Abstract
The advantages of monounsaturated fatty acids (MUFAs) on insulin resistance and type 2 diabetes mellitus (T2DM) have been well established. However, the molecular mechanisms of the anti-diabetic action of MUFAs remain unclear. This study examined the anti-hyperglycemic effect and explored the molecular mechanisms involved in the actions of fish oil- rich in MUFAs that had been acquired from hybrid catfish (Pangasius larnaudii×Pangasianodon hypophthalmus) among experimental type 2 diabetic rats. Diabetic rats that were fed with fish oil (500 and 1,000 mg/kg BW) for 12 weeks significantly reduced the fasting plasma glucose levels without increasing the plasma insulin levels. The diminishing levels of plasma lipids and the muscle triglyceride accumulation as well as the plasma leptin levels were identified in T2DM rats, which had been administrated with fish oil. Notably, the plasma adiponectin levels increased among these rats. The fish oil supplementation also improved glucose tolerance, insulin sensitivity and pancreatic histological changes. Moreover, the supplementation of fish oil improved insulin signaling (p-AktSer473 and p-PKC-ζ/λThr410/403), p-AMPKThr172 and membrane GLUT4 protein expressions, whereas the protein expressions of pro-inflammatory cytokines (TNF-α and nuclear NF-κB) as well as p-PKC-θThr538 were down regulated in the skeletal muscle. These data indicate that the effects of fish oil-rich in MUFAs in these T2DM rats were partly due to the attenuation of insulin resistance and an improvement in the adipokine imbalance. The mechanisms of the anti-hyperglycemic effect are involved in the improvement of insulin signaling, AMPK activation, GLUT4 translocation and suppression of pro-inflammatory cytokine protein expressions.
Keywords: Anti-hyperglycemia, Fish oil, Insulin-signaling, Monounsaturated fatty acids, Type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most common form of diabetes mellitus. Insulin resistance and the impairment of insulin secretion are the major characteristics of the pathology of T2DM leading to hyperglycemia [1]. Chronic hyperglycemia is associated with diabetes complications (microvascular and macrovascular complications) and even death [1,2]. Insulin resistance, the hallmark feature of T2DM, is characterized by a decreased insulin-stimulated glucose uptake due to impaired insulin signaling [3,4]. It is well known that insulin resistance is strongly linked to obesity and the development of T2DM [5]. In addition, obesity is also associated with chronic inflammation that is characterized by an increase in pro-inflammatory cytokine levels and an alteration of adipokine production [6].
Skeletal muscle, the major site of the insulin-stimulated glucose uptake, plays an important role in regulating insulin sensitivity throughout the entire body [4]. Moreover, skeletal insulin resistance is the primary defect in T2DM [4,7]. In the normal physiological state, insulin stimulates glucose uptake through insulin signaling cascade. Briefly, insulin binds to its receptor leading to the tyrosine phosphorylation of the insulin receptor and insulin receptor substrate-1 (IRS-1) and the serine phosphorylation of Akt or the threonine phosphorylation of atypical protein kinase C-ζ/λ (aPKC-ζ/λ) by phosphoinositide 3-kinase (PI3K) activation. Finally, glucose transporter 4 (GLUT4) is translocated to the plasma membrane to uptake glucose into the cells [8]. In the insulin resistant state, muscle insulin-stimulated glucose uptake and glycogen synthesis were markedly reduced via the impairment of the insulin-signaling cascade [9]. Previous studies have shown that lipid metabolites and inflammation are involved in the development of insulin resistance in the skeletal muscle by interfering with the normal insulin-signaling pathway [10,11].
Several studies have demonstrated that dietary fat composition influences insulin sensitivity. Diets comprised of high-saturated fatty acids (SFAs) could induce insulin resistance, while unsaturated fatty acids; monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), have been shown to improve insulin sensitivity [12,13]. The beneficial effects of MUFAs on anti-inflammation, cardio-protective effects as well as the reduction in insulin resistance have been documented [14,15]. Both human and animal models of insulin resistance have clearly shown that the partial replacement of SFAs with MUFAs in the diet actually improved glycemic control and this is accompanied by improved insulin sensitivity [16,17,18,19]. Moreover, our preliminary study showed that fish oil that is rich in MUFAs acquired from hybrid catfish (Pangasius larnaudii× Pangasianodon hypophthalmus) freshwater fish, enhanced insulin-stimulated glucose uptake through the improvement of insulin signaling and translocation of GLUT4 in the diaphragm of normal rats [20]. Supported by the previous study of Moon et al., it has been demonstrated that type 2 diabetic Otsuka Long-Evans Tokushima fatty (OLETF) rats fed with dietary-rich MUFAs acquired from olive oil actually reduced hyperglycemia while improving skeletal muscle insulin sensitivity by conserving the insulin signaling IRS-1/PI3K pathway and the translocation of GLUT4 to the plasma membrane, as compared with an SFAs-rich diet [18]. Furthermore, the anti-inflammatory effect was also found in patients with metabolic syndrome; whereas, a high-MUFAs diet suppressed the nuclear factorkappa B (NF-κB) activity and increased the inhibitory molecule from the NF-κB, inhibitor kappa B-α (IkB-α), and the mRNA levels [21]. Notably, the effects of MUFAs were mostly derived from olive oil supplements while the health benefits of the MUFAs acquired from freshwater fish; hybrid catfish (Pangasius larnaudii×Pangasianodon hypophthalmus), on the type 2 diabetic rat model have not yet been investigated [18,21,22]. Therefore, the present study aims to examine the anti-diabetic effect of fish oil that is rich in MUFAs acquired from the by-products of freshwater hybrid catfish that had been fed with a high-fat diet with streptozotocin (STZ) induced type 2 diabetic rats. Furthermore, this study also evaluate the possible mechanisms involved with the effects of fish oil that is rich in MUFAs on the insulin signaling pathway in the skeletal glucose transport system of type 2 diabetic rats.
METHODS
Preparation of fish oil-rich in MUFAs acquired from hybrid catfish (Pangasius larnaudii×Pangasianodon hypophthalmus)
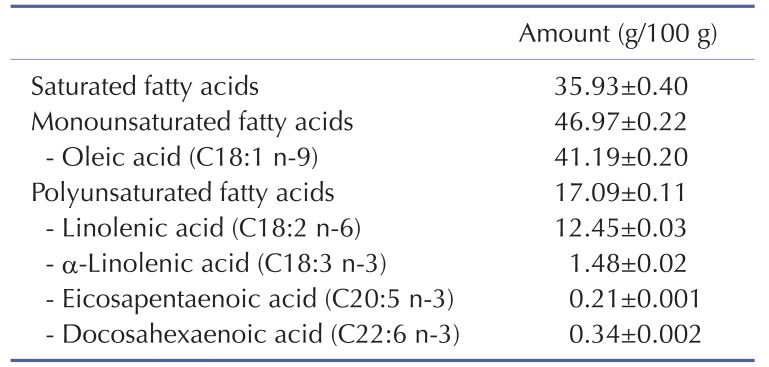
The fish oil that is rich in MUFAs used in this study was provided by the Faculty of Fisheries Technology and Aquatic Resources, Maejo University, Thailand. Briefly, frozen adipose tissue of the hybrid catfish (Pangasius larnaudii×Pangasianodon hypophthalmus) was steamed at 85℃ for 30 minutes. The resulting product was put in a filter sack and then squeezed by screw compressor. To separate the solid particles, the squeezed liquid obtained was subsequently centrifuged at 9,600 g at 4℃ for 20 minutes. The fish oil acquired from the hybrid catfish was sent to the Central Laboratory (Thailand) Co Ltd., Chiang Mai Branch, for fatty acid composition analysis by an in-house method based on AOAC 996.06 [23]. The fatty acid composition of the fish oil that is rich in MUFAs acquired from hybrid catfish is shown in Table 1.
Table 1. Fatty acid composition of fish oil-rich in MUFAs acquired from hybrid catfish.
Values are the mean±SEM of three independently processed samples.
Animals and diet
Adult male Wistar rats (180~200 g) were obtained from the National Laboratory Animal Center, Mahidol University, Salaya Thailand, and housed under the controlled temperature of 25±2℃ with a 12-hour light-dark cycle. Type 2 diabetic rats were induced according to the method of Srinivasan et al. [24]. After the acclimatization period, rats were fed with a normal chow diet (11% energy from fat) or a high-fat diet (58% energy from fat) for the initial period of 2 weeks. Then, the high-fat diet fed rats were injected with STZ 40 mg/kg BW (i.p.), while the normal diet fed rats received citrate buffer (pH 4.4) as a vehicle. After 10 days of STZ injection, rats with a fasting plasma glucose level of ≥250 mg/dl without hypo-insulinemia were classified as T2DM and used in this study. After diabetic induction, type 2 diabetic rats were randomly divided into 5 groups (n=8) and orally administered with vehicle (DMC), fish oil that is rich in MUFAs at a dose of 250 mg/kg BW (DM-FO250), 500 mg/kg BW (DM-FO500), 1,000 mg/kg BW (DM-FO1000) and metformin at a dose of 50 mg/kg BW (DM-Met). Normal rats were randomly separated into 2 groups (n=8); normal control rats (NC) and normal rats that received fish oil-rich in MUFAs at a dose of 1,000 mg/kg BW (ND-FO1000). The normal rats were fed with a normal chow diet while the type 2 diabetic rats were fed with a high-fat diet throughout the experiment. All animals were allowed free access to water and food. Food intake and body weight were recorded weekly. After 12 weeks of treatment, overnight fasted rats were sacrificed with an overdose of Nembutal® (Liboume, France). Blood and tissue samples were collected for further biochemical analyses. The protocols and procedures involved with all animals were performed in accordance with the rules and regulations by the Animal Research Committee of Faculty of Medicine, Chiang Mai University, Thailand.
Oral glucose tolerance test
At week 11 of the treatment period, the oral glucose tolerance test (OGTT) was performed for each rat. All animals were fasted overnight and blood samples were collected as a baseline value (min-0); after which, a glucose solution (2 g/kg BW) was administrated by oral gavage feeding. Blood samples were collected via cutting the tail tip at 15, 30, 60 and 120 minutes after glucose loading and plasma glucose concentrations were measured using a commercial kit. The increments of the plasma glucose concentrations following the glucose loading were expressed in terms of the area under the curve (AUC) for glucose, using the trapezoidal rule [25]. The total AUC (TAUC) was defined as all areas below the curve, which is calculated as:
The basal AUC (BAUC) defined as the area below the baseline value was calcualted as: (120×y0). The incremental AUC (IAUC) value was defined as the area beneath the curve above the fasting glucose level and was calculated as: TAUC - BAUC or TAUC - (120×y0).
Biochemical analysis of plasma
Plasma glucose, triglyceride and total cholesterol concentrations were determined by the enzymatic colorimetric method using a commercial kit (Biotech, Bangkok, Thailand). The plasma insulin, leptin and adiponectin concentrations were determined using the Sandwich ELISA method (Rat/Mouse Insulin ELISA kit, LINCO Research, USA). The insulin resistance was assessed by the homeostasis model assessment of insulin resistance (HOMA-IR). The HOMA-IR was calculated as follows [26]:
Determination of triglyceride accumulation in skeletal muscle
The skeletal muscle triglyceride content was measured in gastrocnemius muscle by a method of Frayn and Maycock [27] with slight modifications. Briefly, the gastrocnemius muscle was minced and put into a glass tube containing 3 ml of chloroformisopropanol 2:3 (v/v). The homogenate was pipetted into a glass tube and evaporated to dryness at 40℃ for 16 hours. The dried residue was dissolved and mixed in 10% bovine serum albumin (BSA). The triglyceride concentrations were analyzed using a commercial colorimetric kit (Biotech, Bangkok, Thailand).
Membrane extraction and total cellular lysate of skeletal muscle
The membrane fraction of the skeletal muscle was prepared based on the method of Mohammad et al. [28]. Briefly, the soleus muscle (100 mg) was homogenated in lysis buffer, and then centrifuged for 10 minutes, 2,000 g at 4℃. The supernatant was centrifuged at 9,000 g for 20 minutes at 4℃. After that, the supernatant was again centrifuged at 180,000 g for 90 minutes at 4℃. The final pellet was re-suspended in a phosphate buffer saline solution (PBS), which represented the membrane fraction and was used to determine membrane GLUT4 protein expression.
The soleus muscle was homogenated in ice-cold lysis buffer. After that, the homogenate was centrifuged at 5,000 g for 20 minutes at 4℃. The supernatant was used as the total cellular lysate. Then, the protein concentrations were determined with a Bradford protein assay reagent kit (Bio-Rad Laboratories, USA).
Nuclear extraction of skeletal muscle
The nuclear fraction of the skeletal muscle was isolated by differential centrifugation as previously described by Rhoads et al. [29] with slight modifications. The gastrocnemius muscle (150 mg) was homogenated in a lysis buffer. The resultant homogenate was then centrifuged at 800 g for 10 minutes at 4℃. The supernatant was considered to be the cytosolic fraction. Subsequently, the nuclear pellet was re-suspended with 200 µl of lysis buffer and rocked for 1 hour at 4℃. Lysed nuclei were centrifuged for 15 minutes, 16,000 g at 4℃. The supernatant of this step was used as the nuclear fraction and employed to determine the nuclear NF-κB protein expression.
Western blot analysis
Aliquots of muscle homogenate were separated by electrophoresis using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis system (SDS-PAGE). In brief, protein samples were electrophoresised on SDS-PAGE gel and subsequently, proteins were blotted to the nitrocellulose membrane and blocked with blocking buffer for 1 hour at room temperature with gentle shaking. Then, the membrane was incubated with commercially available antibodies for Akt (Millipore Corporation, USA), phosphorylation of (p)-AktSer473 (Millipore Corporation, USA), PKC-ζ (Millipore Corporation, USA), p-PKC-ζ/λThr410/403 (Cell signaling Technology, MA, USA), AMP-activated protein kinase-α (AMPK-α) (Millipore Corporation, USA), p-AMPK-αThr172 (Millipore Corporation, USA), GLUT4 (Chemicon International, USA), PKC-θ (Santa Cruz Biotechnology, CA, USA),p-PKC-θThr538 (Santa Cruz Biotechnology, CA, USA), NF-κB (Santa Cruz Biotechnology, CA, USA) and tumor necrosis factor-α (TNF-α) (Cell Signaling Technology, MA, USA) at 4℃ overnight. The membrane was washed and incubated with the secondary antibody conjugated with horseradish peroxidase. The membrane was again washed and immunoreactive proteins were visualized on Kodak Hyperfilm (Godak Rochester, NY) using an enhanced chemiluminescence detection kit (GE Healthcare, Piscataway, NJ). The signal was quantified using Scion Image software and expressed by comparing it with the mean values in the NC group, which was arbitrarily set at 100. The level of the phosphorylated signaling element was expressed relative to the total amount of that protein from the same sample.
Histological study
A splenic portion of pancreatic tissue was removed immediately after the specimens were sacrificed and the portion was rinsed with ice-cold saline. The tissue samples were fixed in 10% neutral formalin and embedded in paraffin blocks. The thin section (5 µm) were de-waxed, dehydrated in a graded series of ethanol, and rehydrated, then stained with hematoxylin and eosin (H&E) for light microscopic examination.
Statistical analysis
The SPSS Advanced Statistics software (version17 SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Data were presented as a mean value±standard error of the mean (SEM). One way analysis of variance (ANOVA) followed by LSD's post-hoc analysis was used to determine significant differences between groups. For all statistical analysis, a p value of less than 0.05 was considered to be a significant difference.
RESULTS
Effects of fish oil-rich in MUFAs acquired from hybrid catfish on the characteristics of type 2 diabetic rats
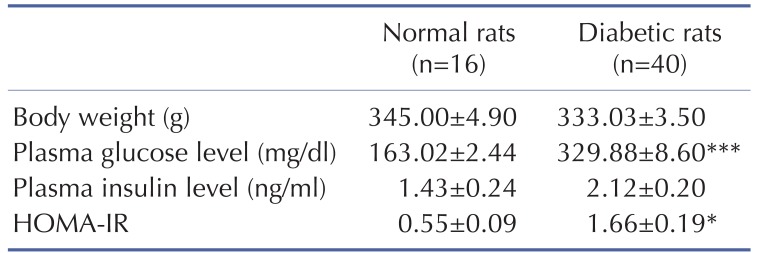
The combination of a high-fat diet and STZ injection successfully induced diabetic rats as is shown in Table 2. The body weight of normal and type 2 diabetic rats were comparable. Type 2 diabetic rats showed higher fasting plasma glucose levels than those in the normal rats, while the plasma insulin levels were not found to be different between the diabetic rats and the normal rats. Furthermore, the HOMA-IR, which indicated the whole body insulin resistance, markedly increased in the diabetic rats compared with the normal rats. These results indicated that these type 2 diabetic rats used in this study showed general characteristics of T2DM, hyperglycemia and insulin resistance, which were close to the characteristics of T2DM patients.
Table 2. General characteristics of type 2 diabetic rats at the beginning of experiment (baseline).
Values are means±SEM. HOMA-IR index, homeostasis model assessment of insulin resistance. *p<0.05, ***p<0.001 vs. normal rats.
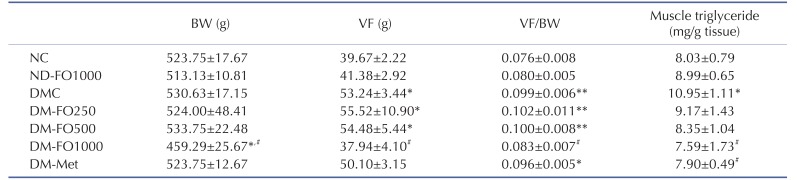
Supplementation with fish oil that is rich in MUFAs for 12 weeks did not affect the body weight, visceral fat weight and visceral fat weight/body weight ratio in normal rats (Table 3). Although the body weight of the DMC and NC groups was not found to be significantly different, but the visceral fat weight and visceral fat weight/body weight ratio were markedly elevated in the DMC group compared with the NC group, which indicates visceral obesity. Interestingly, the body weight, visceral fat weight and visceral fat weight/body weight ratio significantly decreased only in the DM-FO1000 group with respect to the DMC group. In the metformin treatment, the DM-FO250 and DM-FO500 groups failed to reduce the incidence of visceral obesity.
Table 3. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on body weight, visceral fat, visceral fat/ body weight (VF/BW) ratio and muscle triglyceride content in rats at the end of experiment.
Values are means±SEM for 8 animals per group. BW, body weight; VF, visceral fat; NC, normal control rats; ND-FO1000, normal control rats supplemented with fish oil-rich in MUFAs at a dose of 1,000 mg/kg BW; DMC, diabetic control rats; DM-FO250, diabetic rats supplemented with fish oil-rich in MUFAs at a dose of 250 mg/kg BW; DM-FO500, diabetic rats supplemented with fish oil-rich in MUFAs at a dose of 500 mg/kg BW; DM-FO1000, diabetic rats supplemented with fish oil-rich in MUFAs at a dose of 1000 mg/kg BW; DM-Met, diabetic rats treated with metformin at a dose of 50 mg/kg BW. *p<0.05, **p<0.01 vs. NC group; #p<0.05 vs. DMC group.
As shown in Table 3, there was no difference in triglyceride accumulation in the skeletal muscle between the NC and ND-FO1000 groups. On the other hand, the accumulation of skeletal muscle triglycerides was significantly higher in the DMC group than in the NC group. A significant reduction of skeletal muscle triglyceride contents was found only in the DM-FO1000 and DM-Met groups when compared with the DMC group.
Effects of fish oil-rich in MUFAs acquired from hybrid catfish on plasma metabolic parameters
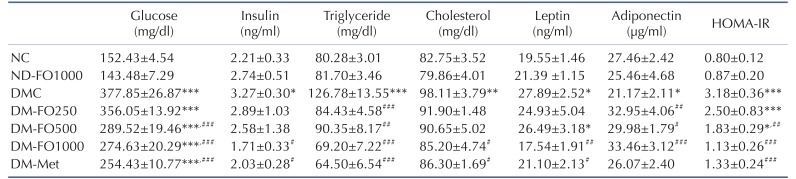
To explore the anti-diabetic effect of fish oil that is rich in MUFAs, the plasma biochemical parameters were measured. As shown in Table 4, the fish oil supplement did not alter the fasting plasma glucose levels among normal rats. Type 2 diabetic rats showed a higher fasting plasma glucose level (+148%) when compared with normal rats. Supplementation with fish oil that is rich in MUFAs at a dose of 500 and 1,000 mg/kg BW successfully reduced the fasting plasma glucose levels in type 2 diabetic rats (–23.38% and –27.32%, respectively). However, diabetic rats treated with metformin have showed the lowest fasting plasma glucose level (–32.67%) among the treated diabetic rats. To investigate whether the glucose-lowering effect of fish oil that is rich in MUFAs is involved in pancreatic insulin secretion, the fasting plasma insulin levels were measured. At the end of the experiment, supplementation with fish oil-rich in MUFAs at a dose of 250 or 500 mg/kg BW did not affect the fasting plasma insulin level in type 2 diabetic rats. Meanwhile, the plasma insulin levels in the DM-FO1000 and DM-Met groups were markedly decreased when compared with the DMC group. Therefore, the decreased fasting plasma glucose levels in type 2 diabetic rats treated with fish oil-rich in MUFAs might be due to the improvement of insulin sensitivity as occurs with metformin treatment. In accordance with these results, the DM-FO500 and DM-FO1000 groups significantly reduced the HOMA-IR when compared with the DMC group. In addition, HOMA-IR significantly decreased in the DM-Met group with respect to the DMC group. These findings indicate that the supplementation of fish oil that is rich in MUFAs reduced the whole body insulin resistance in T2DM rats similar to type 2 diabetic rats treated with metformin.
Table 4. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on plasma metabolic parameters at the end of experiment.
Values are means±SEM for 8 animals per group. HOMA-IR index, homeostasis model assessment of insulin resistance. NC, normal control rats; ND-FO1000, normal control rats supplemented with fish oil-rich in MUFAs at a dose of 1,000 mg/kg BW; DMC, diabetic control rats; DM-FO250, diabetic rats supplemented with fish oil-rich in MUFAs at a dose of 250 mg/kg BW; DM-FO500, diabetic rats supplemented with fish oil-rich in MUFAs at a dose of 500 mg/kg BW; DM-FO1000, diabetic rats supplemented with fish oil-rich in MUFAs at a dose of 1,000 mg/kg BW; DM-Met, diabetic rats treated with metformin at a dose of 50 mg/kg BW. *p<0.05, **p<0.01, ***p<0.001 vs. NC group; #p<0.05, ##p<0.01, ###p<0.001 vs. DMC group.
In the present study, we also examined the hypolipidemic effect of fish oil that is rich in MUFAs on type 2 diabetic rats and those results are shown in Table 4. In normal rats, supplementation with fish oil that is rich in MUFAs did not alter the plasma triglyceride and total cholesterol levels. The plasma lipids, triglyceride and total cholesterol levels were significantly higher in the DMC group than those in the NC group. Remarkably, the administration of fish oil that is rich in MUFAs in all dosages markedly reduced the plasma triglyceride levels in type 2 diabetic rats. The plasma triglyceride concentration also significantly decreased in the DM-Met group. A reduction of the plasma total cholesterol levels was observed only in the DM-FO1000 and DM-Met groups.
To evaluate the relationship of the adipocyte-derived hormone and insulin sensitivity in this experimental model, plasma adiponectin and leptin levels were determined. As shown in Table 4, the plasma adiponectin levels were significantly lower in the DMC group than in the NC group. Interestingly, supplementation with fish oil that is rich in MUFAs in all dosages significantly increased the plasma adiponectin levels when compared with the DMC group. However, treatment with metformin did not augment the plasma adiponectin level in type 2 diabetic rats. Compared with the NC group, the plasma leptin level significantly increased in the DMC group. The plasma leptin levels were significantly lower in the DM-FO1000 and DM-Met groups than in the DMC group.
Effects of fish oil-rich in MUFAs acquired from hybrid catfish on glucose tolerance
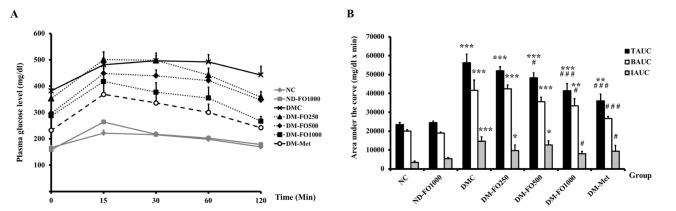
To determine whether fish oil that is rich in MUFAs could affect the whole body insulin sensitivity in type 2 diabetic rats, the OGTT was performed. As shown in Fig. 1A, there were no significant differences in the plasma glucose levels at all time points in the NC and ND-FO1000 groups. In addition, the TAUC, BAUC and IAUC in the NC and ND-FO1000 groups were comparable (Fig. 1B). The plasma glucose levels before and after glucose loading revealed significantly higher values in the DMC group at all time points with respect to the NC group. Compared with the NC group, the TAUC, BAUC and IAUC were markedly increased in the DMC group. Notably, the plasma glucose levels were significantly lower in the DM-FO1000 and DM-Met groups at 30, 60 and 120 minutes after glucose loading when compared with the DMC group. There were significant reductions in the TAUC, BAUC and IAUC values in the DM-FO1000 and DM-Met groups when compared with the DMC group. It is suggested that supplementation with fish oil that is rich in MUFAs at a dose of 1,000 mg/kg BW effectively improved glucose tolerance in T2DM rats, which was similar to the findings of the treatment with metformin.
Fig. 1. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on OGTT in normal and diabetic rats (A) Glucose response (B) Area under the curve for glucose.
TAUC, total area under the curve; BAUC, basal area under the curve; IAUC, incremental area under the curve. Values are means±SEM for 8 animals per group. *p<0.05, **p<0.01, ***p<0.001 vs. NC group; #p<0.05, ###p<0.001 vs. DMC group.
Effects of fish oil-rich in MUFAs acquired from hybrid catfish on insulin signaling and glucose transport system in the skeletal muscle
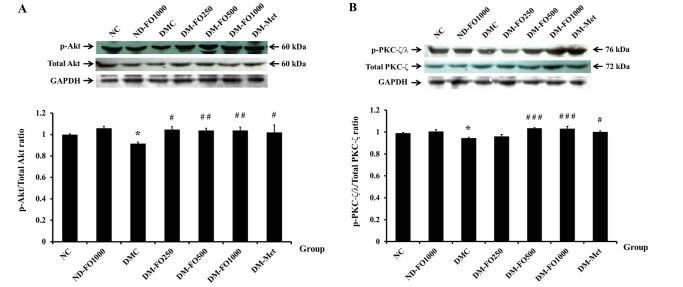
As it has been shown that supplementation with fish oil that is rich in MUFAs has an anti-hyperglycemic effect, we next examined whether fish oil-rich in MUFAs can improve insulin signaling in the skeletal muscle of type 2 diabetic rats. Because the activation of Akt and PKC-ζ/λ plays an important role in the insulin signaling for GLUT4 translocation, we examined the phosphorylation of the two downstream insulin signaling proteins. In normal rats, supplementation with fish oil that is rich in MUFAs did not alter the phosphorylation of AktSer473 with respect to the NC group (Fig. 2A). The phosphorylation of AktSer473 was significantly lowered in the DMC when compared to the NC groups. Interestingly, supplementation of fish oilrich in MUFAs at all dosages as well as metformin treatment significantly increased Akt phosphorylation (Ser473) when compared with the untreated diabetic rats, while the Akt protein expression in all experimental groups was not found to be significantly different. Similarly to Akt, there were no significant differences in the phosphorylation of PKC-ζ/λThr410/403 between the NC and ND-FO1000 groups (Fig. 2B). Compared with the NC group, the p-PKC-ζ/λThr410/403 was significantly decreased in the DMC group. The DM-FO500, DM-FO1000 and DM-Met groups revealed significantly higher phosphorylation of PKC-ζ/λThr410/403 than the DMC group, whereas the PKC-ζ protein expression levels in all experimental groups were similar.
Fig. 2. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on insulin signaling (A) AktSer473 phosphorylation (B) PKC-ζ/λThr 410/403 phosphorylation.
The level of phosphorylated signal elements were expressed relative to total amount of those protein levels from the same sample. Values are means±SEM for 8 animals per group. *p<0.05 vs. NC group; #p<0.05, ##p<0.01, ###p<0.001 vs. DMC group.
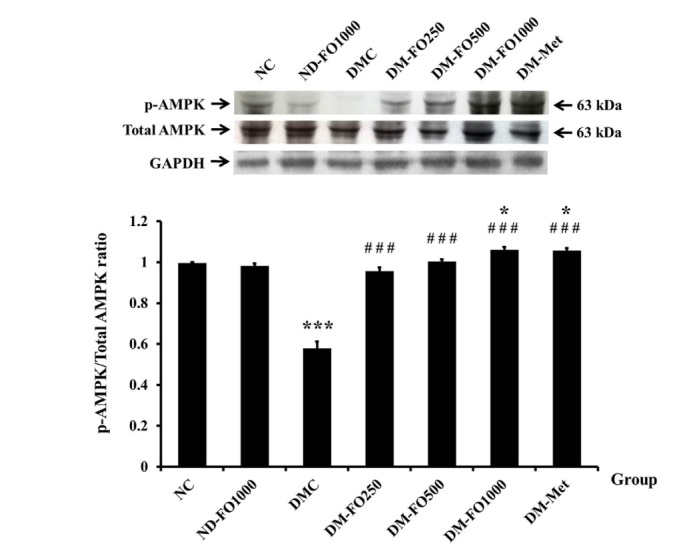
In addition to the insulin signaling proteins, the phosphorylation of AMPK is known to stimulate GLUT4 translocation to the plasma membrane via the insulin-independent pathway in the skeletal muscle [30,31]. Therefore, we further investigated whether fish oil that is rich in MUFAs can induce the phosphorylation of AMPKThr172. As shown in Fig. 3, the p-AMPKThr172 in the NC group was comparable to the value in the ND-FO1000 group. The significant decrease in the p-AMPKThr172 was observed in the DMC group with respect to the NC group. Compared with the DMC group, the p-AMPKThr172 in all groups of the fish oil supplemented specimens with diabetes and the DM-Met group was significantly elevated. On the other hand, the protein expressions of AMPK were not found to be significantly different in all experimental groups.
Fig. 3. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on AMPK-αThr172 phosphorylation.
The p-AMPK-αThr172 was expressed relative to total AMPK-α protein level from the same sample. Values are means±SEM for 8 animals per group. *p<0.05, ***p<0.001 vs. NC group; ###p<0.001 vs. DMC group.
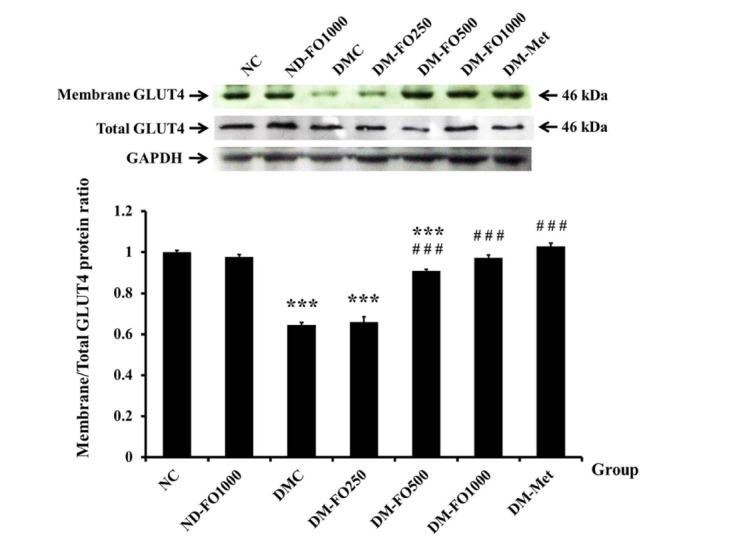
It is well known that insulin stimulates glucose uptake through GLUT4 translocation from cytosol to the plasma membrane. Thus, the rate of glucose uptake depends on the amount of the membrane GLUT4 present. Fig. 4 demonstrates the membrane fraction of GLUT4 protein and total GLUT4 protein expressions. The expression values of GLUT4 protein in the membrane fraction of the NC and ND-FO1000 groups were found to be similar (Fig. 4). As expected, the expression of GLUT4 protein in the membrane fraction of the DMC group significantly decreased when compared with the NC group. The expressions of GLUT4 protein in the membrane fraction were markedly elevated in the DM-FO500, DM-FO1000 and DM-Met groups with respect to the DMC group, while there were no significant differences in the total GLUT4 protein expressions among all experimental groups.
Fig. 4. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on membrane GLUT4 protein expression.
The membrane GLUT4 protein level was expressed relative to the total GLUT4 protein level from the same sample. Values are means±SEM for 8 animals per group. ***p<0.001 vs. NC group; ###p<0.001 vs. DMC group.
Effects of fish oil-rich in MUFAs acquired from hybrid catfish on the inflammation in the skeletal muscle
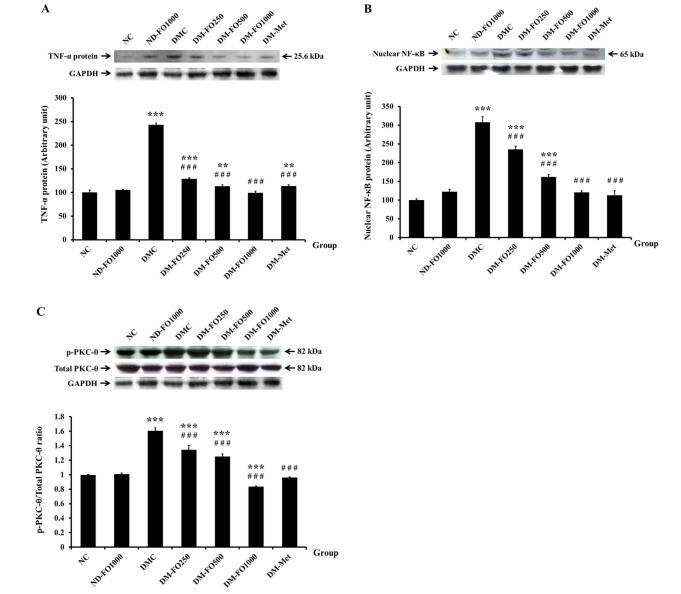
In recent years, it has been recognized that obesity is a chronic type of inflammation involved in the link between obesity-induced inflammation and insulin resistance via the TNF-α and NF-κB/PKC-θ pathway in the skeletal muscle [32,33]. As shown in Fig. 5A, we found that the pro-inflammatory cytokine, TNF-α, protein expression significantly elevated in the DMC group with respect to the NC group, while supplementation with fish oil-rich in MUFAs in normal rats did not affect the TNF-α protein expression values. Compared with the DMC group, the protein expression values of TNF-α were suppressed in the DM-FO250, DM-FO500, DM-FO1000 and DM-Met groups. Furthermore, the protein expression of nuclear NF-κB, which is another inflammatory marker, was also increased in the DMC group compared with the NC group (Fig. 5B). The nuclear NF-κB protein expression was significantly reduced in the DM-FO250, DM-FO500, DM-FO1000 and DM-Met groups compared to the DMC group, suggesting that fish oil that is rich in MUFAs supplementation and metformin treatment attenuated the NF-κB translocation in diabetic rats. Although the protein expressions of PKC-θ in all experimental groups were similar, the activation of PKC-θThr538 was significantly higher in the DMC group than in the NC group (Fig. 5C). As expected, the activation of PKC-θThr538 was markedly decreased in the DM-FO250, DM-FO500, DM-FO1000 and DM-Met groups compared with the DMC group.
Fig. 5. Effects of fish oil-rich in MUFAs acquired from hybrid catfish on inflammatory cytokine protein expressions (A) TNF-α (B) nuclear NF-κB and (C) PKC-θThr538 phosphorylation.
The level of phosphorylated signal elements were expressed relative to total amount of those protein levels from the same sample. Values are means±SEM for 8 animals per group. **p<0.01, ***p<0.001 vs. NC group; ###p<0.001 vs. DMC group.
Effects of fish oil-rich in MUFAs acquired from hybrid catfish on morphological changes of the pancreas
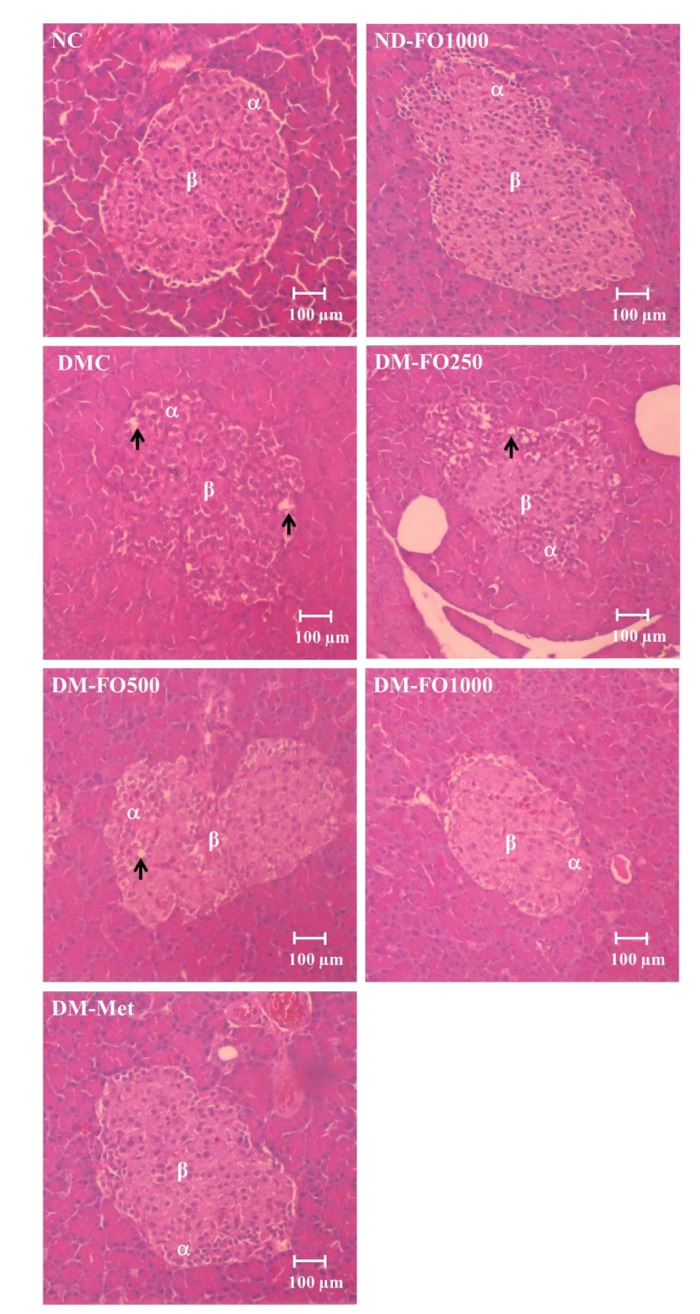
No pancreatic histological changes were observed in the ND-FO1000 group, when compared with normal pancreatic tissue (Fig. 6). Many round-to-elongated islets were found evenly distributed throughout the cytoplasm that were encapsulated in the fibrous membrane and well demarcated. Within the islets, β-cells were evenly distributed and appeared to be normal and healthy in both the NC and ND-FO1000 groups. However, in the DMC group, the pancreatic tissue showed some alterations in the islet morphology. Some islets were smaller than those in the NC group with irregular boundaries, as well as being disorganized and having developed degranulation and vacuolar degeneration. As expected, the histological examination of islets from the DM-FO1000 and DM-Met groups appeared to be much better than that in the DMC group and looked close to normal. The β-cells were more numerous and evenly distributed. This suggests that treatment with metformin or fish oil that is rich in MUFAs at a dose of 1,000 mg/kg BW effectively protected the progressive β-cell damage that is often associated with diabetes.
Fig. 6. Photomicrograph of pancreatic islets of Langerhans.
The beta (β), alpha (α) cells and vacuolization (arrow) were observed.
DISCUSSION
The major challenge in this study is to consider whether fish oil that is rich in MUFAs acquired from freshwater hybrid catfish (Pangasius larnaudii×Pangasianodon hypophthalmus) could ameliorate the hyperglycemia among STZ induced type 2 diabetic rats fed with a high-fat diet. The outcomes of the study provided evidence to demonstrate the anti-hyperglycemic and lipid-lowering effects of fish oil-rich in MUFAs in type 2 diabetic rat model. Nevertheless, the beneficial effects of fish oil that is rich in MUFAs on the glycemic control were not due to the insulinotropic action. Our results clearly revealed that supplementation with fish oil-rich in MUFAs attenuated insulin resistance and improved adipokine imbalance in type 2 diabetic rats. Its antidiabetic effect is partially involved in the improvement of insulin signaling, AMPK activation, GLUT4 translocation and the suppression of pro-inflammatory cytokine protein expressions in the skeletal muscle of T2DM rats.
It has been well established that a high-fat diet combined with low-dose STZ injection has been widely used in experimental animals to induce a T2DM rat model [24]. Type 2 diabetic rats in the present study showed the general characteristics of T2DM including visceral obesity, insulin resistance and hyperglycemia, which are close to type 2 diabetic patients. First, we examined the effects of fish oil-rich in MUFAs on anti-diabetic action. The supplementation of diabetic rats with 500 and 1,000 mg/kg BW of fish oil-rich in MUFAs significantly reduced the fasting plasma glucose levels and also improved insulin sensitivity and glucose tolerance demonstrating its anti-hyperglycemic effect. Only supplementation of fish oil-rich in MUFAs at a dose of 1,000 mg/kg BW significantly reduced the fasting plasma insulin levels, but not at a dose of 500 mg/kg BW. Consistent with several studies, the advantageous effects of MUFAs have been observed on ameliorating hyperglycemia and restoring insulin sensitivity in type 2 diabetic KK-Ay mice and obese individuals [14,19]. Since either insulin deficiency or insulin resistance results appear to elevate the fasting and postprandial plasma glucose levels, the anti-hyperglycemic effect of MUFAs can be explained by several mechanisms such as enhanced insulin sensitivity and glucose uptake, inhibited hepatic gluconeogenesis or relevant insulinotropic actions. However, fish oil-rich in the MUFAs supplement produced a reduction in hyperglycemia incidence without enhancing plasma insulin levels in the same way as metformin, an insulin-sensitizing agent. These results indicate that the anti-hyperglycemic effect of fish oil-rich in MUFAs might be due to the improvement of insulin sensitivity and this notion was supported by the HOMA-IR index and the OGTT. Additionally, the histological examination of the pancreas of diabetic fish oil-rich in MUFAs (1,000 mg/kg BW) treated rats revealed that the severity of the degenerative changes in the islets of Langerhans was less than those in the untreated-diabetic rats. It was likely that the hyperglycemia in diabetes was attenuated due to the treatment of fish oil-rich in MUFAs and further, a lesser degree of hyperglycemia ameliorated the progress of β-cell damage.
Next, we investigated the altering of the plasma adipokine levels in diabetic rats that had been fed a diet supplemented with fish oil-rich in MUFAs. Due to the fact that not only the impairment of insulin sensitivity associated with the pathophysiology of insulin resistance and T2DM, but the adipokine imbalance was also found to be associated [34,35]. Adiponectin and leptin are the adipocyte-derived hormones that play an important role in modulating glucose and lipid metabolisms [36]. Adiponectin acts as an insulin-sensitizer via the activation of AMPK, which serves to enhance fatty acid oxidation, reduce tissue triglyceride accumulation and finally to improve insulin signaling [37,38]. Leptin regulates food intake, energy expenditure, and the regulation of glucose homeostasis by stimulating the PI3K signaling [39,40]. Numerous studies have demonstrated that plasma adiponectin concentration was lower in terms of insulin resistance or T2DM than in the healthy control subjects [34,41], while the plasma leptin concentration had been increased as is known to be a condition called leptin resistance [35,42]. Recent studies have demonstrated that increased adiponectin and decreased leptin concentrations were related with the improvement of insulin sensitivity and a reduction in the plasma glucose and lipid concentrations in insulin resistant or type 2 diabetic rats fed with MUFAs derived from dietary saury oil [19]. Notably, the results from this study exhibited that supplementation with fish oil-rich in MUFAs effectively reversed plasma adiponectin and leptin levels compared with the DMC group. Therefore, the improvement of insulin sensitivity, anti-hyperglycemic as well as the hypolipidemic actions of fish oil-rich in MUFAs has been found to be mediated, at least in part by increased plasma adiponectin and decreased leptin concentrations.
To explore the molecular mechanisms underlying the beneficial effects of fish oil-rich in MUFAs on insulin sensitivity, we have further quantified the insulin signaling and glucose transport system in the skeletal muscle. This is because skeletal muscle plays a crucial role in regulating whole body insulin sensitivity and is the major site of insulin-stimulated glucose disposal [4]. Several studies have reported that the rate of insulin-stimulated glucose uptake depends on the expression of the membrane GLUT4 protein [43,44]. In this present study, we have found that the restoration of membrane GLUT4 protein content in the skeletal muscle of type 2 diabetic rats that had been administered with fish oil-rich in MUFAs (500 and 1,000 mg/kg BW) was close to the results of studies involving diabetic rats treated with metformin. In accordance with previous studies, it has been demonstrated that type 2 diabetic rats fed with a MUFAs enriched high-fat diet revealed an increase in the membrane GLUT4 protein expression through conserving IRS-1/PI3K insulin signaling in the skeletal muscle [18]. Moreover, prolonged exposure of the L6 skeletal muscle cells to palmitoleic acid enhanced glucose uptake via increasing the membrane GLUT1 and GLUT4 protein expressions [45]. It is known that the activation of Akt or PKC-ζ/λ is required for the translocation of GLUT4 to the plasma membrane [44,46]. Consequently, the effects of supplementation with fish oil-rich in MUFAs on those downstream insulin-signaling pathways were examined. As expected, the results showed the restoration of insulin signaling, in terms of both AktSer473 and PKC-ζ/λThr410/403 phosphorylation in the skeletal muscle of type 2 diabetic rats that had been fed a diet supplemented with fish oil-rich in MUFAs either at a dose of 500 and 1,000 mg/kg BW when compared with the untreated diabetic rats. Our results are consistent with several in vitro studies that involved incubating L6 myotubes with oleic acid or palmitoleic acid enhanced insulin-stimulated AktSer473 phosphorylation [45,47]. In addition, Coll et al. demonstrated that C2C12 myotubes co-incubated with palmitic acid and oleic acid reversed the deleterious effects of palmitic acid by restoring AktSer473 phosphorylation [48]. Altogether, our results suggest that fish oilrich in MUFAs supplementation either at a dose 500 or 1,000 mg/kg BW successfully maintained membrane GLUT4 translocation and is associated with the restoration of downstream insulin signaling of p-AktSer473 and p-PKC-ζ/λThr410/403, in the skeletal muscle of type 2 diabetic rats. These changes could contribute to the amelioration of the hyperglycemic effect of fish oil that is rich in MUFAs.
We also investigated the effects of the administration of fish oil-rich in MUFAs on the activation of AMPK, another pathway involved in stimulating GLUT4 translocation [30,31,49]. Recent studies have shown that AMPK activation is recognized as a potential therapeutic target in the prevention and treatment of T2DM [50,51]. Metformin is one of the most commonly used anti-diabetic drugs and exerts its action mainly by activating AMPK [51]. Likewise, this present study identified an increase of AMPK-αThr172 phosphorylation in the skeletal muscle of type 2 diabetic rats after administration of fish oil-rich in MUFAs at doses of 250, 500 and 1,000 mg/kg BW when compared to untreated diabetic rats as well as rats that had been exposed to metformin treatment. These findings were consistent with the previous study that had reported that the reduction of the AMPKThr172 phosphorylation of C2C12 myotubes resulting from palmitic acid incubation was prevented by incubation with oleic acid [48]. However, another study also recorded the enhancement of AMPKThr172 phosphorylation in palmitoleic acid treated 3T3-L1 adipocytes together with an increase in GLUT4 mRNA and protein contents, while the phosphorylation of AktSer473 and AktThr308 did not change [52]. The controversial findings in the study of the phosphorylation of AktSer473 might actually be attributed to the type of fatty acids, type of cells, skeletal muscle cells and the adipocytes. Besides, it has been well established that adiponectin augments insulin sensitivity, stimulates glucose utilization and increases fatty acid oxidation in skeletal muscle via the activation of AMPK [37]. Consequently, an enhancement of AMPKThr172 phosphorylation in this study may contribute to our findings through the supplementation of fish oil that is rich in MUFAs. The AMPK activation present in the skeletal muscle might be an efficient mechanism that could likely explain the anti-diabetic effect of fish oil that is rich in MUFAs.
Although the mechanisms of insulin resistance are not fully understood, lipid- and inflammation-induced insulin resistance is one of the potential candidate mechanisms for insulin resistance [48,53]. Several studies have shown that lipid accumulation such as diacylglycerol (DAG) activates PKC-θ which leads to the impairment of normal insulin signaling by the phosphorylation of IRS-1 at the serine residue [32,54]. The phosphorylation of IRS-1 at the serine residue fails to activate the PI3K/Akt pathway, resulting in a reduced level of insulin action and finally, insulin resistance [55]. In this study, we recorded the reduction of muscle lipid accumulation (triglyceride accumulation) and the suppression of PKC-θThr538 phosphorylation (p-PKC-θThr538) in type 2 diabetic rats administrated with fish oil that is rich in MUFAs or with the treatment of metformin. Thus, the reduced level of muscle lipid accumulation acknowledged in the present study might be involved with the suppression of PKC-θ activation along with an improvement in insulin sensitivity.
Low-grade inflammation has been associated with diabetes mellitus; however, pro-inflammatory cytokines such as TNF-α promoted insulin resistance though NF-κB inflammatory signaling could interfere with normal insulin signaling by IRS-1 serine phosphorylation [11,56]. This is the first study to report on the beneficial effects of supplementation of fish oil that is rich in MUFAs acquired from freshwater hybrid catfish on ameliorating inflammation by down-regulated pro-inflammatory TNF-α and nuclear NF-κB protein expressions in the skeletal muscle of type 2 diabetic rats. Recent studies have reported that the activity of NF-κB was markedly decreased in subjects with metabolic syndrome after long-term consumption of a MUFAs-rich diet [21]. Moreover, in the in vitro study, the co-incubation of skeletal muscle cells treated with palmitic acid and oleic acid decreased the NF-κB binding activity and suppressed the expression of TNF-α and interleukin-6 (IL-6) mRNA [48]. The previous study had reported on the anti-inflammatory action of AMPK activation in the skeletal muscle [57]. Green et al. (2011) demonstrated that AMPK activation ameliorated inflammation through suppressing NF-κB signaling and activity, which were both accompanied by a reduced secretion of TNF-α from obese type 2 diabetic myocytes. Therefore, the increased AMPK activation in all groups of diabetic rats supplemented with fish oil-rich in MUFAs might be involved with the suppression of proinflammatory TNF-α and nuclear NF-κB protein expressions as well as that of PKC-θThr538 phosphorylation.
Our study experienced a few limitations. The fish oil acquired from freshwater hybrid catfish, which was used in this study, was a mixture of heterogeneous fatty acids. Nonetheless, the results from the analysis of the fatty acid compositions demonstrated that oleic acid contains a large quantity of MUFAs (Table 1). Hence, oleic acid might be promising in its ability to exert those beneficial effects of fish oil that is rich in MUFAs. Previous studies have consistently showed that oleic acid attenuates insulin resistance in skeletal muscle cells [45,48].
In conclusion, our results revealed the anti-diabetic action of fish oil-rich in MUFAs acquired from hybrid catfish (Pangasius larnaudii×Pangasianodon hypophthalmus) in type 2 diabetic rats that were induced by a high-fat diet with STZ injection. The advantageous effects of fish oil that is rich in MUFAs on glycemic control are involved in at least the attenuation of plasma adipokine disturbance and the improvement of insulin sensitivity through the conservation of normal insulin signaling, and the suppression of pro-inflammatory signaling along with the enhancement of AMPK activation in the skeletal muscle. Therefore, such benefits have strengthened the significant potential for fish oil-rich in MUFAs to be developed as a dietary supplement for diabetes treatment in the future.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Research Council (NRCT), Thailand.
Footnotes
Author contributions: W.K. performed the animal handling, western blot experiment and wrote the manuscript. S.A. performed the animal handling. D.A. performed the extraction and analysis of fish oil. N.L. performed assay measurement, supervised and coordinated the study.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys. 2007;48:103–113. doi: 10.1007/s12013-007-0030-9. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 6.Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7:14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaag A, Henriksen JE, Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:782–788. doi: 10.1172/JCI115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 12.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 13.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–456. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Due A, Larsen TM, Hermansen K, Stender S, Holst JJ, Toubro S, Martinussen T, Astrup A. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am J Clin Nutr. 2008;87:855–862. doi: 10.1093/ajcn/87.4.855. [DOI] [PubMed] [Google Scholar]
- 15.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011;46:209–228. doi: 10.1007/s11745-010-3524-y. [DOI] [PubMed] [Google Scholar]
- 16.Paniagua JA, de la Sacristana AG, Sánchez E, Romero I, Vidal-Puig A, Berral FJ, Escribano A, Moyano MJ, Peréz-Martinez P, López-Miranda J, Pérez-Jiménez F. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr. 2007;26:434–444. doi: 10.1080/07315724.2007.10719633. [DOI] [PubMed] [Google Scholar]
- 17.Shah M, Adams-Huet B, Brinkley L, Grundy SM, Garg A. Lipid, glycemic, and insulin responses to meals rich in saturated, cis-monounsaturated, and polyunsaturated (n-3 and n-6) fatty acids in subjects with type 2 diabetes. Diabetes Care. 2007;30:2993–2998. doi: 10.2337/dc07-1026. [DOI] [PubMed] [Google Scholar]
- 18.Moon JH, Lee JY, Kang SB, Park JS, Lee BW, Kang ES, Ahn CW, Lee HC, Cha BS. Dietary monounsaturated fatty acids but not saturated fatty acids preserve the insulin signaling pathway via IRS-1/PI3K in rat skeletal muscle. Lipids. 2010;45:1109–1116. doi: 10.1007/s11745-010-3475-3. [DOI] [PubMed] [Google Scholar]
- 19.Yang ZH, Miyahara H, Takemura S, Hatanaka A. Dietary saury oil reduces hyperglycemia and hyperlipidemia in diabetic KKAy mice and in diet-induced obese C57BL/6J mice by altering gene expression. Lipids. 2011;46:425–434. doi: 10.1007/s11745-011-3553-1. [DOI] [PubMed] [Google Scholar]
- 20.Keapai W, Apichai S, Pongchaidecha A, Amonlerdpison D, Lailerd N. Enhancing of skeletal glucose uptake by fish oil-derived MUFAs through glucose transporter 4 translocation in rat diaphragm; Proceeding of the International Graduate Research Conference; 2014 Dec 12; Chiang Mai, Thailand. pp. HS7–HS12. [Google Scholar]
- 21.Cruz-Teno C, Pérez-Martínez P, Delgado-Lista J, Yubero-Serrano EM, García-Ríos A, Marín C, Gómez P, Jiménez-Gómez Y, Camargo A, Rodríguez-Cantalejo F, Malagón MM, Pérez-Jiménez F, Roche HM, López-Miranda J. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: the LIPGENE study. Mol Nutr Food Res. 2012;56:854–865. doi: 10.1002/mnfr.201200096. [DOI] [PubMed] [Google Scholar]
- 22.Martín-Peláez S, Covas MI, Fitó M, Kušar A, Pravst I. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res. 2013;57:760–771. doi: 10.1002/mnfr.201200421. [DOI] [PubMed] [Google Scholar]
- 23.AOAC. Official method 996.06: fat (total, saturated, and unsaturated) in foods. 18th ed. AOAC International; 2005. pp. 20–25. Ch4. [Google Scholar]
- 24.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res. 1980;21:139–144. [PubMed] [Google Scholar]
- 28.Mohammad A, Sharma V, McNeill JH. Vanadium increases GLUT4 in diabetic rat skeletal muscle. Mol Cell Biochem. 2002;233:139–143. doi: 10.1023/a:1015558328757. [DOI] [PubMed] [Google Scholar]
- 29.Rhoads MG, Kandarian SC, Pacelli F, Doglietto GB, Bossola M. Expression of NF-kappaB and IkappaB proteins in skeletal muscle of gastric cancer patients. Eur J Cancer. 2010;46:191–197. doi: 10.1016/j.ejca.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musi N, Goodyear LJ. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol Scand. 2003;178:337–345. doi: 10.1046/j.1365-201X.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 31.Hilder TL, Baer LA, Fuller PM, Fuller CA, Grindeland RE, Wade CE, Graves LM. Insulin-independent pathways mediating glucose uptake in hindlimb-suspended skeletal muscle. J Appl Physiol (1985) 2005;99:2181–2188. doi: 10.1152/japplphysiol.00743.2005. [DOI] [PubMed] [Google Scholar]
- 32.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 33.Jové M, Planavila A, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology. 2006;147:552–561. doi: 10.1210/en.2005-0440. [DOI] [PubMed] [Google Scholar]
- 34.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 35.Al-Shoumer KA, Al-Asousi AA, Doi SA, Vasanthy BA. Serum leptin and its relationship with metabolic variables in Arabs with type 2 diabetes mellitusd. Ann Saudi Me. 2008;28:367–370. doi: 10.5144/0256-4947.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 38.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens) 2012;11:8–20. doi: 10.1007/BF03401534. [DOI] [PubMed] [Google Scholar]
- 39.Paz-Filho G, Mastronardi C, Wong ML, Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocrinol Metab. 2012;16(Suppl 3):S549–S555. doi: 10.4103/2230-8210.105571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Zhang Q, Zhang L, Li C, Jiang H. Expression of ghrelin and leptin during the development of type 2 diabetes mellitus in a rat model. Mol Med Rep. 2013;7:223–228. doi: 10.3892/mmr.2012.1154. [DOI] [PubMed] [Google Scholar]
- 43.Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- 44.Michelle Furtado L, Poon V, Klip A. GLUT4 activation: thoughts on possible mechanisms. Acta Physiol Scand. 2003;178:287–296. doi: 10.1046/j.1365-201X.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 45.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J. 2006;399:473–481. doi: 10.1042/BJ20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavaré JM, Fletcher LM, Oatey PB, Tyas L, Wakefield JG, Welsh GI. Lighting up insulin action. Diabet Med. 2001;18:253–260. doi: 10.1046/j.1464-5491.2001.00540.x. [DOI] [PubMed] [Google Scholar]
- 47.Nardi F, Lipina C, Magill D, Hage Hassan R, Hajduch E, Gray A, Hundal HS. Enhanced insulin sensitivity associated with provision of mono and polyunsaturated fatty acids in skeletal muscle cells involves counter modulation of PP2A. PLoS One. 2014;9:e92255. doi: 10.1371/journal.pone.0092255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitateinduced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283:11107–11116. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 49.Friedrichsen M, Mortensen B, Pehmøller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013;366:204–214. doi: 10.1016/j.mce.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 51.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–89. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Bolsoni-Lopes A, Festuccia WT, Chimin P, Farias TS, Torres-Leal FL, Cruz MM, Andrade PB, Hirabara SM, Lima FB, Alonso-Vale MI1. Palmitoleic acid (n-7) increases white adipocytes GLUT4 content and glucose uptake in association with AMPK activation. Lipids Health Dis. 2014;13:199. doi: 10.1186/1476-511X-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 55.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 56.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 57.Green CJ, Pedersen M, Pedersen BK, Scheele C. Elevated NF-κB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes. 2011;60:2810–2819. doi: 10.2337/db11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]