Abstract
Objective
The purpose of this study was to investigate the effect of dry needling (DN) on pain intensity and pressure pain threshold (PPT) compared with ischemic compression (IC) immediately and 48 hours after each treatment session in individuals with myofascial trigger points in the upper trapezius muscle.
Methods
Thirty-one patients with myofascial trigger points in the upper trapezius muscle participated in this study. Patients were randomly assigned to a standard (N = 17) or experimental group (N = 14). The treatment protocol for the standard group consisted of IC, whereas the patients in the experimental group received DN.
Results
The results indicated that the effect size of the DN methods for pain intensity and PPT was considerably greater after 2 days compared with immediately after the treatment session. In contrast, the effect of the IC for PPT was greater immediately after treatment compared with the measures after 2 days. There was also no noticeable difference in the effect size for IC on pain intensity between the scores obtained immediately and 2 days after treatment. However, our data also revealed a greater effect size for DN on PPT after 2 days compared with the IC technique.
Conclusions
In this study, DN improved the pain intensity and PPT after 2 days. However, it had no clinical improvement immediately after application because of muscle soreness. Thus, assessment of the effect of DN immediately after application can be criticized, and the results should be interpreted with caution.
Key Indexing Terms: Trigger Point, Upper Trapezius, Dry Needling, Ischemic Compression
Introduction
Musculoskeletal pain is a major cause of morbidity in today’s societies.1, 2, 3 About one-third of the patients with musculoskeletal pain meet the diagnostic criteria for myofascial pain syndrome.1 A myofascial trigger point (MTP) has been described as a hyperirritable spot located in a taut band of muscle, or a small pea or ropelike nodular or crepitant (crackling, grating) area within the muscle that is painful to palpation or compression and refers pain, tenderness, or an autonomic response to a remote area.4
Previous studies have indicated that MTPs are the primary source of musculoskeletal pain in patients. The prevalence of trigger point varies from 21% of patients seen in a general orthopedic clinic, to 30% of general medical clinic patients with regional pain, to as high as 85% to 93% of patients presenting to specialty pain management centers.5, 6 It has detrimental effects on people’s social and work-related activities, has a significant impact on the quality of life, and causes pain and functional disability in the neck and shoulder areas.2, 3
Some chemical changes, such as increased levels of bradykinin, substance P, and calcitonin gene-related peptide and lowered pH, have been reported in MTP.7, 8 Investigators established that the local oxygen saturation at an MTP site is less than 5% of normal.8 Hypoxia leads to a drop in tissue pH and the release of several nociceptive chemicals, including bradykinin, calcitonin gene-related peptide, and substance P.8 Local tenderness and referred pain are common with MTPs as muscle nociceptors are stimulated in response to reduced oxygen levels and lowered pH and increased inflammatory chemicals. Histologic studies have confirmed the presence of extreme sarcomere contractions, resulting in localized tissue hypoxia.8
The upper trapezius (UT) muscle was determined to be often affected by MTPs.4, 9
The common symptoms and pain pattern in participants with MTPs in the UT muscle are taut and painful muscle, tension headache, neck pain, dizziness or vertigo, and limited neck and shoulder range of motion.4, 9
One of the unique characteristics of an MTP is the local twitch response (LTR) phenomenon, which is an involuntary spinal cord reflex contraction of the contracted muscle fibers in a taut band after palpation or needling of the taut band in MTPs.8, 10, 11
Several treatment protocols have been suggested for MTPs.1, 12, 13 Physical therapy programs play a significant role in treatment and improvement of symptoms in patients with MTP. Ischemic compression (IC) is one of the most common treatment methods currently used for patients with MTP attending physical therapy clinics.1, 12, 13
More recently, there has been an increased interest in the use of dry needling (DN) by therapists to treat MTP.8 Dry needling, also referred to as intramuscular stimulation, is an invasive procedure in which an acupuncture needle is inserted into the skin and muscle.11 The objectives of DN include inactivating the MTP, normalizing the chemical environment of active MTPs, releasing muscle shortening, removing the source of muscle irritation, normalizing peripheral nerve sensitization, promoting self-healing of the injured tissue, and decreasing spontaneous muscle activity.8
Investigators have attributed the therapeutic effects of DN to various mechanisms, such as mechanical, neurophysiologic, and chemical effects.8, 10, 11 It is thought that DN provides a mechanical localized stretch to the shortened sarcomeres and contracted cytoskeletal structures within the MTP.8, 10, 11 Dry needling effects may also stimulate Aδ nerve fibers (group III), which in turn may activate the enkephalinergic inhibitory dorsal horn interneurons, resulting in opioid-mediated pain suppression and pain relief.8, 11 Some studies have reported that DN may influence the microcirculation in skin or muscle blood flow and levels of chemical properties at the MTP area.14, 15
Although some previous studies have assessed the effect of DN on MTP in UT muscles, a review of the published reports determined that some randomized clinical trials have been conducted to determine the effectiveness of DN in the treatment of MTP in UT muscle.16, 17, 18, 19, 20, 21 However, with the use of different designs, samples, and testing procedures, controversial results have been reported regarding the effect of DN on MTP in UT muscles.16, 17, 18, 19, 20, 21 Most of the previous studies have assessed the clinical effectiveness of DN immediately after treatment procedures.17, 19, 20, 21
Muscle soreness is a common report after DN application.20 Typically, after DN technique the muscle soreness lasts and may be felt for a few hours up to 24 to 48 hours.20 Considering this soreness after DN, the results collected immediately after treatment, such as pain intensity and pressure pain threshold (PPT), may be affected by soreness caused by needle insertion to the muscle. Because of the risk of muscle soreness, assessment of only immediate effects of DN can be criticized, and the results from the studies that measured the variables immediately after needling should be interpreted with caution.
To our knowledge, no study has assessed and compared the effect of DN in the treatment of MTP in UT muscle immediately and 48 hours after DN, when soreness has been relieved.14, 17, 18, 19, 20, 21, 22 The purpose of this study was to investigate the effect of DN compared with IC on pain intensity and PPT immediately after each treatment session compared with the measurements obtained 48 hours after each treatment session in individuals with MTP in the UT muscle.
Materials and Methods
General Design
A randomized controlled trial was performed (registered with the ClinicalTrials.gov, NCT 02107456) to investigate the clinical effect of DN compared with IC on pain intensity and PPT immediately and 48 hours after each treatment session in patients with MTP in the UT muscle. The study protocol was approved by human research ethics committee of the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran (reference no. 100-201; 2013). Before participating in the study, all participants signed an informed consent form approved by the human participants committee.
Participants
A total of 31 nonpregnant women with MTPs in the UT muscle, who had been referred for outpatient physical therapy evaluation and intervention, participated in this study. The patient population in this study was a sample of convenience made up of participants aged between 20 and 48 years. They were consecutive patients who agreed to participate and fulfilled the inclusion criteria. The inclusion criteria for having active MTP in the UT muscle were as follows23, 24:
-
1.
Presence of palpable taut band in muscle.
-
2.
Presence of a hypersensitive tender spot in the taut band.
-
3.
Reproduction of the typical referred pain pattern of the MTP in response to compression. For third criteria (detecting active MTP), MTP pressure tolerance was assessed using a mechanical pressure algometer. The investigator applied continuous pressure with the algometer at a pressure of approximately 2.5 kg/cm2.
-
4.
Spontaneous presence of the typical referred pain pattern or patient recognition of the referred pain as familiar.
-
5.
Pain of at least 30 mm on a numeric pain scale (NPS).24
The selected MTP of the UT muscle was located in the middle of the more nearly horizontal fibers of the UT.19
Patients were also excluded if they had a history of fibromyalgia syndrome, whiplash injury, cervical spine surgery and fracture, cervical radiculopathy, having MTP therapy within the past month before the study, or if they had a diagnosis of any systematic disease such as rheumatism, tuberculosis, cervical myelopathy, or multiple sclerosis.25, 26, 27, 28, 29 The patients, who underwent DN, also had no contraindication for needling such as local infection, pregnancy with threatened abortion, taking anticoagulants (eg, warfarin), or long-term steroid use. After the initial screening, 31 patients fulfilled all inclusion criteria. Before participating in the study, all participants signed an informed consent form approved by the human participants committee at the University of Social Welfare and Rehabilitation Sciences.
Patients were randomly assigned to a standard (IC) group (N = 17, mean age = 26.69 ± 9.4 years) or an experimental (DN) group (N = 14, mean age = 30.78 ± 10.39 years).
The coin toss method was used for randomization. Once a participant, after screening, was registered for trial, a coin was tossed to decide the allocation to group and systematic assignment was used to restrict groups. Power analysis was used to determine the sample size. Physical characteristics of the patients in each group are shown in Table 1.
Table 1.
Demographic Data of Participants (Mean ± SD)
| Variables | IC (n = 17) | DN (n = 14) | P | |
|---|---|---|---|---|
| Age, y | 526.6 ± 9.4 | 30.07 ± 10.39 | .35 | |
| Weight, kg | 55.5 ± 5.27 | 59.8 ± 7.32 | .31 | |
| Height, cm | 163.6 ± 4.93 | 163.9 ± 7.03 | .95 | |
| Affected side | Right | (n = 7) | (n = 6) | |
| Left | (n = 10) | (n = 8) | ||
DN, dry needling; IC, ischemic compression; SD, standard deviation.
Procedure
The procedure for both groups was as follows.
The treatment protocol for the standard group consisted of the IC technique on MTPs in UT muscle. The patients in the experimental group received DN for UT muscle.
Patients in each group received 1-week treatment sessions. Treatment frequency was 3 times per week (48-hour interval between sessions) for each group. The outcome measures were collected before treatment and immediately and 2 days after each treatment session in both groups. The measures after 2 days for each treatment session were the same as the measures of before-treatment session scores for the next treatment session.
Ischemic Compression
In the IC group, the patient was supine or prone with the cervical spine in a neutral position. The therapist manually applied gradually increasing pressure to the MTP until the onset of a sensation of pressure and pain. At that moment, the pressure was maintained until the discomfort or pain was relieved by around 50%, as perceived by the patient. At that time, the pressure was increased until discomfort was felt again. This process was repeated for 90 seconds.24, 25
Dry Needling
The DN for MTP was performed with disposable stainless steel needles (50 × 0.3 mm). The participant was asked to lie in prone position. The overlying skin was cleaned with alcohol. The taut band, localized between the thumb and index finger, was needled forward and backward perpendicularly to the tissue with no rotation to get an LTR. The procedure was repeated until there was no more LTR.17 However, the speed of needle insertion was not controlled.
Outcome Measures
Pain intensity and PPT were measured before treatment and immediately and 2 days after each treatment session in both groups.
Assessment of Pain Intensity
To evaluate pain intensity, a pressure of 25 N was exerted on the MTP using an algometer and patients were asked to show their pain on the NPS after pressure application. The NPS is a sensitive and reproducible instrument commonly used for the assessment of variations in intensity of pain. In clinical practice, the amount of pain relief is often considered as a measure of the efficacy of treatment. The NPS was a 10-cm horizontal line divided into 10 equal parts. To evaluate the pain intensity, the NPS was used simultaneously with the algometer application.24, 25, 30
Assessment of PPT
A pressure threshold algometer (Lutron electronic, FG5005, RS232) was used to measure PPT in the MTP of the UT muscle before and after treatment. At first, the procedure was clearly explained to the patient. The algometer was applied to the MTP area with the metal rod perpendicular to the skin surface. The patient was asked to say “pain” as soon as any increase in pain intensity or discomfort occurred. The compression was stopped when the participant reported “pain.” The average value of the 3 repetitive measurements with an interval of 30 to 60 seconds (expressed as kilograms per square centimeter) was taken for data analysis of the PPT.19, 26
Data Analysis
Kolmogrov-Smirnov test was used to assess the normality of distribution for tested variables before and after treatment. Normal distribution was observed for tested variables in both groups. Repeated measures analysis of variance was used to determine any significant change in the tested variables (NPS, PPT) immediately and 2 days after each treatment session in standard and experimental group. A P value < .05 was considered statistically significant. To measure the magnitude of the treatment effect, Cohen effect size was calculated. Power of test analysis and sample size estimation was performed by PS software (PS Power and Sample Size Calculations, Version 3.0). The power of the study was considered to be 80%.
Results
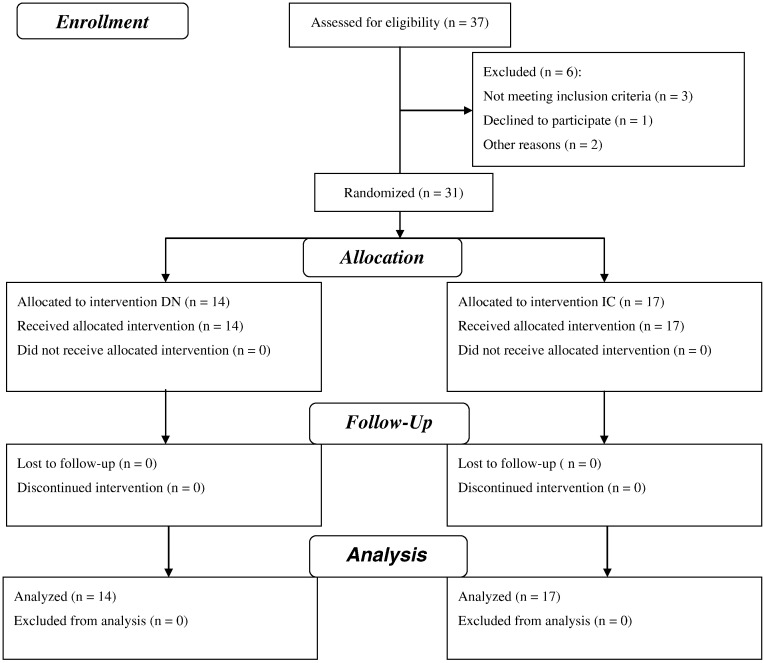
The participant flow diagram provided in Figure 1 reports the number of participants who were registered and those who received allocated intervention, assignment, and measurements for each group.
Fig 1.
Flow diagram.
Demographic data (mean ± standard deviation) for the participants in both groups are presented in Table 1. No statistically significant differences were found between the 2 groups in age, weight, and height.
Table 2 presents the descriptive statistics (mean ± standard deviation) for the measures (NPS, PPT) obtained before and immediately after each treatment session in both groups.
Table 2.
Descriptive Statistics (Mean ± SD) for the Measures (NPS, PPT) Before and Immediately After Each Treatment Session in Both Groups
| Variable | Group | First Session |
Second Session |
Third Session |
Fourth Session | |||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||
| NPS | DN | 7.96 ± 1.52 | 7.85 ± 2.24 | 6.42 ± 1.98 | 7.5 ± 1.95 | 5.2 ± 2.45 | 6.07 ± 2.01 | 4.78 ± 2.4 |
| IC | 8.26 ± 1.71 | 7.55 ± 2.17 | 7.35 ± 1.94 | 6.35 ± 2.05 | 5.91 ± 2.3 | 5.17 ± 2.65 | 4.88 ± 2.34 | |
| PPT | DN | 10.61 ± 4.01 | 10.46 ± 4.88 | 13.07 ± 4.88 | 11.47 ± 3.7 | 13.94 ± 4.89 | 12.87 ± 3.7 | 16.43 ± 4.66 |
| IC | 10.87 ± 3.9 | 12.63 ± 4.36 | 12.43 ± 4.25 | 13.97 ± 4.60 | 13.46 ± 4.77 | 15.55 ± 5.33 | 14.5 ± 4.44 | |
DN, dry needling; IC, ischemic compression; NPS, numeric pain scale; PPT, pressure pain threshold (higher is better); SD, standard deviation.
Table 3 shows the result of repeated measures analysis of variance to compare the change in tested variables immediately and 2 days after each treatment session in both standard and experimental groups. The effect size to assess clinical effectiveness of each method has been reported as well. The results indicate that the effect size of the DN methods for NPS and PPT was considerably greater after 2 days compared with immediately after treatment session. However, there was no noticeable difference in the effect size of IC for NPS between the scores obtained immediately and 2 days after treatment. More interestingly, the effect of the IC method for PPT was greater immediately after treatment compared with the measures after 2 days.
Table 3.
Pairwise Comparison of the Change in Tested Variables Immediately and 2 Days After Each Treatment Session in Both Groups
| Variable | Group | Session | Session | Mean Difference | Effect Size | P | 95% Confidence Interval for Difference |
|
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| NPS | DN | 1 | 2 | 0.11 | 0.057 | .76 | –0.64 | 0.86 |
| 1 | 3 | 1.54 | 0.87 | .000 | 0.54 | 2.52 | ||
| 3 | 4 | –1.07 | –0.54 | .01 | –1.83 | –0.30 | ||
| 3 | 5 | 1.21 | 0.54 | .000 | 0.36 | 2.06 | ||
| 5 | 6 | –0.85 | –0.38 | .11 | –1.96 | 0.24 | ||
| 5 | 7 | 0.42 | 0.17 | .39 | –0.62 | 1.48 | ||
| IC | 1 | 2 | 0.706 | 0.37 | .000 | 0.20 | 1.20 | |
| 1 | 3 | 0.91 | 0.49 | .000 | 0.28 | 1.5 | ||
| 3 | 4 | 1.00 | 0.50 | .000 | 0.44 | 1.55 | ||
| 3 | 5 | 1.44 | 0.67 | .000 | 0.62 | 2.25 | ||
| 5 | 6 | 0.73 | 0.29 | .01 | 0.17 | 1.29 | ||
| 5 | 7 | 1.02 | 0.44 | .01 | 0.22 | 1.18 | ||
| PPT | DN | 1 | 2 | 0.14 | –0.03 | .87 | –1.75 | 2.03 |
| 1 | 3 | –2.46 | 0.55 | .000 | –3.72 | –1.19 | ||
| 3 | 4 | 1.6 | –0.36 | .13 | –0.59 | 3.80 | ||
| 3 | 5 | –0.86 | 0.17 | .44 | –3.25 | 1.52 | ||
| 5 | 6 | 1.07 | –0.24 | .38 | –1.51 | 3.65 | ||
| 5 | 7 | –2.49 | 0.52 | .04 | –4.97 | –0.11 | ||
| IC | 1 | 2 | –1.75 | 0.42 | .000 | –2.53 | –0.98 | |
| 1 | 3 | –1.55 | 0.38 | .04 | –3.05 | –0.04 | ||
| 3 | 4 | –1.54 | 0.34 | .09 | –3.35 | 0.27 | ||
| 3 | 5 | –1.02 | 0.22 | .18 | –2.61 | 0.55 | ||
| 5 | 6 | –2.09 | 0.41 | .000 | –3.46 | –0.72 | ||
| 5 | 7 | –1.04 | 0.22 | .04 | –2.031 | –0.056 | ||
1, before treatment; 2, immediately after treatment session; 3, 2 days after first treatment/before second treatment session; 4, immediately after second treatment session; 5, 2 days after second treatment/before third treatment session; 6, immediately after third treatment; 7, 2 days after third treatment session; DN, dry needling; IC, ischemic compression; PPT, pressure pain threshold (higher is better).
Our data also revealed a greater effect size for DN on PPT after 2 days compared with the IC technique. No similar trend was found for NPS.
Discussion
The results of this study indicate that the effect size of the DN methods for NPS and PPT was considerably greater after 2 days compared with immediately after treatment session. However, there was no noticeable difference in the effect size of IC for NPS between the scores obtained immediately and 2 days after treatment.
The results of this study indicate a significant change in pain intensity 2 days after treatment compared with pretreatment scores in both groups and a significant change in PPT 2 days after treatment only in DN.
Review of the published reports indicated that with the use of different designs and testing procedures, several studies have assessed the effect of DN on MTPs.16, 17, 18, 19, 20, 21
It has been suggested that DN may influence the microcirculation. Several investigators have reported that needle insertion in the muscles increased both skin and muscle blood flow in the stimulated region.14, 15 A change in inflammatory mediators has been reported after DN of the UT muscle, which suggested increasing local blood flow to the MTP region.14, 15 It is thought that DN provides a mechanical localized stretch to the shortened sarcomeres and contracted cytoskeletal structures within the MTP.8, 10, 11 It may also stimulate the Aδ nerve fibers and activate the enkephalinergic inhibitory dorsal horn interneurons. Some studies have reported changes in the chemical properties at the MTP combined with eliciting an LTR after DN.7, 8
Our data also revealed significant difference in the NPS and PPT immediately and 2 days after IC. Several studies have indicated immediate and short-term effect of IC on pain intensity in participants with MTPs.25, 30, 31 Investigators attributed the effect of IC to vasodilation and change in blood flow and cellular metabolism at the site of the MTP.31
The effect size of IC on NPS and PPT was greater than DN immediately after treatment. However, the effect size of DN on NPS and PPT was greater than IC 2 days after treatment. This might be due to the muscle soreness. After DN, the muscle soreness generally may be felt for a few hours up to 24 to 48 hours.20 Considering this soreness after DN, the results collected immediately after treatment technique can be criticized and should be interpreted with caution. One explanation for the lack of effect of DN immediately after treatment could be the fact that in all patients, needling of local MTPs might provoke a short contraction of muscle fibers, which is known as an LTR of muscle bands and causes a typical soreness in the treated region for some hours. This soreness can overlie the original pain and might have influenced patients’ ratings immediately after treatment.20
Our data also revealed a greater effect size for DN on PPT after 2 days compared with IC. However, changes in NPS and PPT after 2 days were not statistically different between the 2 groups.
Study Limitations
One of the limitations of this study was sample size. We suggest that this study could be done on a larger sample size to provide more insight regarding the effect of DN on MTPs. Another area of concern in our study was the fact that only women participated in the study.
Conclusions
According to the present study, the application of DN seemed to produce an improvement in the pain intensity and PPT after 2 days. However, it did not improve pain intensity and PPT immediately after application because of muscle soreness. Thus, assessment of the effect of DN immediately after application can be criticized, and the results should be interpreted with caution.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): M.R.N., A.M.A.
Design (planned the methods to generate the results): A.M.A., M.Z., M.R.N.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): A.M.A., M.Z., M.R.N.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): A.M.A., M.Z.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): A.M.A., M.Z., M.R.N.
Literature search (performed the literature search): A.M.A., M.Z.
Writing (responsible for writing a substantive part of the manuscript): A.M.A., M.Z.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): A.M.A., M.Z.
Practical Applications
-
•
Dry needling improved pain and PPT after 2 days.
-
•
Dry needling had no immediate effect on pain and PPT.
-
•
Immediate effect of DN might be criticized because of muscle soreness.
References
- 1.Rickards LD. The effectiveness of non-invasive treatments for active myofascial trigger point pain: a systematic review of the literature. Int J Osteopath Med. 2006;9(4):120–136. [Google Scholar]
- 2.Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome—evidence from a review of the literature. Clin J Pain. 2007;23(3):278–286. doi: 10.1097/AJP.0b013e31802fda7c. [DOI] [PubMed] [Google Scholar]
- 3.Yap EC. Myofascial pain—an overview. Ann Acad Med Singapore. 2007;36(1):43–48. [PubMed] [Google Scholar]
- 4.Hanten WP, Olson SL, Butts NL, Nowicki AL. Effectiveness of a home program of ischemic pressure followed by sustained stretch for treatment of myofascial trigger points. Phys Ther. 2000;80(10):997–1003. [PubMed] [Google Scholar]
- 5.Borg-Stein J, Simons DG. Focused review: myofascial pain. Arch Phys Med Rehabil. 2002;83(3 Suppl. 1):S40–S47. doi: 10.1053/apmr.2002.32155. S48-S49. [DOI] [PubMed] [Google Scholar]
- 6.Shah JP, Danoff JV, Desai MJ. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Shah JP. Integrating dry needling with new concepts of myofascial pain, muscle physiology, and sensitization. In: Audette J.F., Bailey A., editors. Integrative Pain Medicine. Humana Press; Totowa, NJ: 2008. pp. 107–121. [Google Scholar]
- 8.Dommerholt J. Dry needling in orthopedic physical therapy practice. Orthop Pract. 2004;16(3):15–20. [Google Scholar]
- 9.Fernandez-de-las-Penas C, Alonso-Blanco C, Miangolarra JC. Myofascial trigger points in participants presenting with mechanical neck pain: a blinded, controlled study. Man Ther. 2007;12(1):29–33. doi: 10.1016/j.math.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Rickards LD. Therapeutic needling in osteopathic practice: an evidence-informed perspective. Int J Osteopath Med. 2009;12(1):2–13. [Google Scholar]
- 11.Dommerholt J, del Moral OM, Gröbli C. Trigger point dry needling. In: Dommerhold J., Huijbregts P., editors. Myofascial Trigger Points: Pathophysiology and Evidence-Informed Diagnosis and Management. Jones and Bartlett Publishers; Sudbary, MA: 2010. pp. 159–190. [Google Scholar]
- 12.Chaitow L, Fritz S, King RK, Chambers G. Churchill Livingstone Elsevier; London: 2006. A Massage Therapist’s Guide to Understanding, Locating and Treating Myofascial Trigger Points. [Google Scholar]
- 13.de las Peñas CF, Sohrbeck Campo M, Fernández Carnero J, Miangolarra Page JC. Manual therapies in myofascial trigger point treatment: a systematic review. J Bodyw Mov Ther. 2005;9(1):27–34. [Google Scholar]
- 14.Cagnie B, Barbe T, De Ridder E, Van Oosterwijck J, Cools A, Danneels L. The influence of dry needling of the trapezius muscle on muscle blood flow and oxygenation. J Manipulative Physiol Ther. 2012;35(9):685–691. doi: 10.1016/j.jmpt.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12(4):371–384. doi: 10.1016/j.jbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Ziaeifar M, Arab AM, Karimi N, Nourbakhsh MR. The effect of dry needling on pain, pressure pain threshold and disability in patients with a myofascial trigger point in the upper trapezius muscle. J Bodyw Mov Ther. 2014;18(2):298–305. doi: 10.1016/j.jbmt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Ga H, Choi JH, Park CH, Yoon HJ. Dry needling of trigger points with and without paraspinal needling in myofascial pain syndromes in elderly patients. J Altern Complement Med. 2007;13(6):617–624. doi: 10.1089/acm.2006.6371. [DOI] [PubMed] [Google Scholar]
- 18.Ilbuldu E, Cakmak A, Disci R, Aydin R. Comparison of laser, dry needling, and placebo laser treatments in myofascial pain syndrome. Photomed Laser Surg. 2004;22(4):306–311. doi: 10.1089/pho.2004.22.306. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CT, Hsieh LF, Kuan TS, Kao MJ, Chou LW, Hong CZ. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil. 2010;89(2):133–140. doi: 10.1097/PHM.0b013e3181a5b1bc. [DOI] [PubMed] [Google Scholar]
- 20.Irnich D, Behrens N, Gleditsch JM. Immediate effects of dry needling and acupuncture at distant points in chronic neck pain: results of a randomized, double-blind, sham-controlled crossover trial. Pain. 2002;99(1-2):83–89. doi: 10.1016/s0304-3959(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Edwards J, Knowles N. Superficial dry needling and active stretching in the treatment of myofascial pain—a randomised controlled trial. Acupunct Med. 2003;21(3):80–86. doi: 10.1136/aim.21.3.80. [DOI] [PubMed] [Google Scholar]
- 22.Leon-Hernandez JV, Martin-Pintado-Zugasti A, Frutos LG, Alguacil-Diego IM, de la Llave-Rincon AI, Fernandez-Carnero J. Immediate and short-term effects of the combination of dry needling and percutaneous TENS on post-needling soreness in patients with chronic myofascial neck pain [e-pub ahead of print]. Braz J Phys Ther. doi: 10.1590/bjpt-rbf.2014.0176. Accessed July 11, 2016. [DOI] [PMC free article] [PubMed]
- 23.Aguilera FJ, Martin DP, Masanet RA, Botella AC, Soler LB, Morell FB. Immediate effect of ultrasound and ischemic compression techniques for the treatment of trapezius latent myofascial trigger points in healthy subjects: a randomized controlled study. J Manipulative Physiol Ther. 2009;32(7):515–520. doi: 10.1016/j.jmpt.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Gemmell H, Miller P, Nordstrom H. Immediate effect of ischaemic compression and trigger point pressure release on neck pain and upper trapezius trigger points: a randomised controlled trial. Clin Chiropract. 2008;11(1):30–36. [Google Scholar]
- 25.Fernandez-de-las-Penas C, Alonso-Blanco C, Fernandez-Carnero J, Carlos Miangolarra-Page J. The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study. J Bodyw Mov Ther. 2006;10(1):3–9. [Google Scholar]
- 26.Hsieh YL, Kao MJ, Kuan TS, Chen SM, Chen JT, Hong CZ. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil. 2007;86(5):397–403. doi: 10.1097/PHM.0b013e31804a554d. [DOI] [PubMed] [Google Scholar]
- 27.Blikstad A, Gemmell H. Immediate effect of activator trigger point therapy and myofascial band therapy on non-specific neck pain in patients with upper trapezius trigger points compared to sham ultrasound: a randomised controlled trial. Clin Chiropract. 2008;11(1):23–29. [Google Scholar]
- 28.Ruiz-Saez M, Fernandez-de-las-Penas C, Blanco CR, Martinez-Segura R, Garcia-Leon R. Changes in pressure pain sensitivity in latent myofascial trigger points in the upper trapezius muscle after a cervical spine manipulation in pain-free subjects. J Manipulative Physiol Ther. 2007;30(8):578–583. doi: 10.1016/j.jmpt.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch Phys Med Rehabil. 2002;83(10):1406–1414. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 30.Sarrafzadeh J, Ahmadi A, Yassin M. The effects of pressure release, phonophoresis of hydrocortisone, and ultrasound on upper trapezius latent myofascial trigger point. Arch Phys Med Rehabil. 2012;93(1):72–77. doi: 10.1016/j.apmr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Moraska AF, Hickner RC, Kohrt WM, Brewer A. Changes in blood flow and cellular metabolism at a myofascial trigger point with trigger point release (ischemic compression): a proof-of-principle pilot study. Arch Phys Med Rehabil. 2013;94(1):196–200. doi: 10.1016/j.apmr.2012.08.216. [DOI] [PMC free article] [PubMed] [Google Scholar]