Abstract
Hypertension can be caused by various factors while the predominant causes include increase in body fluid volume and resistance in the circulatory system that elevate the blood pressure. Consumption of probiotics has been proven to attenuate hypertension; however, the effect is much strain-dependent. In this study, a newly isolated Lactobacillus casei (Lb. casei) strain C1 was investigated for its antihypertensive properties in spontaneously hypertensive rats (SHR). Lactic acid bacteria (LAB) suspension of 11 log colony-forming unit (CFU) was given to SHR (SHR+LAB, n=8), and phosphate buffer saline (PBS) was given as a control in SHR (SHR, n=8) and in Wistar rats as sham (WIS, n=8). The treatment was given via oral gavage for 8 weeks. The results showed that the weekly systolic blood pressure (SBP), mean arterial pressure (MAP), diastolic blood pressure (DBP) and aortic reactivity function were remarkably improved after 8 weeks of bacterial administration in SHR+LAB. These effects were mostly attributed by restoration of wall tension and tensile stress following the bacterial treatment. Although not statistically significant, the level of malondialdehye (MDA) in SHR+LAB serum was found declining. Increased levels of glutathione (GSH) and nitric oxide (NO) in SHR+LAB serum suggested that the bacterium exerted vascular protection through antioxidative functions and relatively high NO level that induced vasodilation. Collectively, Lb. casei strain C1 is a promising alternative for hypertension improvement.
Keywords: Antioxidant, Blood pressure, Nitric oxide, Probiotic, Vascular reactivity
INTRODUCTION
Probiotic microorganisms provide health benefits to hosts when taken in sufficient quantity [1]. They assist in balancing host gut microbiota and avoid harmful pathogenic infections. Several scientific reports have revealed that probiotic bacteria can be remedial for irritable bowel syndrome, eczema and allergies [2,3,4]. Lactic acid bacteria (LAB) are among the extensively studied probiotic microorganisms [5]. Oral administration of Lactobacillus GG helped to reduce oxidative stress and thus prevented alcoholic liver disease in rat model with alcoholic steatohepatitis [6]. Intake of Lactobacillus casei (Lb. casei) activated the intestinal mucosal immune system by increasing the expression level of inflammatory receptors namely CD-206 and Toll-like receptor 2 (TLR-2) [7]. Recently, 12 Lactobacillus strains were isolated from probiotic drinks in Malaysia [8]. These strains were shown to possess significant antimicrobial properties and able to withstand extreme gastric pH. Among the isolates, Lb. casei strain C1 exhibited the most significant antimicrobial effects against both gram-positive and gram-negative bacteria hence its remarkable probiotic potentials.
Hypertension is a public health problem worldwide and highly associated with chronic cardiovascular diseases such as heart failure, ischemic heart disease and stroke [9]. One of the key features of primary hypertension is thickening and hardening of the arterial wall that is predominantly due to structural remodeling [10]. Structural remodeling is often coupled with impaired vascular reactivity. This phenomenon was clearly demonstrated in rats undergoing chronic nicotine administration [11]. One of the key factors that results in hypertensive vessels and impaired vascular reactivity is low bioavailability of nitric oxide (NO) [12].
Interestingly, a few probiotic strains were reported to exhibit antihypertensive effects. For instance, intake of dairy products containing a mixture of Enterococcus faecium and two strains of Streptococcus thermophiles for 8 weeks lowered low-density lipoprotein cholesterol (LDL) level and systolic blood pressure [13]. Administration of Lactobacillus plantarum 299v for 6 weeks was also found to reduce systolic blood pressure in heavy smokers [14]. Besides, consumption of probiotics-fermented potato yoghurt could debilitate hypertension-induced cardiac apoptosis and therefore enabled cardiac protection against hypertension [15]. Lb. casei and Streptococcus thermophilus TMC 1543 were proven to lower systolic blood pressure and risk factors that caused ischemic heart disease [14]. However, antihypertensive effects of LAB are highly strain-specific [16], thus it is not known if Lb. casei is capable of exerting similar antihypertensive effects as the other members of LAB.
Therefore, this study aimed to investigate antihypertensive functions of Lb. casei strain C1 in spontaneously hypertensive rats (SHR) for 8 weeks. Essentially, Lb. casei strain C1 helped lower blood pressure via aortic structural adaptation and promoted the expression of endothelium-derived relaxing factor (EDRF) such as NO as part of the underlying mechanisms of antihypertension.
METHODS
Preparation of Lb. casei strain C1 suspension
Lb. casei strain C1 was prepared as described previously [8]. A volume of 10 µL of Lb. casei strain C1 was cultured on a deMan, Rogosa and Sharpe (MRS) agar plate (Sigma-Aldrich, St. Louis, United States) for 48 hours at 37℃. A bacterial colony was selected and inoculated into MRS broth and incubated for 24 hours at 37℃. The culture was centrifuged at 5,000 g at 4℃ for 10 minutes. The bacterial pellet was then recovered at 11 log colonyforming unit (CFU)/0.5 ml phosphate buffer saline (PBS) for feeding.
Animals
A total of 24 male rats aged 8 weeks old were used in this study. All protocols employed in animal handling were performed in accordance with the guidelines issued by the National University of Malaysia (UKM) Animal Ethics Committee. All animals were housed under ambient room temperature and lighting (12 hour light/dark cycle) and were fed with standard laboratory pellet diet and water ad libitum. Animals were acclamatized for 7 days prior to the experiment. Wistar (WIS) and spontaneously hypertensive rats (SHR) (n=8 in each group) were given 0.5 ml/kg body weight (b.w) of PBS. SHR+LAB group (n=8) was given Lb. casei strain C1 suspension (11 log CFU in PBS) daily [5] at the same dosage for 8 weeks [13].
Measurement of blood pressure
Rats were restrained and placed on a heating plate (30~32℃) and their tails were occluded with cuffs which were connected to CODA II ™ Non-Invasive Blood Pressure System (Kent Scientific, US). Each measurement encompassed five acclimatization cycles followed by 15 measurement cycles. Measurements were taken at weekly intervals throughout the study.
Assessment of aortic reactivity
After 8 weeks, thoracic aorta was dissected meticulously from rats under urethane anesthesia (1 g/kg). The aorta was cut transversely into 4-mm rings and mounted in 50 ml of Krebs-Henseleit buffer [NaCl (118 mM), KCl (4.7 mM), CaCl2 · 2H2O (2.5 mM), MgSO4 · 7H2O (1.2 mM), KH2PO4 (1.2 mM), NaHCO3 (25.0 mM), and glucose (11.7 mM)], pH 7.4 before mounting it on the organ bath. The tissues were aerated continuously with 95% O2 and 5% CO2 at 37℃ and equilibrated with an initial resting tension of 1.5 g for 45 min. Isometric contraction, data processing and analysis were performed as described [12]. After equilibration, the tissues were primed with 6 ml of 120 mM KCl, followed by a washout of KCl and then the addition of phenylephrine (PE, 10–9 to 10–5 M) to test the adrenergic response of the smooth muscle. Tissue contraction was expressed as percentage of maximal KCl-induced contraction. Endothelium-dependent relaxation was tested by adding acetylcholine (ACh, 10–9 to 10–5 M) to the tissues following PE-induced precontraction. Relaxation of aortic tissues was expressed as percentage of maximal PE-induced contraction.
Collection of blood serum
Blood was drawn into 15-ml plain tubes via orbital sinus and then left to clot at room temperature. Serum was obtained by centrifugation at 1,000 g for 10 minutes at 4℃ and stored at –80℃ until use.
Biochemical analysis of serum
NO level in the serum was measured using the spectrophotometric Griess assay with sodium nitrite (NaNO2) as a reference standard [17,18,19]. Levels of NO, malondialdehye (MDA) and glutathione (GSH) in the serum were derived from standard curves and expressed as per mg of serum protein.
Histological analysis of aortic tissues
A 5-mm aortic ring was fixed in 10% (v/v %) neutrally buffered formalin and subsequently processed in paraffin. Thin tissue sections (3~5 µm) were then rehydrated and stained with haematoxylin and eosin (H&E). Digital images of aortic sections were captured and analyzed with the Image-J software. Measurements of intima-media thickness (IMT), intima-media area (IMA), lumen diameter, circumferential wall tension (CWT), and tensile stress (TS) were performed as described [20]. Four measurements of IMT were obtained at 0°, 90°, 180° and 270° to derive the mean IMA. Lumen area (a) was estimated by drawing a line over the circle at the boundary of the inner intima. The lumen diameter (d) was calculated as d=(2√a)/π where π=3.14, d was expressed in mm2. The mean cross-sectional area of the tunica intima and tunica media (intima-media area, IMA)=[π(d/2+IMT)2]–[π(d/2)2]. CWT=MSBP×(d/2), where d was expressed in cm meanwhile CWT and MSBP (mean systolic blood pressure) were expressed in dyne/cm and dynes/cm2, respectively. Tensile stress (TS), TS=CWT/IMT; units for TS and IMT were dyne/cm2 and cm, respectively.
Statistical analysis
Statistical analysis was performed using One-way ANOVA in GraphPad PRISM Version 6.07. All data were expressed as mean±standard error mean (SEM). Significance of data was determined at p<0.05.
RESULTS
Body weight gain
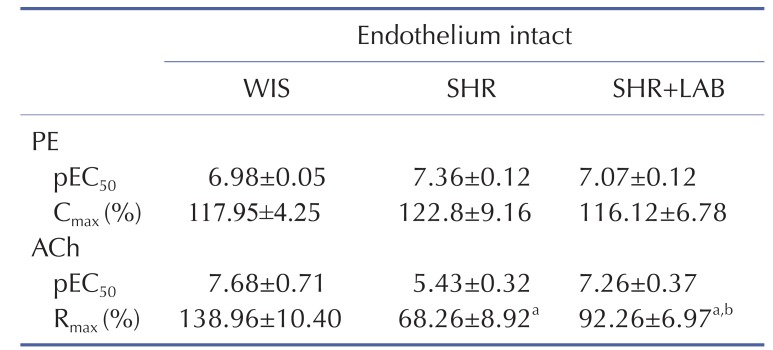
Fig. 1 shows body weight gains in rats across the eight weeks of Lb. casei strain C1 administration. However, there were no significant differences in weight gains for SHR compared to SHR+LAB. This implies that Lb. casei strain C1 administration in SHR did not influence diet intake by the rats.
Fig. 1. Body weight gain of rats. SHR+LAB and SHR showed lower weight gains than WIS.
Body weight gain is expressed as mean±SEM for n=6 each group. asignificant difference (p<0.05) in body weight gains between SHR and WIS. bsignificant difference (p<0.05) in body weight gains between SHR+LAB and WIS.
Lb. casei strain C1 lowered blood pressure in SHR
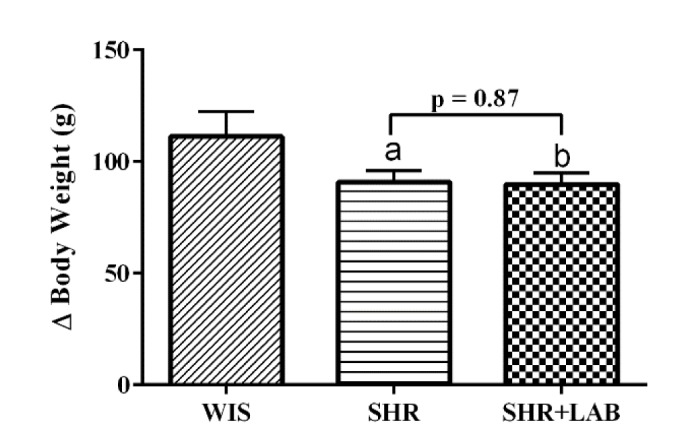
Changes in blood pressure (BP) throughout the eight experimental weeks are summarized in Fig. 2. At the end of the experiment, systolic blood pressure (SBP) of SHR (Fig. 2A) increased significantly (p<0.05) as much as 27.07±4.57 mmHg compared to WIS which only increased 8.74±0.97 mmHg from the baseline. The relatively high SBP was reverted from 205.97±6.81 mmHg to 168.41±35 mmHg (p<0.05) following the bacterial administration. A similar pattern was observed in diastolic blood pressure (DBP) (Fig. 2B). There was a significant increase of 19.26 mmHg (p<0.05) in SHR as compared to WIS (4.78±1.39 mmHg). The relatively high DBP were lowered significantly in SHR+LAB from 173.57±5.11 to 131.67±1.77 mmHg (p<0.05). In Fig. 2C, the mean arterial pressure (MAP) of WIS remained rather constant for the whole eight weeks whereas a significant increase (p<0.05) of 15.07±3.68 mmHg was observed in SHR. The relatively high MAP was then restored in SHR+LAB with a reduction of 20.86±9.22 mmHg.
Fig. 2. Changes in (A) SBP (B) DBP (C) MAP in rats during the 8 weeks of Lb. casei strain C1 administration.
Values are given as mean with SEM for n=6 each group. asignificant in relative to WIS (p<0.05), bsignificant in relative to SHR (p<0.05).
Lb. casei strain C1 prevented impairment in vascular reactivity
Vascular Contraction
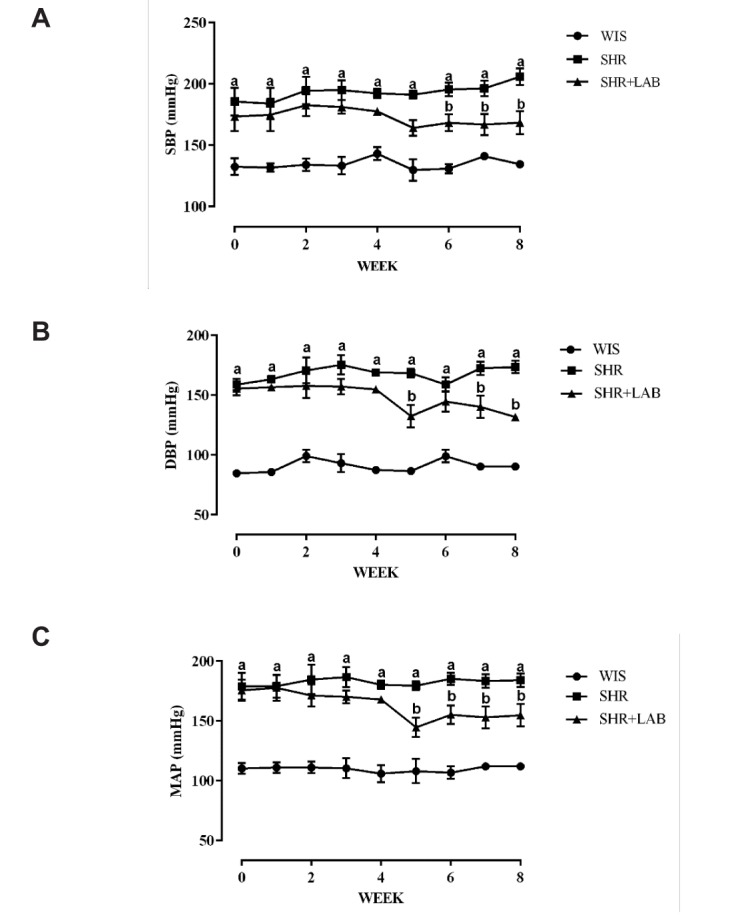
The aortic rings of all rat groups contracted gradually with the increasing concentrations of PE (Fig. 3A). A higher pEC50 (50% effective concentration) of 7.36±0.12 (Table 1) was recorded in SHR although it was not significantly different from the other rat groups. In terms of the maximum contraction (Cmax), SHR+LAB and WIS showed very similar Cmax which were 116.12±6.78 and 117.95±4.25%, respectively (Table 1).
Fig. 3. Concentration-dependent responses of aortic rings to (A) PE and (B) ACh.
Values are presented as mean with SEM for n=6 in each group. asignificant relative to WIS group (p<0.05), bsignificant relative to SHR group (p<0.05), using One-way ANOVA plus Tukey multiple comparison test.
Table 1. Contraction and relaxation responses in aortic rings.
Values are given as mean with SEM for n=6 each group. asignificant relative to WIS group (p<0.05), bsignificant relative to SHR group (p<0.05), using One-way ANOVA plus Tukey multiple comparison test.
Vascular Relaxation
Fig. 3B shows an increasing relaxation pattern among the three rat groups, with WIS showing the greatest relaxation followed by SHR+LAB and then SHR. The least sensitivity in response to ACh was exhibited by SHR with an pEC50 of 5.43±0.32 (Table 1). This observation was further ascertained by the lowest maximum relaxation (Rmax) achieved by SHR (68%) compared to that observed in WIS (139%) and SHR+LAB (92%). The differences were statistically significant (p<0.05).
Lb. casei strain C1 reduced systemic oxidative stress
In Fig. 4A, SHR serum contained higher MDA level than that found in the WIS serum. Although there was no significant difference in the MDA level between SHR+LAB and SHR, the MDA level in SHR+LAB declined after eight weeks of Lb. casei C1 administration.
Fig. 4. Levels of (A) MDA, (B) GSH and (C) NO in the rat serum.
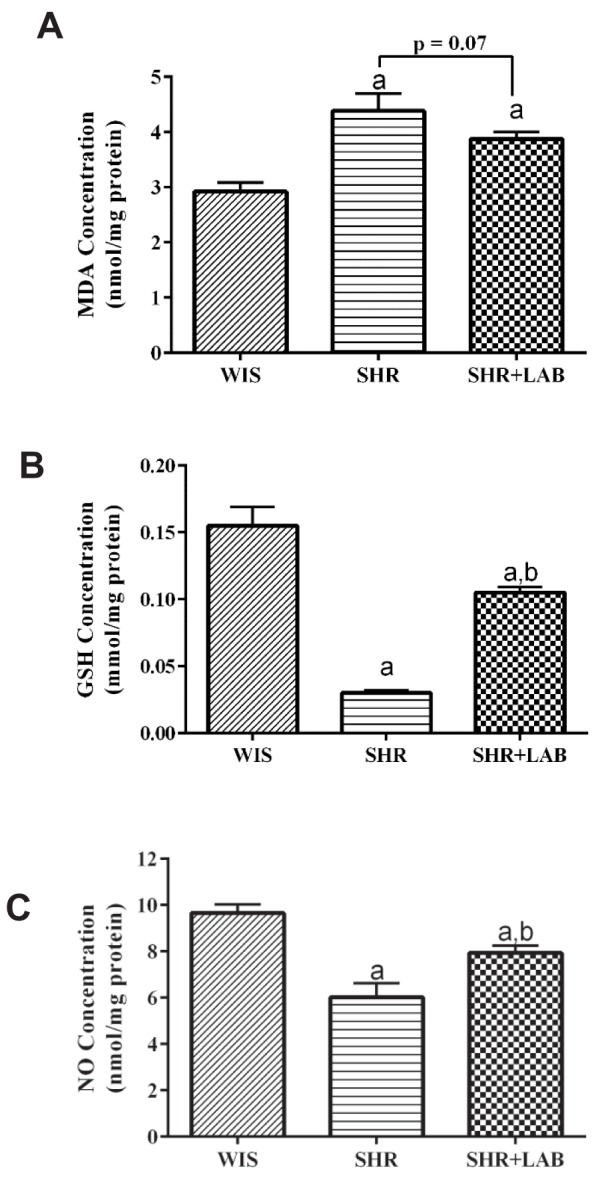
Values are presented as mean with SEM for n=6 in each group. asignificant in relative to WIS group (p<0.05), bsignificant in relative to SHR group (p<0.05).
Hypertension inhibits the production of endogenous antioxidant, GSH potentially. This is particularly evident in this study in which the GSH level in SHR serum was significantly lower than that found in WIS (Fig. 4B). However, by feeding Lb. casei strain C1 to SHR+LAB, the GSH production was recovered more than three folds higher than that found in SHR.
Theoretically, an antihypertensive substance is able to stimulate higher NO production which in turn regulates vasodilation and relieves hypertension in succumbed individuals. This concept was well substantiated in this study. In the SHR serum, the NO level was found to be approximately two folds less than that in the WIS serum (Fig. 4C). However, the NO level was successfully raised in SHR+LAB. The increase was found to be statistically significant in relative to the NO level in SHR serum.
Lb. casei strain C1 prevented structural changes
Fig. 5 shows the H&E-stained microscopic images of aorta for WIS, SHR and SHR+LAB. Aortic tissue of WIS contained organized tunica layers with orderly arrayed elastic lamellae and nuclei. Meanwhile, in the aortic tissue of SHR, the tunica layers remained visible but the structure was rather disorganized. The elastic lamellae were distorted with nuclei scattered in the tunica media.
Fig. 5. Representative microscopic images of H&E-stained aortic wall structures for each group.
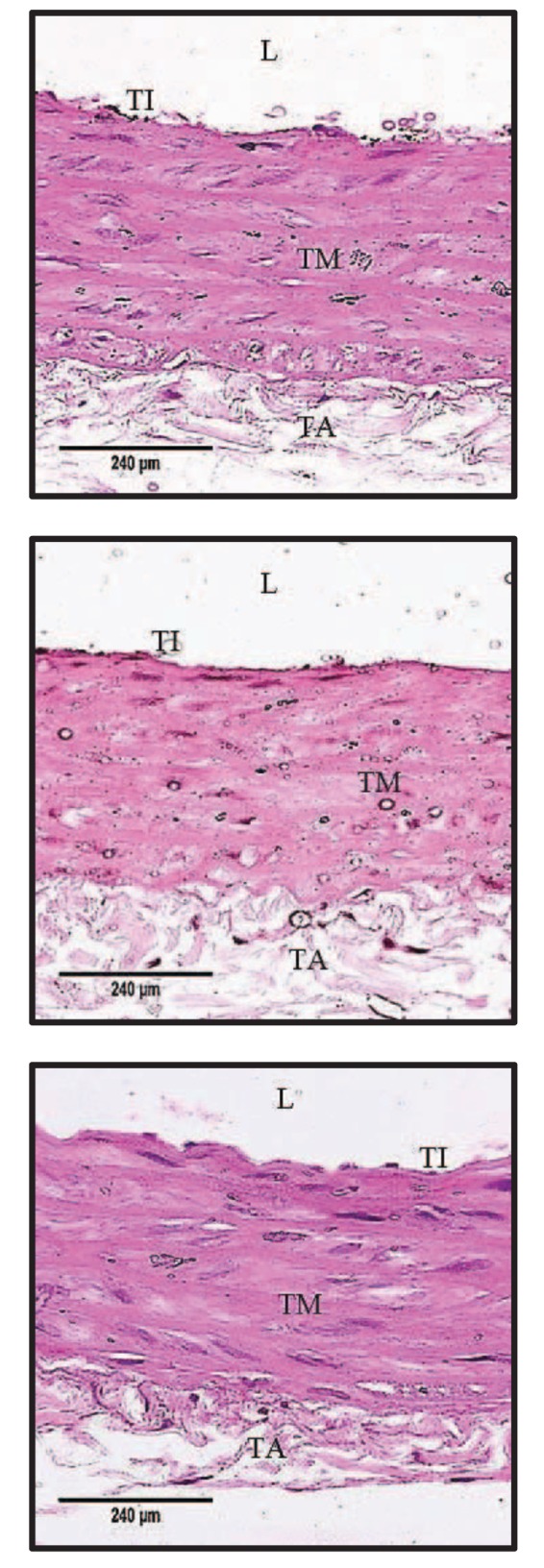
(A) WIS group displayed normal arrangement of elastic lamella at the tunica media layer. (B) SHR showed disorganized tunica media layer with increased interlamellar space. (C) SHR+LAB group showed a more organized arrangement of elastic lamella than SHR group at the tunica media. L, lumen; TI, tunica intima; TM, tunica media; TA, tunica adventitia (400x magnification).
To better describe histological changes in the tissues, IMT and IMA were compared. There were no discernible changes observed in the IMT (Fig. 6A) and the IMA (Fig. 6B) among the three groups. However, there was a significant reduction (p<0.05) in the lumen diameter of SHR aorta in comparison to that of WIS (Fig. 6C). This could be a contributing factor to the relatively high blood pressure in SHR. The lumen size was then enlarged to that similar to WIS after eight weeks of bacterial administration in SHR+LAB. The increment was found to be statistically significant as compared to the lumen size of SHR aorta. Administration of Lb. casei strain C1 in SHR+LAB also helped reduce the CWT significantly from 1.2×104 (SHR) to 0.8×104 (SHR+LAB) dyne/cm (Fig. 6D). However, it only induced slight reduction in TS in SHR+LAB (Fig. 6E). Overall, this study provided some insights on the functions of Lb. casei strain C1 in improving structural changes that caused by hypertension.
Fig. 6. Measurements for (A) IMT, (B) IMA, (C) lumen diameter, (D) CWT and (E) TS were constructed to evaluate degree of vascular remodelling.
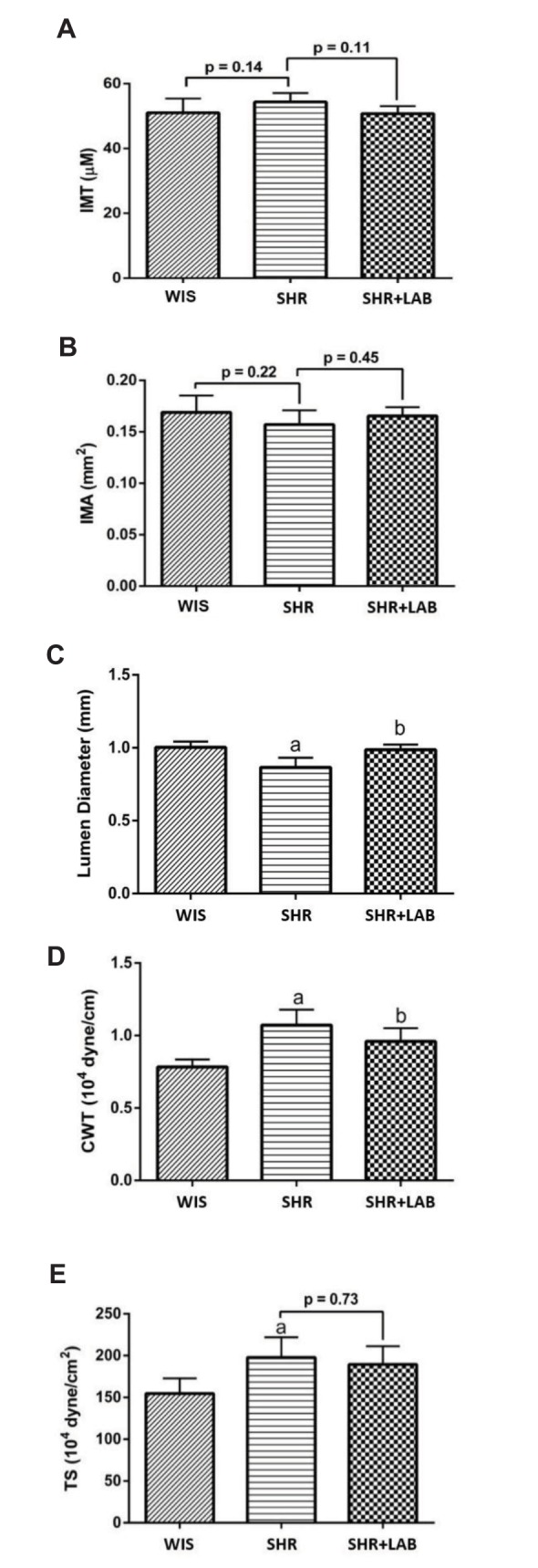
Values are stated as mean with SEM for n=6 in each group. asignificant in relative to (WIS) (p<0.05), bsignificant in relative to SHR group (p<0.05).
DISCUSSION
A former study reported that probiotics could reduce blood pressure in SHR within eight weeks of administration [13]. For this reason, Lb. casei strain C1 was fed to SHR for eight weeks in this study in order to produce plausible antihypertensive effects. SHR were given Lb. casei strain C1 at a dosage of 11 log CFU/0.5 ml PBS which was proven to be effective in improving hypertension [5]. In our study, Lb. casei strain C1 administration conferred antihypertensive effects by lowering the SBP, DBP and MAP in SHR+LAB significantly. Similar phenomena were also reported in previous studies [14,21]. Probiotics have been shown to lower vascular pro-oxidative state which in turn reduces blood pressure and maintains vasodilation and constriction at the normal state [22]. In addition, probiotic strains such as Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4 were also shown to produce angiotensin-converting enzyme (ACE) inhibitory peptide, mostly β-casein, in fermented milk that caused relaxation of blood vessels and decreased blood volume. These activities are essential in lowering blood pressure and decreasing oxygen demand from the heart [23].
Reactivity towards vasoconstrictor agonist, PE, and endothelium-dependent vasorelaxant agonist, ACh, determines sensitivity of the aorta [11]. Hypertension causes relatively high aorta contractility but low aorta relaxation in response to PE and ACh, respectively. The aorta ring of SHR showed higher sensitivity to PE (high pEC50 and Cmax) but a poorer response to ACh (low pEC50 and Rmax) than WIS. However, this situation was improved in SHR+LAB. Furthermore, in hypertension models, endothelium dysfunction is often associated with decreased level of NO. Vasodilation is improved following an increase in the NO level in the circulation [24]. This phenomenon was clearly demonstrated in this study where the NO level was successfully hoisted following eight weeks of Lb. casei strain C1 administration in SHR+LAB. The improved vasodilation response and high circulating NO level could prevent aortic tissue remodeling [25] and therefore reduces hypertension.
Intensified stress exerted by hypertension promotes the generation of reactive oxygen species (ROS) that elicits oxidative stress [24]. The imbalanced oxidative stress destroys endothelial membrane hence lipid peroxidation which is best indicated by the presence of high level of MDA. Intake of Lactobacillus coryniformis CECT5711 was able to attenuate the production of ROS [22]. The attenuation is normally associated with reduced NADPH oxidase activity and restoration of antioxidant enzymes [26]. This is in line with our findings that administration of Lb. casei strain C1 in SHR+LAB brought down the MDA level. The reduction is expected to be more significant with a longer administration of the since relatively longer antihypertensive treatment duration has been proven be more beneficial [27]. However, the precise mechanism of action of Lb. casei strain C1 in preventing systemic hypertension is still unknown. According to the results, the bacterium helped restore normal barrier function in SHR model by debilitating endothelial dependent-oxidative stress and thus attenuated hypertension in the rats.
To counterbalance hypertension-induced oxidative stress, endogenous antioxidants are generated, for instance, the antioxidative GSH. SHR serum contained relatively lower level of GSH hence higher oxidative stress. Following eight weeks of Lb. casei strain C1 administration in SHR+LAB, a higher GSH level was produced, suggesting that the bacterial administration helped restore the GSH level in SHR which in turn reduced oxidative stress resulted by hypertension. Restoration of GSH by Lb. casei strain C1 could be a consequence of glutamate-cysteine-ligase activity in the pancreatic cells [28].
Chronic high blood pressure causes aorta remodeling that is characterized by structural disorientation and damages in the aorta [29]. Such structural disorientation could be restored by the administration of probiotic bacteria in experimental mice [15]. Structural disorientation and lumen size were restored in SHR+LAB and the improvement helped reduce CWT and TS in the SHR+LAB. In addition, improvement in CWT and TS has been proven to be positively correlated with higher NO level in the circulation [12,30]. The higher NO level in SHR+LAB serum is, therefore, a contributing factor to the improved structural orientation and enlarged lumen size in the rat aortic tissues. The IMT and IMA did not vary significantly among the three rat groups. This is explainable by relatively shorter treatment duration in this study.
CONCLUSION
In conclusion, administration of Lb. casei strain C1 conferred effective vascular protection to SHR. There are several ways on how the bacterium exerts its protective functions in SHR: (i) reduces systolic and diastolic blood pressure, (ii) reverts aorta remodeling, (iii) counterbalances oxidative stress by promoting GSH biosynthesis and reducing MDA, and (iv) promotes NO production that improves vasodilation. In the nutshell, this study adds to the benefits of Lb. casei strain C1 as a potential probiotic strain in preventing hypertension.
Acknowledgements
This research was funded by the Ministry of Higher Education Malaysia with Grant No.: FRGS/1/2014/SG05/UKM/02/4. We thank Dr. Muhizam Mustafa from the Universiti Sains Malaysia and Mr Anand Ramalingam for proof-reading.
Footnotes
Author contributions: F.M.A. performed the LAB preparation and vascular reactivity. Y.C.L. performed the histomorphometry and statistical analysis. Y.W.B. and S.Z. supervised and coordinated the study. F.M.A, Y.W.B and S.Z. wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, Bruce AW. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35:131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 2.Ouwehand AC. Antiallergic effects of probiotics. J Nutr. 2007;137(3 Suppl 2):794S–797S. doi: 10.1093/jn/137.3.794S. [DOI] [PubMed] [Google Scholar]
- 3.Quigley EM. Probiotics in irritable bowel syndrome: an immunomodulatory strategy? J Am Coll Nutr. 2007;26:684S–690S. doi: 10.1080/07315724.2007.10719648. [DOI] [PubMed] [Google Scholar]
- 4.Tappenden KA, Deutsch AS. The physiological relevance of the intestinal microbiota--contributions to human health. J Am Coll Nutr. 2007;26:679S–683S. doi: 10.1080/07315724.2007.10719647. [DOI] [PubMed] [Google Scholar]
- 5.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 6.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galdeano CM, Perdigón G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yap WB, Sujang R, Tan TS. Identification and characterization of Lactic Acid Bacteria (LAB) isolated from probiotic drinks in Malaysia. J Sains Kesihatan Malaysia. 2015;13:23–31. [Google Scholar]
- 9.Persell SD1. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 11.Zainalabidin S, Budin SB, Ramalingam A, Lim YC. Aortic remodelling in chronic nicotine-administered rat. Korean J Physiol Pharmacol. 2014;18:411–418. doi: 10.4196/kjpp.2014.18.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, Joannidès R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- 13.Agerholm-Larsen L, Raben A, Haulrik N, Hansen AS, Manders M, Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur J Clin Nutr. 2000;54:288–297. doi: 10.1038/sj.ejcn.1600937. [DOI] [PubMed] [Google Scholar]
- 14.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76:1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 15.Lin PP, Hsieh YM, Kuo WW, Lin YM, Yeh YL, Lin CC, Tsai FJ, Tsai CH, Huang CY, Tsai CC. Probiotic-fermented purple sweet potato yogurt activates compensatory IGF-IR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int J Mol Med. 2013;32:1319–1328. doi: 10.3892/ijmm.2013.1524. [DOI] [PubMed] [Google Scholar]
- 16.Rowland I, Capurso L, Collins K, Cummings J, Delzenne N, Goulet O, Guarner F, Marteau P, Meier R. Current level of consensus on probiotic science--report of an expert meeting--London, 23 November 2009. Gut Microbes. 2010;1:436–439. doi: 10.4161/gmic.1.6.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leelarungrayub D, Sawattikanon N, Klaphajone J, Pothongsunan P, Bloomer RJ. Coenzyme Q10 supplementation decreases oxidative stress and improves physical performance in young swimmers: a pilot study. Open Sports Med J. 2010;4:1–8. [Google Scholar]
- 18.Stocks J, Dormandy TL. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes-Santos C, de Souza Mendonça L, Mandarim-de-Lacerda CA. Favorable cardiac and aortic remodeling in olmesartan-treated spontaneously hypertensive rats. Heart Vessels. 2009;24:219–227. doi: 10.1007/s00380-008-1104-3. [DOI] [PubMed] [Google Scholar]
- 21.Lye HS, Kuan CY, Ewe JA, Fung WY, Liong MT. The improvement of hypertension by probiotics: effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci. 2009;10:3755–3775. doi: 10.3390/ijms10093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla MP, Garrido-Mesa N, Rodríguez-Cabezas ME, Olivares M, Gálvez J, Duarte J. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci (Lond) 2014;127:33–45. doi: 10.1042/CS20130339. [DOI] [PubMed] [Google Scholar]
- 23.Gobbetti M, Ferranti P, Smacchi E, Goffredi F, Addeo F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl Environ Microbiol. 2000;66:3898–3904. doi: 10.1128/aem.66.9.3898-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001;16(Suppl 1):60–62. doi: 10.1093/ndt/16.suppl_1.60. [DOI] [PubMed] [Google Scholar]
- 25.Guo Z, Wang J, Yan L, Chen W, Liu X, Zhang H. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT-Food Sci Technol. 2009;42:1640–1646. [Google Scholar]
- 26.Ran X, Zhao W, Li W, Shi J, Chen X. Cryptotanshinone inhibits TNF-α-induced LOX-1 expression by suppressing reactive oxygen species (ROS) formation in endothelial cells. Korean J Physiol Pharmacol. 2016;20:347–355. doi: 10.4196/kjpp.2016.20.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, Kurabayashi M, Masuda I, Sakuma I, Yamazaki T, Yokoi H, Yoshida M. Effect of long-term intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness--Justification for Atherosclerosis Regression Treatment (JART) extension study. Circ J. 2013;77:1526–1533. doi: 10.1253/circj.cj-12-1149. [DOI] [PubMed] [Google Scholar]
- 28.Lutgendorff F, Trulsson LM, van Minnen LP, Rijkers GT, Timmerman HM, Franzén LE, Gooszen HG, Akkermans LM, Söderholm JD, Sandström PA. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1111–G1121. doi: 10.1152/ajpgi.00603.2007. [DOI] [PubMed] [Google Scholar]
- 29.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant. 2008;23:1892–1901. doi: 10.1093/ndt/gfm861. [DOI] [PubMed] [Google Scholar]
- 30.Sobko T, Reinders CI, Jansson E, Norin E, Midtvedt T, Lundberg JO. Gastrointestinal bacteria generate nitric oxide from nitrate and nitrite. Nitric Oxide. 2005;13:272–278. doi: 10.1016/j.niox.2005.08.002. [DOI] [PubMed] [Google Scholar]