Graphical abstract
Keywords: Acaricidal effect, Cattle ticks, Essential oil, Natural product, Control, Boophilus microplus
Abstract
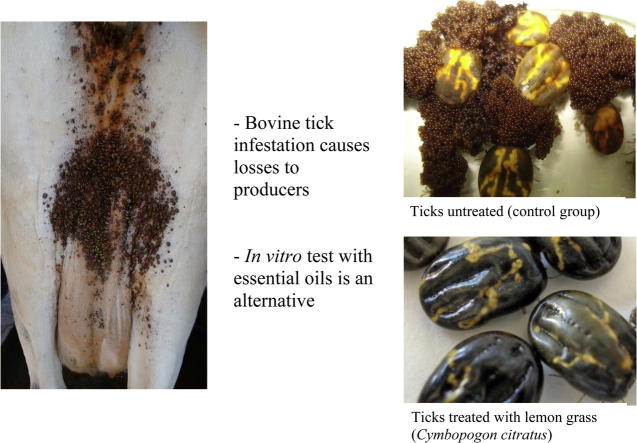
The acaricidal effect of seven essential oils was examined in vitro against the cattle tick (Rhipicephalus microplus). Engorged female ticks were manually collected in farms of Southern Brazil and placed into petri dishes (n = 10) in order to test the following oils: juniper (Juniperus communis), palmarosa (Cymbopogon martinii), cedar (Cedrus atlantica), lemon grass (Cymbopogon citratus), ginger (Zingiber officinale), geranium (Pelargonium graveolens) and bergamot (Citrus aurantium var bergamia) at concentrations of 1%, 5%, and 10% each. A control group was used to validate the tests containing Triton X-100 only. Treatment effectiveness was measured considering inhibition of tick oviposition (partial or total), egg’s weight, and hatchability. C. martinii, C. citratus and C. atlantica essential oils showed efficacy higher than 99% at all concentrations tested. In addition, J. communis, Z. officinale, P. graveolens, and C. aurantium var bergamia oils showed efficiency ranging from 73% to 95%, depending on the concentration tested, where higher concentrations showed greater efficacy. It was concluded that essential oils can affect tick reproduction in vitro by inhibiting oviposition and hatchability.
Introduction
The cattle tick Rhipicephalus microplus stands out as the most harmful pest for cattle, causing animal stress, lower growth, and poor performance, in addition to higher production costs due to constant anti-parasitic treatments [1], [2]. The economic impact caused by cattle ticks in Brazil is of approximately $3.24 billion dollars a year [1] since climatic conditions are favorable to their survival and development [3], increasing control costs with synthetic acaricides [4]. Moreover, restrictions on the use of insecticides and acaricides, such as organophosphates due to their effects on human and animal health [5], and the environment [6] have enhanced the development of effective alternatives for control, including essential oils.
The essential oils used in this study have exhibited several biological activities as previously described in the literature. Essential oils from Cymbopogon citratus (Poaceae family), Cymbopogon martinii (Gramineae family) and Juniperus communis (Cupressaceae family) have showed antioxidant [7], antimicrobial, antifungal and anthelmintic properties [8], [9]. Cedrus atlantica (Pinaceae family) is the plant with fewer studies, even though its analgesic property has been described [10]. In vitro, Zingiber officinale (Zingiberaceae family) extract was able to reduce Streptococcus mutans and Streptococcus sanguinis growth with minimum inhibitory concentration of 0.02 mg/mL and 0.3 mg/mL, respectively [11]. The Pelargonium graveolens essential oil has been used due to its hypoglycemic and antioxidant [12] properties, and exhibits also antifungal and insecticidal activities against Rhizoctonia solani and Rhysopertha dominica, respectively [13]. The use of Citrus aurantium essential oil by Homa et al. [14] revealed the antifungal activity against different isolates of Fusarium keratitis, antibacterial activity against Vibrio species [15], as well as insecticidal activity against Aedes aegypti and Anopheles dirus [16]. As mentioned above, there are many properties of these essential oils, but there are only few studies on their acaricidal effects despite the great interest on finding alternative control methods. Therefore, this study aimed to evaluate the in vitro effect of essential oils (C. martinii, C. citratus, C. atlantica, J. communis, Z. officinale, P. graveolens, and Citrus aurantium var bergamia) on cattle tick R. microplus.
Material and methods
Essential oils
Seven essential oils were used to test the reproduction of engorged R. microplus females. The oils used were as follows: juniper (J. communis), palmarosa (C. martinii), cedar (C. atlantica), lemon grass (C. citratus), ginger (Z. officinale), geranium (P. graveolens), and bergamot (C. aurantium var bergamia). Three concentrations (1%, 5%, and 10%, i.e. 1v/99v, 5v/95v, and 10v/90v, respectively) were evaluated and Triton X-100 (Sigma Aldrich®, São Paulo, Brazil) was used as surfactant (1v/v), in addition to distilled water [17]. The essential oils of juniper, palmarosa, and lemon grass were acquired from BioEssencia® (São Paulo, Brazil), while essential oils of cedar, ginger, geranium, and bergamot were acquired from Phytoterápica® (São Paulo, Brazil).
Gas chromatography-flame ionization detector (GC-FID) of essential oils
The gas chromatography (GC) analyses were carried out using an 6890N GC-FID system equipped with DB-5 capillary column (30 m × 0.25 mm; film thickness of 0.25 mm) (Agilent Technologies, Santa Clara, United States) connected to an FID detector. The injector and detector temperatures were set at 280 °C at a rate of 5 °C/min. The carrier gas was helium (>99.2% purity) at a flow rate of 1.3 mL/min. All samples were analyzed in duplicate. Relative component concentrations were calculated based on GC peak areas without using correction factors [18].
Gas chromatography–mass spectrometry (GC–MS)
GC–MS analyses were performed on Agilent Technologies AutoSystem XL GC–MS operated in the EI mode at 70 eV (Hewlett Packard, Palo Alto, CA, USA) equipped with a splitless injector (250 °C). The transfer line temperature was 280 °C. Helium was used as the carrier gas (1.3 mL/min) and the capillary columns were DB-5 and HP5 MS (30 m × 0.25 mm; film thickness of 0.25 mm). Column temperature was programmed on 40–220 °C at 3 °C/min. The oils were diluted in hexane (1:5, v/v) and 1 μL was injected.
Identification of the constituents was performed on the basis of retention index (RI) on DB-5 capillary column, determined in relation to homologous series of n-alkanes (C7–C30) with those reported in the literature. Fragmentation patterns in the mass spectra library search (NIST and Wiley) were compared with those stored on databases [19]. The quantification of the compounds was performed on the basis of their relative peak areas on DB-5.
Ticks
The ticks were collected from dairy cows naturally infested in farms located in Quilombo city, Santa Catarina State, Southern Brazil. These animals did not receive any acaricidal treatment in the last 50 days prior to the beginning of the study in order to avoid any negative interference. The engorged female ticks were stored in plastic bottles, packed in a cooler (±15 °C), and immediately transported to the laboratory where the bioassays were conducted.
Bioassays
In the laboratory, engorged females ticks with similar weights were randomly distributed, placed into covered petri dishes during the incubation period. The experimental design was completely randomized with three replicates per oil concentration, and 10 ticks for each petri dish (total of 30 ticks per oil tested). The tests were performed according to the methodology described by Drummond et al. [20], where ticks were immersed for five minutes in the test solutions with essential oils at concentrations of 1%, 5%, and 10%. After that, they were dried and incubated under controlled conditions (25 °C; 75% relative humidity (RH)) for 14 days. Subsequently, oviposition was recorded as total, partial or absent and their eggs were weighted. Laid eggs were placed into glass tubes and incubated for 30 days in order to verify hatchability, which was measured considering the number of remaining eggs that did not hatch and the number of shells, versus the number of larvae (active or inactive) [21].
A control group containing only the diluents (water + Triton X-100) at concentration of 10% of Triton was used. The results were tabulated and reproductive efficiency (RE) and effectiveness of the treatment (ET) were calculated as described by Drummond et al. [20] [RE = egg weight × % of hatchability × 20,000/weight of engorged female ticks; ET = (RE control − RE treatment) × 100/RE control].
Statistical analysis
The data collected were subjected to normality test which showed normal distribution. Then, the data were analyzed statistically by analysis of variance (one-way ANOVA) and Duncan’s test. The results were considered significant when P < 0.05.
Results
In vitro test
The number of ticks that had partial or total oviposition, as well as egg weight, and percentage of hatched larvae is shown in Table 1. All results were compared to the control group that showed total oviposition and 86.3% of hatchability. The use of J. communis oil caused partial oviposition of smaller eggs (P = 0.0032) in all concentrations tested, even though it was unable to inhibit hatchability. On the contrary, the use of C. martinii oil (1%) led to lower egg hatchability (P = 0.0012), in addition to lower oviposition and egg weight, on a dose-dependent effect. C. atlantica, C. citratus, Z. officinale, and P. graveolens essential oils tested at 1% were unable to reduce the number of ticks that showed oviposition, i.e. these oils did not cause any effect on reproduction (P = 0.142), which was not observed at concentrations of 5 and 10% (P = 0.092). C. atlantica and C. citratus oils were able to inhibit hatchability, an effect not seen for Z. officinale and P. graveolens oils. C. aurantium var bergamia oil was able to reduce the number of ticks that performed oviposition and the weight of eggs at all concentrations, but did not inhibit hatchability.
Table 1.
Mean and standard deviation of the weight of engorged tick, number of postures by treatment, egg weight, and hatchability after treatment with essential oils of juniper (J. communis), palmarosa (C. martinii), cedar (C. atlantica), lemon grass (C. citratus), ginger (Z. officinale), geranium (P. graveolens) and bergamot (C. aurantium bergamia).
| Treatment | Engorged tick weight (g) | Number posture by treatment⁎ (n = 10) | Weighing eggs per treatment (g) | Hatchability (%) |
|---|---|---|---|---|
| Control | 0.190 ± 0.016 | 10.0a ± 0.0 | 0.96a ± 0.03 | 90 |
| Juniper 1% | 0.198 ± 0.021 | 8.0b ± 1.1 | 0.35c ± 0.01 | 38 |
| Juniper 5% | 0.201 ± 0.011 | 7.0bc ± 1.5 | 0.28d ± 0.03 | 10 |
| Juniper 10% | 0.187 ± 0.018 | 7.0bc ± 0.2 | 0.25de ± 0.02 | 08 |
| Palmarosa 1% | 0.177 ± 0.019 | 5.0d ± 1.1 | 0.14ef ± 0.01 | 03 |
| Palmarosa 5% | 0.203 ± 0.013 | 2.0e ± 1.0 | 0.06g ± 0.01 | 00 |
| Palmarosa 10% | 0.192 ± 0.022 | 0.3f ± 0.5 | 0.06g ± 0.01 | 00 |
| Cedar 1% | 0.196 ± 0.016 | 8.7ab ± 1.1 | 0.51b ± 0.04 | 00 |
| Cedar 5% | 0.184 ± 0.020 | 6.6bc ± 0.5 | 0.35c ± 0.05 | 00 |
| Cedar 10% | 0.188 ± 0.012 | 4.6d ± 1.1 | 0.06g ± 0.01 | 00 |
| Lemon grass 1% | 0.204 ± 0.018 | 8.6ab ± 1.1 | 0.27d ± 0.02 | 00 |
| Lemon grass 5% | 0.179 ± 0.015 | 5.6cd ± 1.5 | 0.27d ± 0.03 | 00 |
| Lemon grass 10% | 0.192 ± 0.017 | 4.3d ± 1.2 | 0.12f ± 0.01 | 00 |
| Ginger 1% | 0.185 ± 0.014 | 8.6ab ± 1.5 | 0.42bc ± 0.06 | 15 |
| Ginger 5% | 0.194 ± 0.016 | 7.0bc ± 1.7 | 0.13f ± 0.04 | 06 |
| Ginger 10% | 0.205 ± 0.019 | 4.3d ± 0.6 | 0.20e ± 0.01 | 05 |
| Geranium 1% | 0.191 ± 0.013 | 9.0ab ± 1.0 | 0.42bc ± 0.04 | 13 |
| Geranium 5% | 0.188 ± 0.017 | 6.3cd ± 2.0 | 0.16e ± 0.02 | 09 |
| Geranium 10% | 0.199 ± 0.015 | 5.3d ± 1.2 | 0.09fg ± 0.01 | 05 |
| Bergamot 1% | 0.178 ± 0.010 | 7.3bcd ± 1.1 | 0.36c ± 0.05 | 20 |
| Bergamot 5% | 0.197 ± 0.014 | 6.3cd ± 0.6 | 0.26d ± 0.03 | 11 |
| Bergamot 10% | 0.180 ± 0.013 | 6.3cd ± 1.5 | 0.29cd ± 0.04 | 08 |
Note: Means followed by the same letter in the same column do not differ statistically among themselves, the significance level of 5% (P > 0.05).
Number of engorged females (ticks) that perform posture (partial or total) per treatment, and “n” by repeating 10 specimens (test performed in triplicate).
Data on tick reproductive efficiency and oil treatment efficacy are shown in Table 2. Oil treatment was able to significantly reduce tick reproductive efficiency compared to the control group (P = 0.0001). Regarding C. atlantica and C. citratus oils, all concentrations tested interfered with the reproduction of cattle ticks (100% efficacy) similar to C. martinii oil at 5% and 10%. The C. aurantium var bergamia, Z. officinale, J. communis, and P. graveolens oils at concentration 10%, exhibited an approximate efficiency of 90%, 94%, 96%, and 97%, respectively.
Table 2.
Reproductive efficiency and effectiveness of treatment of seven essential oils against cattle tick Rhipicephalus microplus.
| Treatment | Reproductive efficiency (%) | Treatment efficacy (%) |
|---|---|---|
| Control | 81.0 | 0.0 |
| Juniper 1% | 23.3 | 73.8 |
| Juniper 5% | 4.2 | 95.2 |
| Juniper 10% | 3.6 | 96.3 |
| Palmarosa 1% | 1.8 | 99.7 |
| Palmarosa 5% | 0.0 | 100.0 |
| Palmarosa 10% | 0.0 | 100.0 |
| Cedar 1% | 0.0 | 100.0 |
| Cedar 5% | 0.0 | 100.0 |
| Cedar 10% | 0.0 | 100.0 |
| Lemon grass 1% | 0.0 | 100.0 |
| Lemon grass 5% | 0.0 | 100.0 |
| Lemon grass 10% | 0.0 | 100.0 |
| Ginger 1% | 5.7 | 85.7 |
| Ginger 5% | 2.9 | 92.6 |
| Ginger 10% | 2.5 | 94.0 |
| Geranium 1% | 5.6 | 85.9 |
| Geranium 5% | 3.3 | 91.6 |
| Geranium 10% | 1.2 | 97.0 |
| Bergamot 1% | 5.1 | 84.9 |
| Bergamot 5% | 5.3 | 86.6 |
| Bergamot 10% | 3.7 | 90.5 |
Oil composition
The major components found in each oil were as follows: linalool (J. communis; 18.07%), geraniol (C. martini; 35.27%), α-himachalene (C. atlantica; 19.74%), geranial (C. citratus; 46.51%), α-zingiberene (Z. officinale; 26.47%), citronellol (P. graveolens; 31.37%), and limonene (C. aurantium var bergamia; 30.17%) (Suppl. Table 1).
Discussion
In this study, it was observed that the J. communis oil was able to partially inhibit oviposition, and therefore, reduce tick reproductive efficiency. Carrol et al. [22] reported repellent action of juniper oil against two species of ticks (Amblyomma americanum and Ixodes scapularis). Studies conducted by Dietrich et al. [23] and Dolan et al. [24] have reported that the J. communis oil is a rich source of anti-tick compounds with well-known repellent and insecticidal activities. Researchers also found 43.2% of repellent effect for juniper oil against A. aegypti after 210 min of application [25].
Additionally, C. citratus oil showed 100% efficacy against R. microplus, similar to those findings reported by other authors [26], [27]. The effectiveness of C. citratus oil on ticks, according to Tchoumbougnang et al. [28] may be due to its geraniol content, measured as 47%. C. martinii oil at 5% and 10% showed 100% efficacy against adult ticks in this current study, and this oil has been studied for its repellent activity to insects [29], [30] and antifungal actions [31], but it had not been tested on cattle ticks yet.
Z. Officinale belongs to Zingiberceae family, an aromatic plant used as spice and in medicine. According to the literature, the Z. officinale oil showed bactericidal effect on Staphylococcus aureus [32], repellent activity against mosquitoes of the species Culex quinquefasciatus [33], as well as repellent effect against Leptotrombidium deliense larvae, a species of mite [34], similar to the cattle tick used in this study.
The C. aurantium var bergamia oil negatively affected the reproduction of cattle tick. According to the literature, some compounds present in Citrus sp. essential oils showed repellant effect against mosquitoes and ticks [35]. Already, the Cedrus deodara oil demonstrated strong effect against cattle ticks [36], similar to what was observed in this study, even though a different kind of C. atlantica was used in this current study. Another study also reported efficacy to control cattle tick using a herbal preparation containing extracts of C. deodara, Azadirachta indica, and Embelia ribes [37], and according to these authors, these extracts have acaricidal effect against larvae, nymphs, and adult stages of ticks.
The P. graveolens oil showed some effect on tick oviposition (inhibited or reduced), but it did not interfere on hatchability. Tabanca et al. [38] tested ten essential oils of P. graveolens and demonstrated repellent activities against nymphs of the medically important lone star tick, A. americanum. Researchers described that P. graveolens oil showed 100% repellency against host-seeking nymphs of Ixodes ricinus [39].
Conclusions
Based on these in vitro results it is possible to conclude that C. martinii, C. citratus, and C. atlantica oils may interfere on cattle tick reproduction. The essential oils of J. communis, Z. officinale, P. graveolens, and C. aurantium var bergamia also caused a negative effect on tick reproduction, but they were unable to inhibit hatchability. The use of essential oils in the control of R. microplus shows great potential for the future as an alternative method besides chemical products. Note that more tests, especially in vivo, are needed, in order to conclude whether such oils could be used as an alternative for the control of cattle ticks, and this is the main perspective of our research group.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgment
The authors thank Professor Athayde M.L. (in memoriam) for his technical assistance.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jare.2016.05.003.
Appendix A. Supplementary material
References
- 1.Grisi L., Leite R.C., Martins J.R.S., Barros A.T.M., Andreotti R., Cançado P.H.D. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet. 2014;23(2):150–156. doi: 10.1590/s1984-29612014042. [DOI] [PubMed] [Google Scholar]
- 2.Monteiro C.M.D., Prata M.C.D., Furlong J., Faza A.P., Mendes A.S., Andalo V. Heterorhabditis amazonensis (Rhabditidae: Heterorhabditidae), strain RSC-5, for biological control of the catle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Parasitol Res. 2010;106:821–826. doi: 10.1007/s00436-010-1720-6. [DOI] [PubMed] [Google Scholar]
- 3.Rocha CMBM. Caracterização da percepção dos produtores de leite do município de Divinolópis/MG sobre a importância do carrapato Boophilus microplus e fatores determinantes das formas de combate utilizadas. 1995. 205f. Dissertação (Mestrado em Medicina veterinária preventiva e Epidemiologia) – Curso de pós-graduação em Medicina Veterinária, Universidade Federal de Minas Gerais; 1995.
- 4.Ellse L., Burden F., Wall R. Pyrethroid tolerance in the chewing louse Bovicola (Werneckiella) ocellatus. Vet Parasitol. 2012;188:134–139. doi: 10.1016/j.vetpar.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Kolaczinski J.H., Curtis C.F. Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem Toxicol. 2004;4:697–706. doi: 10.1016/j.fct.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Ramwell C.T., Sinclair C.J., Van Beinum G.W., Bryning G. Management of the environmental inputs and risks of cypermethrin based sheep dips. Central Sci Lab Rep. 2009;1:35–43. [Google Scholar]
- 7.Andrade B.F.M.T., Braga C.P., dos Santos K.C., Barbosa L.N., Rall V.L.M., Sforcin J.M. Effect of inhaling Cymbopogon martinii essential oil and geranial on serum biochemistry parameters and oxidative stress. Biochem Res Int. 2014;2014:493183. doi: 10.1155/2014/493183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katiti L.M., Chagas A.C., Bizzo H.R., Ferreira A.J., Amarante A.F. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Vet Parasitol. 2011;183:103–108. doi: 10.1016/j.vetpar.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Gemeda N., Woldeamanuel Y., Asrat D., Debella A. Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: a potential source of botanical food preservative. Asian Pac J Trop Biomed. 2014;4:S373–S381. doi: 10.12980/APJTB.4.2014C857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins D.F., Emer A.A., Paula Batisti A., Donatello N., Mazzardo-Martins L., Venzke D. Inhalation of Cedrus atlantica essential oil Alleviates pain bevavior through activation of descending pain modulation Pathways in a mouse model of postoperative pain. J Ethnopharmacol. 2015;175:30–38. doi: 10.1016/j.jep.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 11.Azizi A., Aghayan S., Zaker S., Shakeri M., Entezari N., Lawaf S. In vitro effect of Zingiber officinale extract on growth of Streptococcus mutans and Streptococcus sanguinis. Int J Dent. 2015;2015:489842. doi: 10.1155/2015/489842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boukhris M., Bouaziz M., Feki I., Jemai H., El Feki.A., Sayadi S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induce diabetic rats. Lipids Health Dis. 2012;11:81. doi: 10.1186/1476-511X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouzenna H., Krichen L. Pelargonium graveolens L’Hér. and Artemisia arborescens L. essential oils: chemical composition, antifungal activity against Rhizoctonia solani and insecticidal activity against Rhysopertha dominica. Nat Prod Res. 2013;27:841–846. doi: 10.1080/14786419.2012.711325. [DOI] [PubMed] [Google Scholar]
- 14.Homa M., Fekete I.P., Boszorményi A., Singh Y.R., Selvam K.P., Shobana C.S. Antifungal effect of essential oils against Fusarium keratitis isolates. Planta Med. 2015;81:1277–1284. doi: 10.1055/s-0035-1546272. [DOI] [PubMed] [Google Scholar]
- 15.Tomotake H., Koga T., Yamato M., Kassu A., Ota F. Antibacterial activity of Citrus fruit juices against Vibrio species. J Nutrit Sci Vitaminol. 2006;52:157–160. doi: 10.3177/jnsv.52.157. [DOI] [PubMed] [Google Scholar]
- 16.Auysawasdi N., Chuntranuluck S., Phasomkusolsil S., Keeratinijakal V. Improving the effectiveness of three essential oils against Aedes aegypti (Linn.) and Anopheles dirus (Peyton and Harrison) Parasitol Res. 2016;115(1):99–106. doi: 10.1007/s00436-015-4725-3. [DOI] [PubMed] [Google Scholar]
- 17.Pazinato R., Klauck V., Volpato A., Tonin A.A., Santos R.C., Souza M.E. Influence of tea tree oil (Melaleuca alternifolia) on the cattle tick Rhipicephalus microplus. Exp Appl Acarol. 2014;63:77–83. doi: 10.1007/s10493-013-9765-8. [DOI] [PubMed] [Google Scholar]
- 18.Boligon A.A., Kubiça T.K., Mario D.B., Brum T.F., Piana M., Weiblen R. Antimicrobial and antiviral activity-guided fractionation from Scutia buxifolia Reissek extracts. Acta Physiol Plant. 2013;35:2229–2239. [Google Scholar]
- 19.Adams R.P. Allured Publishing Corporation; Illinois (USA): 1995. Identification of essential oil components by Gas Chromatography/Mass spectroscopy; p. 456p. [Google Scholar]
- 20.Drummond R.O., Ernst S.E., Trevino J.L., Gladney W.J., Graham O.H. Boophilus annulatus and Boophilus microplus: Laboratory tests of insecticides. J Econ Entomol. 1973;66:130–133. doi: 10.1093/jee/66.1.130. [DOI] [PubMed] [Google Scholar]
- 21.Buzzati A., Sprenger L.K., Kucharsky T., Molento M.B. Ação do óleo de nim frente à teleóginas de Rhipicephalus (Boophilus) microplus em testes in vitro. Arch Vet Sci. 2013;18:7–12. [Google Scholar]
- 22.Carrol J.F., Tabanca N., Kramer M., Elejalde N.M., Wedge D.E., Bemier U.R. Essential oils of Cupressus funebris, Juniperus communis, and J. chinensis (Cupressaceae) as repellents against ticks (Acari: Ixodidae) and mosquitoes (Diptera: Culicidae) and as toxicants against mosquitoes. J Vector Ecol. 2011;2:258–268. doi: 10.1111/j.1948-7134.2011.00166.x. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich G., Dolan M.C., Peralta-Cruz J., Schimidt J., Piesman J., Eisen R.J. Repellent activity of fractioned compounds from Chamaecyparis nootkatensis essential oil against nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2006;43:957–961. doi: 10.1603/0022-2585(2006)43[957:raofcf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Dolan M.C., Jordan R.A., Schulze T.L., Schulze C.J., Manning M.C., Ruffolo D. Ability of two natural products, nootkatone and carvacrol, to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J Econ Entomol. 2009;102:2316–2324. doi: 10.1603/029.102.0638. [DOI] [PubMed] [Google Scholar]
- 25.Amer A., Mehlkorn H. Repellency effect of forty one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res. 2006;99:478–490. doi: 10.1007/s00436-006-0184-1. [DOI] [PubMed] [Google Scholar]
- 26.Agnolin C.A., Olivo C.J., Parra C.L.C. Efeito do óleo de capim limão (Cymbopogon flexuosus Stapf) no controle do carrapato dos bovinos. Rev Bras Pl Med. 2014;16:77–82. [Google Scholar]
- 27.Silva W.W., Athayde A.C.R., Rodrigues O.G., Araújo G.M.B., Santos V.D., Neto A.B.S. Efeitos do neem (Azadirachta indica A. Juss) e do capim santo [Cymbopogon citratus (DC) Stapf] sobre os parâmetros reprodutivos de fêmeas ingurgitadas d Boophilus microplus e Rhipicephalus sanguineus (Acari: Ixodidae) no semiárido paraibano. Rev Bras Pl Med. 2007;9:1–5. [Google Scholar]
- 28.Tchoumbougnang F., Amvam Zollo P., Dagne E., Mekonnen Y. In vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimum on mice infected with Plasmodium berghei. Plant Med. 2005;71:20–23. doi: 10.1055/s-2005-837745. [DOI] [PubMed] [Google Scholar]
- 29.Makhaik M., Narayana S.N., Tewary D. Evaluation of anti-mosquito properties of essential oils. J Sci Ind Res. 2005;64:129–133. [Google Scholar]
- 30.Kumar R., Srivastava M., Dubey N.K. Evaluation of Cymbopogon martinii oil extract for control of postharvest insect deterioration in cereals and legumes. J Food Protect. 2007;70:172–178. doi: 10.4315/0362-028x-70.1.172. [DOI] [PubMed] [Google Scholar]
- 31.Duarte M.C.T., Figueira G.M., Sartoratto A., Rehder V.L., Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005;97:305–311. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Silva W.C., Marins J.R.S., Souza E.M., Heinzen H., Cesio M.V., Mato M. Toxicity of piper aduncum L. (Piperales: Piperaceae) from the Amazon Forest for the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Vet Parasitol. 2009;164:267–274. doi: 10.1016/j.vetpar.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Nerio L.S., Olivero-Verbe J., Stashenko E. Repellet activity of essential oils: a review. Biores Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Hanifah A.L., Ming H.T., Narainasamy V.V., Yusoff A.T. Laboratory evaluation of six crude plant extracts as repellents against larval Leptotrombidium deliense (Acari: Trombiculidae) Asian Pacif J Trop Biomed. 2012;2:257–259. [Google Scholar]
- 35.Weldon P.J., Carroll J.F., Kramer M., Bedoukian R.H., Coleman R.E., Bernier U.R. Anointing chemicals and hematophagous arthropods: responses by ticks and mosquitoes to Citrus (Rutaceae) peel exudates and monoterpene components. J Chem Ecol. 2011;34:348–359. doi: 10.1007/s10886-011-9922-7. [DOI] [PubMed] [Google Scholar]
- 36.Slathia P.S., Bhagat G.R., Singh S., Kher S.K., Paul N. Traditional knowledge on utility of Cedrus deodara (Roxb.) Loud in Doda district of Jammu province. Indian J Trad Know. 2007;6:518–520. [Google Scholar]
- 37.Maske D.K., Bhilegaonkar N.G., Jangde C.R. Treatment of tick infestation in cattle with pestoban. J Indian Indig Med. 1996;17:81–83. [Google Scholar]
- 38.Tabanca N., Wang M., Avonto C., Chittiboyina A.G., Parcher J.F., Carroll J.F. Bioactivity-guided investigation of geranium essential oils as natural tick repellents. J Agric Food Chem. 2013;61:4101–4107. doi: 10.1021/jf400246a. [DOI] [PubMed] [Google Scholar]
- 39.Jaenson T.G., Garboui S., Palsson K. Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J Med Entomol. 2006;4:731–736. doi: 10.1603/0022-2585(2006)43[731:rooole]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.