Abstract
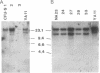
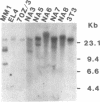
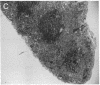
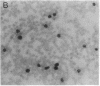
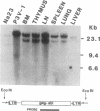
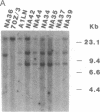
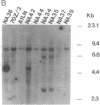
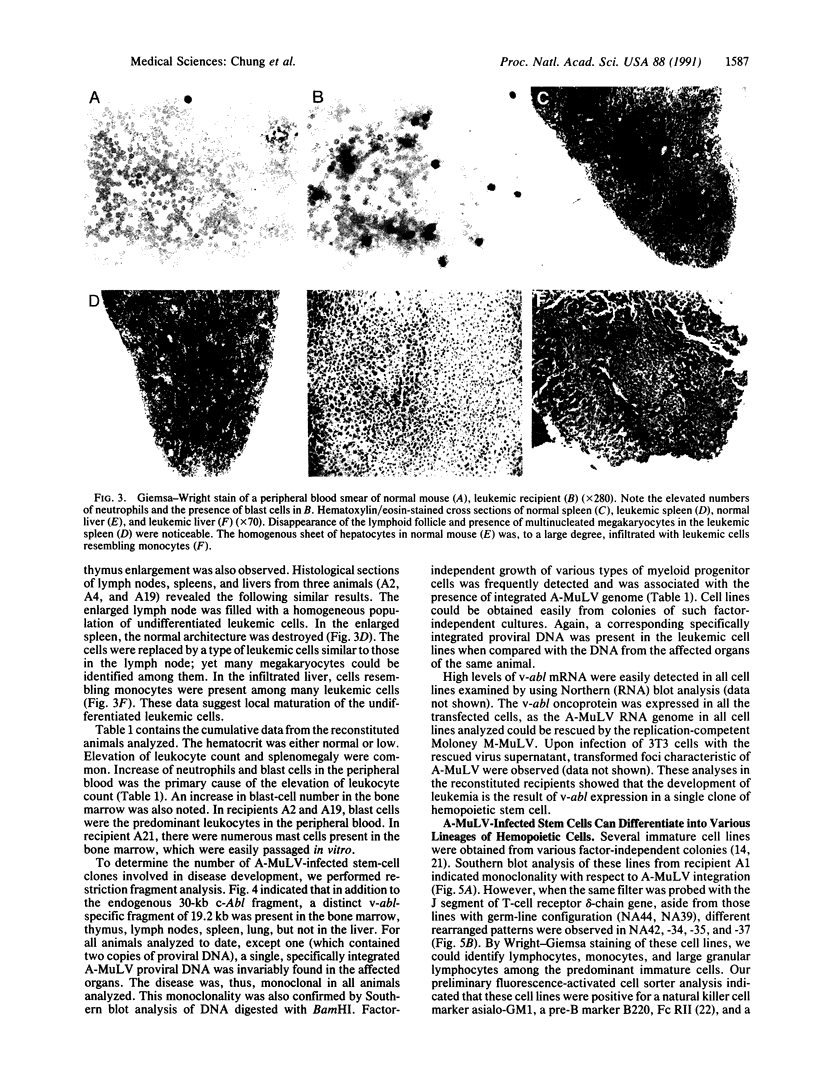
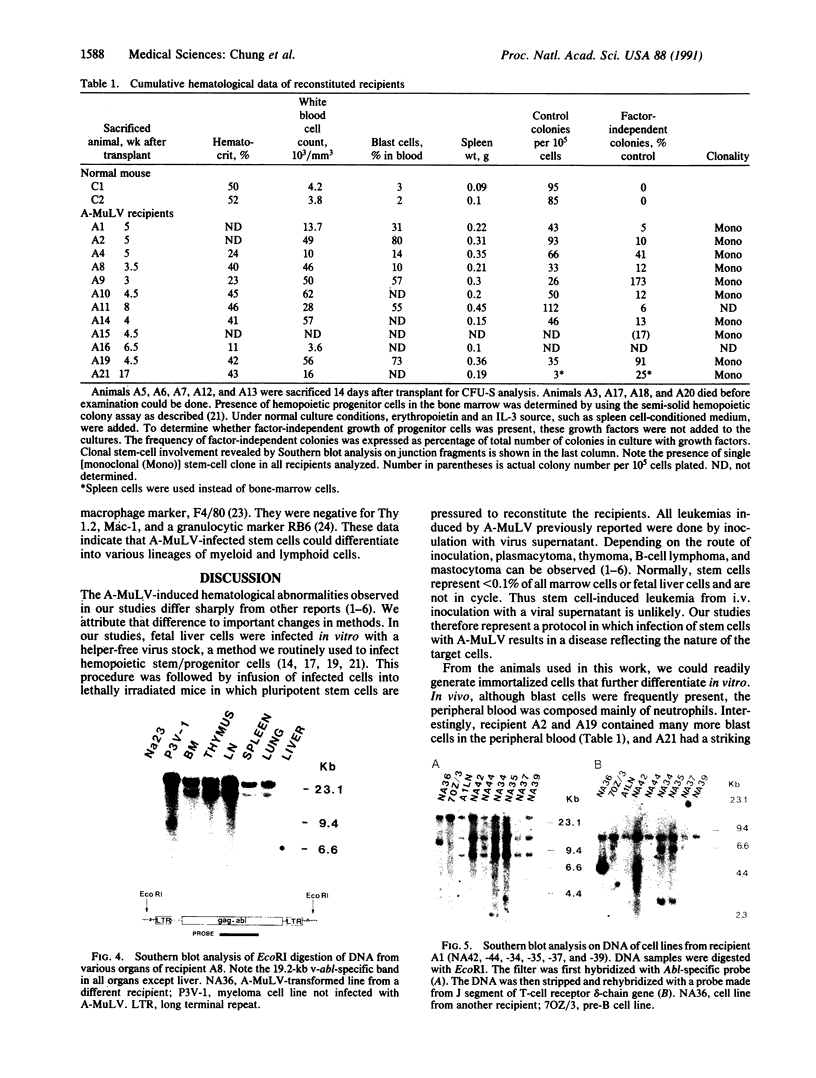
We report a mouse model with which to study leukemogenesis initiated by a specific genetic change introduced into a primary lymphoid-myeloid pluripotent stem cell. Fetal liver hemopoietic cells were infected with a high titer of helper-free Abelson murine leukemia virus (A-MuLV) and were used to reconstitute lethally irradiated mice. Two weeks later, progenies of a single primitive hemopoietic stem cell carrying a specifically integrated A-MuLV proviral DNA could be detected in both colony-forming units in spleen and myeloid colony-forming cells in the bone marrow. Beginning at 3 weeks after transplantation, the recipients developed elevated leukocyte counts, splenomegaly, and increase of blast cells in the peripheral blood. Multiple clones of A-MuLV-infected cells were infused into each recipient. However, in the same animal, DNA extracted from various affected organs and from factor-independent lymphoid and myeloid immortalized cells all contained an identical, specifically integrated proviral genome. The A-MuLV-infected stem cells differentiated into various lineages of hemopoietic cells. Our data show that the expression of the v-abl oncogene in a primary lymphoid-myeloid hemopoietic stem cell directly initiates leukemogenesis by stimulating factor-independent growth. The monoclonal-type disease development seen in these animals may require the occurrence of an additional genetic event.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Gonda T. J., Johnson G. R. Long-term exposure to retrovirally expressed granulocyte-colony-stimulating factor induces a nonneoplastic granulocytic and progenitor cell hyperplasia without tissue damage in mice. J Clin Invest. 1989 Nov;84(5):1488–1496. doi: 10.1172/JCI114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Lang R. A., Gonda T. J., Johnson G. R. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989 May 1;73(6):1487–1497. [PubMed] [Google Scholar]
- Chung S. W., Ruscetti S., Wong P. M. Formation of factor-independent hematopoietic multilineage colonies after Abelson virus infection. Blood. 1988 Apr;71(4):973–977. [PubMed] [Google Scholar]
- Chung S. W., Wong P. M., Shen-Ong G., Ruscetti S., Ishizaka T., Eaves C. J. Production of granulocyte-macrophage colony-stimulating factor by Abelson virus-induced tumorigenic mast cell lines. Blood. 1986 Nov;68(5):1074–1081. [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Timmons M., Pendergast A. M., Ben-Neriah Y., Dow L. W., Crist W., Rovera G., Smith S. D., Witte O. N. Expression of a distinctive BCR-ABL oncogene in Ph1-positive acute lymphocytic leukemia (ALL). Science. 1988 Feb 12;239(4841 Pt 1):775–777. doi: 10.1126/science.3422516. [DOI] [PubMed] [Google Scholar]
- Cook W. D. Rapid thymomas induced by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2917–2921. doi: 10.1073/pnas.79.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Langdon W. Y., Fredrickson T. N., Coffman R. L., Hoffman P. M., Hartley J. W., Morse H. C., 3rd Analysis of neoplasms induced by Cas-Br-M MuLV tumor extracts. J Immunol. 1986 Jul 15;137(2):679–688. [PubMed] [Google Scholar]
- Humphries R. K., Abraham S., Krystal G., Lansdorp P., Lemoine F., Eaves C. J. Activation of multiple hemopoietic growth factor genes in Abelson virus-transformed myeloid cells. Exp Hematol. 1988 Oct;16(9):774–781. [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Najfeld V., Hansen J. A., Penfold G. K., Jacobson R. J., Fialkow P. J. Involvement of the B-lymphoid system in chronic myelogenous leukaemia. Nature. 1980 Sep 4;287(5777):49–50. doi: 10.1038/287049a0. [DOI] [PubMed] [Google Scholar]
- Mendoza G. R., Metzger H. Disparity of IgE binding between normal and tumor mouse mast cells. J Immunol. 1976 Nov;117(5 Pt 1):1573–1578. [PubMed] [Google Scholar]
- Micklem H. S., Lennon J. E., Ansell J. D., Gray R. A. Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as functions of injected cell dose. Exp Hematol. 1987 Mar;15(3):251–257. [PubMed] [Google Scholar]
- Mori T., Nakazawa S., Nishino K., Sugita K., Takane K., Mori M., Sagawa K., Hayashi Y., Sakurai M. Ph1-positive CML-derived myeloid-monocytoid precursor cell line producing substance(s) that stimulates normal CFU-C. Leuk Res. 1987;11(3):241–249. doi: 10.1016/0145-2126(87)90047-6. [DOI] [PubMed] [Google Scholar]
- Poirier Y., Jolicoeur P. Distinct helper virus requirements for Abelson murine leukemia virus-induced pre-B- and T-cell lymphomas. J Virol. 1989 May;63(5):2088–2098. doi: 10.1128/jvi.63.5.2088-2098.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Dunbar C. E., Bodine D. M., Ruscetti S., Nienhuis A. W. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989 Feb;9(2):798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Raefsky E., Eaves C. J., Nienhuis A. W. Blast colonies containing hemopoietic progenitor cells can give rise to Abelson virus (A-MuLV)-transformed cell lines. Exp Hematol. 1988 Jan;16(1):5–11. [PubMed] [Google Scholar]