failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1996;39:933–938. doi: 10.1097/00006123-199611000-00010. discussion 938-940. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Mejia RO, Limbo M, Cheng JS, Camara J, Ward MM, Barbaro NM. Recurrent or refractory trigeminal neuralgia after microvascular decompression, radiofrequency ablation, or radiosurgery. Neurosurg Focus. 2005;18:e12. doi: 10.3171/foc.2005.18.5.13. [DOI] [PubMed] [Google Scholar]
- 33.Tyler-Kabara EC, Kassam AB, Horowitz MH, Urgo L, Hadjipanayis C, Levy EI, et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia : comparison of results following microvascular decompression. J Neurosurg. 2002;96:527–531. doi: 10.3171/jns.2002.96.3.0527. [DOI] [PubMed] [Google Scholar]
- 34.Walchenbach R, Voormolen JH. Surgical treatment for trigeminal neuralgia. BMJ. 1996;313:1027–1028. doi: 10.1136/bmj.313.7064.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidmann MJ. Trigeminal neuralgia. Surgical treatment by microvascular decompression of the trigeminal nerve root. Med J Aust. 1979;2:628–630. doi: 10.5694/j.1326-5377.1979.tb127241.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilkins RH. Surgical therapy of neuralgia : vascular decompression procedures. Semin Neurol. 1988;8:280–285. doi: 10.1055/s-2008-1041390. [DOI] [PubMed] [Google Scholar]
- 37.Wilson CB, Yorke C, Prioleau G. Microsurgical vascular decompression for trigeminal neuralgia and hemifacial spasm. West J Med. 1980;132:481–487. [PMC free article] [PubMed] [Google Scholar]
- 38.Xu-Hui W, Chun Z, Guang-Jian S, Min-Hui X, Guang-Xin C, Yong-Wen Z, et al. Long-term outcomes of percutaneous retrogasserian glycerol rhizotomy in 3370 patients with trigeminal neuralgia. Turk Neurosurg. 2011;21:48–52. [PubMed] [Google Scholar]
- 39.Yang DB, Jiang DY, Chen HC, Wang ZM. Second microvascular decompression for trigeminal neuralgia in recurrent cases after microvascular decompression. J Craniofac Surg. 2015;26:491–494. doi: 10.1097/SCS.0000000000001523. [DOI] [PubMed] [Google Scholar]
- 40.Zakrzewska JM. Surgical management of trigeminal neuralgia. Br Dent J. 1991;170:61–62. doi: 10.1038/sj.bdj.4807418. [DOI] [PubMed] [Google Scholar]
- 41.Zakrzewska JM, Lopez BC. Trigeminal neuralgia. Clin Evid. 2006;(15):1827–1835. [PubMed] [Google Scholar]
- 42.Ziccardi VB, Braun TW, Ochs MW. Trigeminal neuralgia : review of etiologies and treatments. Compendium. 1993;14:1256, 1258–1262. quiz 1264. [PubMed] [Google Scholar]
- 43.Zuniga JR. Surgical management of trigeminal neuropathic pain. Atlas Oral Maxillofac Surg Clin North Am. 2001;9:59–75. [PubMed] [Google Scholar]