Abstract
Oscillatory activity of retinal ganglion cell (RGC) has been observed in various species. It was reported such oscillatory activity is raised within large neural network and involved in retinal information coding. In the present research, we found an oscillation-like activity in ON–OFF RGC of bullfrog retina, and studied the mechanisms underlying the ON and OFF activities respectively. Pharmacological experiments revealed that the oscillation-like activity patterns in both ON and OFF pathways were abolished by GABA receptor antagonists, indicating GABAergic inhibition is essential for generating them. At the meantime, such activities in the ON and OFF pathways showed different responses to several other applied drugs. The oscillation-like pattern in the OFF pathway was abolished by glycine receptor antagonist or gap junction blocker, whereas that in the ON pathway was not affected. Furthermore, the blockade of the ON pathway by metabotropic glutamate receptor agonist led to suppression of the oscillation-like pattern in the OFF pathway. These results suggest that the ON pathway has modulatory effect on the oscillation-like activity in the OFF pathway. Therefore, the mechanisms underlying the oscillation-like activities in the ON and OFF pathways are different: the oscillation-like activity in the ON pathway is likely caused by GABAergic amacrine cell network, while that in the OFF pathway needs the contributions of GABAergic and glycinergic amacrine cell network, as well as gap junction connections.
Keywords: ON–OFF retinal ganglion cell, Oscillation, Inhibitory pathway, Gap junction
Introduction
Neural oscillation is widely observed in various nervous systems, including visual (Gray and Singer 1989; Neuenschwander and Singer 1996; Ishikane et al. 2005), olfactory (Bressler and Freeman 1980; Laurent 2002; Rojas-Libano and Kay 2008), somatosensory (Ahissar et al. 2000) and auditory (Eguiluz et al. 2000; Roberts and Rutherford 2008) systems. Previous studies suggested that neural oscillations encode specific types of information about the stimulus (Koepsell et al. 2010) and might contribute to perception, attention and memory (Eckhorn et al. 1988; Engel et al. 2001; Fries et al. 2001). It was suggested that inhibitory interneurons and gap junction may play certain roles in generating oscillatory activities in the central nervous system (Ritz and Sejnowski 1997; Traub et al. 2001; LeBeau et al. 2002; Li et al. 2011; Xie and Wang 2013).
Light-evoked oscillation in RGC has functional significance. It was reported that it may serve to perceptual integration (Singer and Gray 1995) and carry necessary information which is crucial for animal’s behavior (Ishikane et al. 2005). The generation of oscillation in a certain type of frog RGC depends on the size of light stimulus, and it was suggested that the oscillation is raised within large neural network and facilitates synchronization between remotely separated RGCs (Ishikane et al. 1999). It was also suggested that the generation of the oscillatory activity is dependent on rhythmic synaptic inputs rather than the neuron’s own active membrane properties (Arai et al. 2004). As for the mechanism underlying oscillation, GABAergic inhibition has been proved to play an important role (Ishikane et al. 1999, 2005; Arai et al. 2004). However, how neural network generates oscillation and whether other neurotransmitters are involved in generating oscillation are still not clear. Thus further pharmacological experiments are required to explore the mechanisms.
In addition, there exist some differences between oscillations in the ON and OFF pathways. It was reported that the oscillatory frequency of ON response was higher than that of OFF response in cat RGCs (Neuenschwander et al. 1999). The ON and OFF pathways in retinas are traditionally considered as two parallel pathways, which means that they are structurally and functionally symmetric. However, many recent researches have proved that the ON and OFF pathways are asymmetric in some aspects. It was reported that there are inhibitory interactions between the ON and OFF pathways. In mammals, the ON bipolar cell electrically couples to the glycinergic AII amacrine cell, which in turn has inhibitory effect on the activities of OFF bipolar and ganglion cells (Pang et al. 2007a; Manookin et al. 2008; Liang and Freed 2010). In amphibian, such as salamander and frog, there is also crossover inhibition between ON and OFF pathways, which is mediated by amacrine cell (Popova et al. 2000; Pang et al. 2007b; Chen et al. 2014). In addition, there is excitatory crosstalk between these two pathways. Ackert et al. (2009) found that blockade of GABAergic inhibition reveals an OFF response in ON direction-selective ganglion cell. Farajian et al. (2011) reported that GABA blockade unmasks an ON response in OFF α-ganglion cell. These studies all suggest that the ON and OFF pathways are not fully independent and there are interactions between them. It is thus intriguing whether the asymmetry and the interaction account for the differences between oscillations in the ON and OFF pathways.
In the present research, we observed an oscillation-like activity in ON–OFF RGC of bullfrog retina, and investigated the properties of it. Given that γ-aminobutyric acid (GABA) and glycine are major inhibitory neurotransmitters in retinas (Wassle et al. 1998), we purposed to investigate whether GABA, glycine and gap junction are involved in generating the oscillation-like activities of ON and OFF responses in RGCs. In addition, there existed difference between oscillation-like activities in the ON and OFF pathways, which might be related with the interaction of the two pathways. Thus which cross circuit is involved in generating oscillation-like activity, and how this circuit contributes to the differences between the activities in the ON and OFF pathways are also our study aims. Our results showed that in bullfrog ON–OFF RGCs, the oscillatory frequency of the ON response was higher than that of the OFF response. Oscillation-like activity patterns of both ON and OFF responses were abolished by GABA receptor antagonists. Interestingly, oscillation-like pattern of the OFF response was abolished by glycine receptor antagonist or gap junction blocker, whereas that of the ON response was not affected. Furthermore, when the ON pathway was blocked by metabotropic glutamate receptor agonist, oscillation-like pattern of the OFF response was also eliminated. Our results suggest that GABAergic inhibition was essential for generating oscillation-like activities in ON and OFF pathways of ON–OFF RGCs, and the oscillation-like activity in the OFF pathway might be modulated by the ON pathway through a glycinergic inhibitory cross circuit. This might provide us a good insight into the underlying mechanism of oscillation.
Materials and methods
Preparation
Bullfrogs were used for electrophysiological experiments (Jing et al. 2010). After being dark adapted for 30 min, a bullfrog was pithed and eyes were enucleated under dim red light. The eyeball was hemisected and the eyecup was cut into several pieces. A piece of isolated retina (around 4 × 4 mm2) was transferred onto a multi-electrode array (MEA) with the ganglion cell layer contacting the electrodes. During recording, the retina was perfused with oxygenated Ringer’s solution (95 % O2 and 5 % CO2) consisting of (in mM): NaCl 100.0, KCl 2.5, MgCl2 1.6, CaCl2 2.0, NaHCO3 25.0, glucose 10.0. For pharmacological experiments, the following drugs were applied by being added to the Ringer’s solution: picrotoxin (100 μM), bicuculline (10 μM), strychnine (2 μM), meclofenamic acid (MFA, 100 μM), l-(+)-2-Amino-4-phosphonobutyric acid (l-AP4, 100 μM). All drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA).
All experimental procedures were performed according to the humane treatment and use of animals as prescribed by the Association for Research in Vision and Ophthalmology, and were approved by the Ethic Committee, School of Biomedical Engineering, Shanghai Jiao Tong University.
Extracellular recording
Neuronal responses were recorded by the MEA (64 electrodes, 8 × 8 array, electrode size 8 μm in diameter, interpolar distance 150 μm, CNNS UNT, USA) and then amplified (1000×) and bandpass filtered from 0.1 to 8 kHz by a 64-channel amplifier (MEA workstation, Plexon Inc. Texas, USA). Signals from each channel were sampled at a rate of 20 kHz, along with the stimulation. Spikes generated by ganglion cells were sorted by principal component analysis (PCA) and the commercial software OfflineSorter (Plexon Inc. Texas, USA).
Visual stimulation
Light stimulus presented on a computer monitor was projected onto the retina through a lens system. The stimulation protocol applied in our experiments included two parts: (1) a full field sustained dim white light (15.2 cd/m2) with duration of 30 s was used to adjust the RGCs’ sensitivity to similar levels; (2) repeated stimulation with 1-s light ON duration (30.4 cd/m2) followed by 1-s light OFF interval (0 cd/m2) was given with 30 trials to evoke neuronal spike discharges.
Data analysis
Autocorrelation of spike trains was calculated, with the autocorrelation being defined as:
where x n and x n+m are the values of spike trains x at moment n and n + m, respectively; R is the normalizing factor, C xx(m) presents the similarity between spike trains as a function of time lag m. To estimate the possible oscillatory frequency of spike trains, the power spectra of the correlograms were computed (Fig. 1a). Statistical difference between groups was assessed with t test.
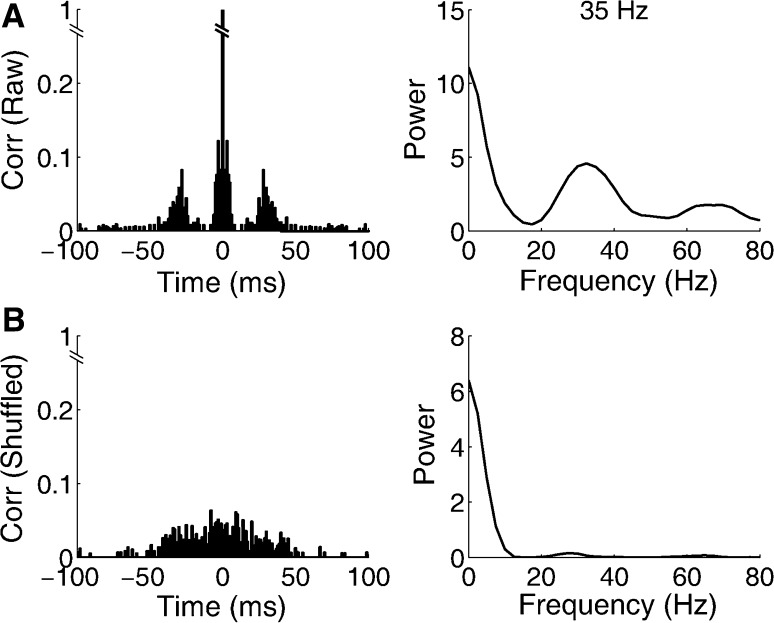
Fig. 1.
An example of data analysis. a The raw autocorrelation (left) and the corresponding power spectral analysis (right), b the shuffled autocorrelation (left) and the corresponding power spectral analysis (right)
To make a quantitative measurement, we compute an oscillation index which was previously proposed (Ishikane et al. 1999). In this method, the raw autocorrelation and the shuffled autocorrelation of spike trains were calculated (Fig. 1). The correlation value of the highest side peak within ±50 ms next to the center peak in the raw autocorrelation (P), and the mean (M) and standard deviation (SD) of correlation value between ±50 ms time shift in the shuffled autocorrelation were measured. If (P − M) > 2 * SD, this means that the side peak level was significantly higher than the noise level, and the oscillation index was calculated as (P − M)/M.
Results
Experiments were performed on isolated bullfrog retinas. According to photo-response properties, bullfrog RGCs can be classified into four subtypes: dimming detector, moving-edge detector, contrast detector and convexity detector (Lettvin et al. 1959). In the present study, we focused on the moving-edge detector which generates transient spike discharges at both onset and offset of full field light stimulation and is thus identified as ON–OFF RGC.
Oscillation-like activities in the ON and OFF pathways
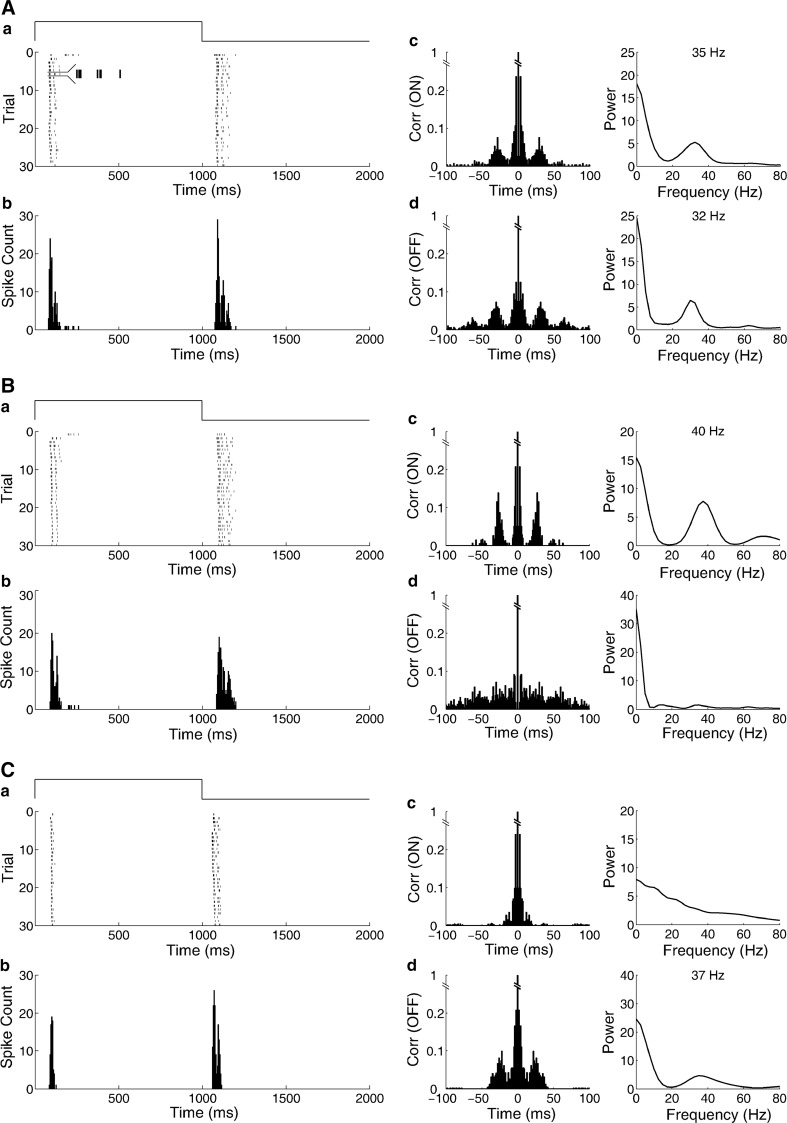
Bullfrog retina was stimulated with full field illumination. Raster plots and peri-stimulus time histogram (PSTH) of example ON–OFF cells are shown in Fig. 2. Transient spike charges at the light onset and offset were observed. Autocorrelation analysis was used to explore time-domain properties of spike trains. As the ON–OFF RGC illustrated in Fig. 2A, there were large side peaks in the autocorrelogram, and the corresponding power spectrum showed a peak in γ range (20–80 Hz), which approximated the oscillatory behavior of dimming detectors’ spike trains (around 30 Hz) (Ishikane et al. 1999, 2005; Arai et al. 2004). Further inspection into the spike trains of the ON–OFF RGC revealed that the trains were mostly composed of a few recurrent spike events with regular interval (Fig. 2Aa inset), thus we call it oscillation-like activity.
Fig. 2.
The oscillation-like activities of the ON and OFF responses of bullfrog’s ON–OFF RGCs. There were three different response patterns. A The first example cell in which both ON (35 Hz) and OFF (32 Hz) responses had oscillation-like activities. B The second example cell in which oscillation-like activity only existed in the cell’s ON response (40 Hz) but not in the OFF response. C The third example cell in which oscillation-like activity only existed in the cell’s OFF response (37 Hz) but not in the ON response. a Raster plots, b Peri-stimulus histogram (5-ms bin width), c Autocorrelation (left) and power spectral analysis (right) of the ON response, d Autocorrelation (left) and power spectral analysis (right) of the OFF response
In our present study, the autocorrelations of the ON and OFF responses were calculated respectively, and three different response patterns were obtained as presented in Fig. 2. In the cell illustrated in Fig. 2A, the autocorrelations of both ON and OFF responses showed oscillation-like activities. The power spectral analysis showed clear peaks at approximately 35 and 32 Hz for the ON and OFF responses respectively, indicating the existence of γ-range component in the ON and OFF pathways. In the cell illustrated in Fig. 2B, the oscillation-like activity was only obtained for the ON response (40 Hz) but not for the OFF response. In Fig. 2C, the oscillation-like activity only existed in the cell’s OFF response (37 Hz) but not in the ON response.
Among 100 ON–OFF RGCs from 24 retinas, 57 RGCs had oscillation-like activity in both ON and OFF responses, 29 RGCs had oscillation-like activity only in ON response and 14 RGCs had oscillation-like activity only in OFF response. Statistical results showed that the oscillatory frequency of the ON response (37.5 ± 0.6 Hz, mean ± s.e.m., N = 86) was higher than that of the OFF response (33.7 ± 0.7 Hz, mean ± s.e.m., N = 71) (p < 0.01, t test).
Effects of GABA receptor antagonists on the oscillation-like activities
It was suggested that inhibitory interneurons may play a role in generating oscillatory activities in the central nervous system (Ritz and Sejnowski 1997). In retinas, γ-aminobutyric acid (GABA) and glycine are major inhibitory neurotransmitters. Previous studies have shown that oscillations in OFF RGCs of bullfrog’s retina can be suppressed by the GABA receptor antagonists bicuculline or picrotoxin (Ishikane et al. 1999, 2005; Arai et al. 2004). Therefore we tried to test whether GABAergic activity was involved in the oscillation-like activities of ON–OFF RGCs.
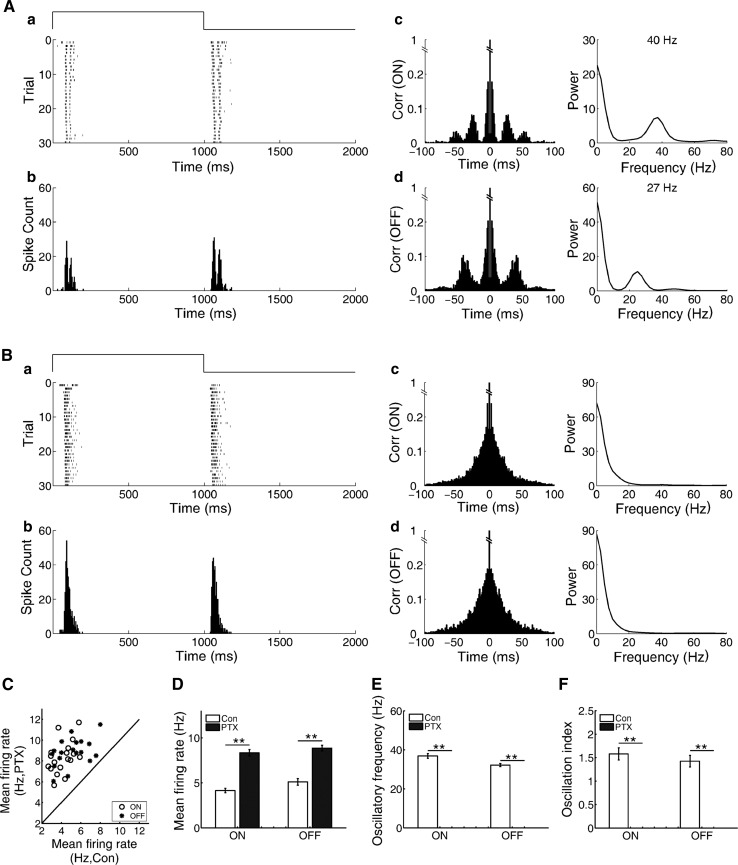
In the example ON–OFF cell illustrated in Fig. 3A, autocorrelograms of both ON and OFF responses showed oscillation-like activities in the control condition. The oscillatory frequency of the ON response (40 Hz) was higher than that of the OFF response (27 Hz). When picrotoxin (100 μM) was applied to the Ringer’s solution, the spike discharges of both ON and OFF responses were increased. However, the oscillation-like activity patterns of both ON and OFF responses were eliminated (Fig. 3B). Observations made from 18 RGCs (7 retinas) with both ON- and OFF-oscillation presented that all recorded oscillation-like patterns were abolished by picrotoxin. In addition, for cells which only had oscillation-like activity in the ON or OFF response, these oscillation-like patterns were also eliminated by picrotoxin (7 retinas, 9 RGCs with ON-oscillation and 3 RGCs with OFF-oscillation).
Fig. 3.
Effects of picrotoxin on the oscillation-like activities of the ON and OFF responses. A In control, the oscillatory frequency was 40 and 27 Hz for the ON and OFF responses, respectively. B The oscillation-like activity patterns of both ON and OFF responses were eliminated by picrotoxin (100 μM). C, D Scatter and bar plots for the mean firing rate during control and picrotoxin. E Oscillatory frequency. F Oscillation index (N = 18 neurons from 7 retinas, bar plot: mean ± s.e.m., *p < 0.05; **p < 0.01; t test)
GABAA receptor blocker bicuculline (10 μM) had similarly effect on the oscillation-like activities of both ON and OFF responses. Observations made from 12 ON–OFF RGCs (2 retinas, 7 RGCs with ON- and OFF-oscillation, 4 RGCs with ON-oscillation and 1 RGC with OFF-oscillation) presented that all recorded oscillation-like patterns were abolished by bicuculline (data not shown). These results indicate that GABAergic inhibition is involved in generating oscillation-like activities in ON and OFF pathways of ON–OFF RGCs.
Effects of glycine receptor antagonist on the oscillation-like activities
Given that GABAergic inhibition was involved in generating the oscillation-like activities in ON and OFF pathways of ON–OFF RGCs, we next tested whether glycine contributed to the oscillation-like activities. As the example cell shown in Fig. 4, both ON and OFF responses had oscillation-like activities in the control condition. The oscillatory frequency of the ON response (40 Hz) was higher than that of the OFF response (32 Hz). After applying the glycine antagonist strychnine (2 μM), the spike discharges of both ON and OFF responses were increased slightly. However, strychnine had different effects on the oscillation-like activities of the ON and OFF responses. For the ON response, strychnine did not induce any obvious changes in the autocorrelogram or peak frequency of oscillation-like activity. However, for the OFF response, the autocorrelation analysis and power spectrum analysis demonstrated that the oscillation-like pattern was totally eliminated by strychnine. Observations made from 18 ON–OFF RGCs with oscillation-like activities (4 retinas, 9 RGCs with both ON- and OFF-oscillation, 6 RGCs with ON-oscillation and 3 RGCs with OFF-oscillation) presented that the oscillation-like pattern of the OFF response was abolished by strychnine, while the oscillation-like activity of the ON response still existed with its oscillatory frequency unchanged. These results indicate that glycine, unlike GABA, is involved in generating the oscillation-like activity in the OFF pathway, whereas it does not contribute to that activity in the ON pathway.
Fig. 4.
Effects of strychnine on the oscillation-like activities of the ON and OFF responses. A In control, the oscillatory frequency was 40 and 32 Hz for the ON and OFF responses, respectively. B The oscillation-like activity of the OFF response was abolished by strychnine (2 μM), while the oscillation-like activity of the ON response was not affected (N = 9 neurons from 4 retinas)
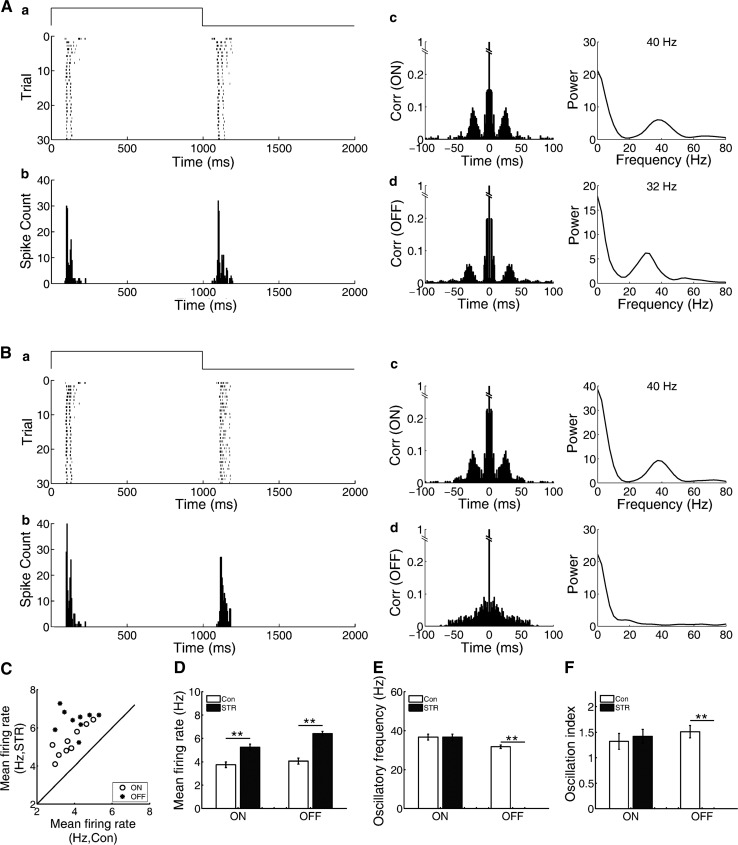
Effects of gap junction blocker on the oscillation-like activities
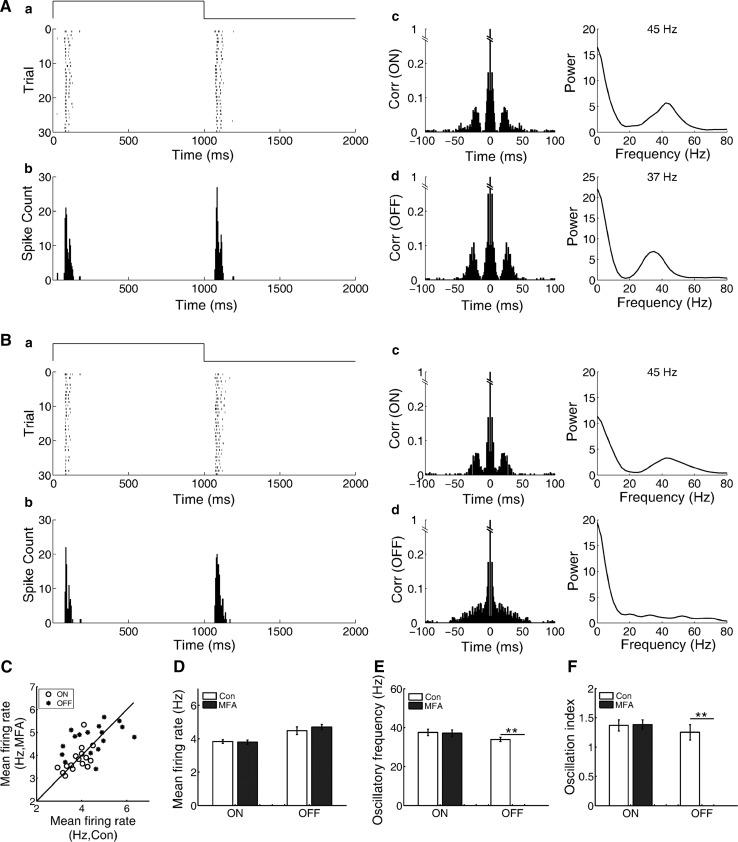
It was proposed that electrical synapses are involved in generating oscillatory activities, and γ oscillations in CNS can be abolished by gap junction blockers (Traub et al. 2001; LeBeau et al. 2002). It was reported that gap junctions containing connexin 36 (Cx36) and Cx45 are critical for the generation of oscillatory activities in mouse RGCs (Borowska et al. 2011; Trenholm et al. 2012; Margolis et al. 2014). To test whether gap junction was involved in generating the oscillation-like activities of bullfrog ON–OFF RGCs, we examined the effects of a specific gap junction blocker MFA (100 μM) which blocks Cx36 and Cx45 gap junctions (Pan et al. 2007; Veruki and Hartveit 2009). It was found that the effects of MFA on the oscillation-like activities in the ON and OFF pathways were different (Fig. 5). For the ON response, MFA did not induce any obvious changes in the autocorrelogram or the peak frequency of oscillation-like activity. However, for the OFF response, the autocorrelation analysis and power spectrum analysis demonstrated that the oscillation-like pattern was totally eliminated by MFA. Observations made from 31 ON–OFF RGCs with oscillation-like activities (5 retinas, 17 RGCs with both ON- and OFF-oscillation, 10 RGCs with ON-oscillation and 4 RGCs with OFF-oscillation) presented that the oscillation-like pattern of the OFF response was abolished by MFA, while the oscillation-like activity of the ON response survived with the oscillatory frequency unaltered. These results may suggest that gap junction consisting Cx36/Cx45 is essential for generating the oscillation-like activity in the OFF pathway, whereas it does not contribute to that in the ON pathway.
Fig. 5.
Effects of MFA on the oscillation-like activities of the ON and OFF responses. A In control, the oscillatory frequency was 45 and 37 Hz for the ON and OFF responses, respectively. B The oscillation-like activity pattern of the OFF response was abolished by MFA (100 μM), while the oscillation-like activity of the ON response was not affected (N = 17 neurons from 5 retinas)
Effects of metabotropic glutamate receptor agonist on the oscillation-like activities
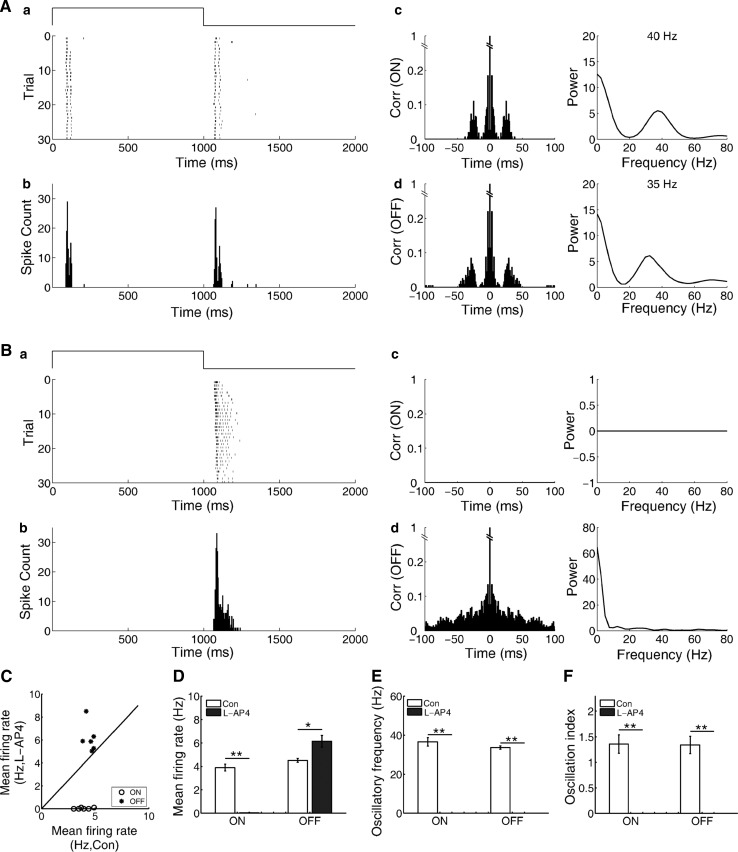
As the results above, there were differences between the oscillation-like activities in the ON and OFF pathways. Although the segregation of the ON and OFF pathways is demonstrated by both morphological and physiological data, there is emerging evidence for “crossover” between the two pathways (Zaghloul et al. 2003; Renteria et al. 2006; Liang and Freed 2010). It was reported that the ON pathway has crossover inhibition onto the OFF pathway in frog and salamander retinas (Popova et al. 2000; Pang et al. 2007b; Chen et al. 2014). And in degenerating mouse retina, spontaneous oscillatory activity is transferred from the ON pathway to the OFF pathway via glycinergic synapse (Poria and Dhingra 2015). Therefore we tried to test whether the ON pathway had any effects on the oscillation-like activity in the OFF pathway. The metabotropic glutamate receptor agonist l-AP4 (100 μM), which can hyperpolarize ON bipolar cell, was applied to the Ringer’s solution.
As the example cell shown in Fig. 6, both ON and OFF responses had oscillation-like activities in the control condition. Application of l-AP4 not only eliminated the ON response, but also slightly increased firing rate of the OFF response as previously reported (Popova et al. 2000). However, the oscillation-like pattern of the OFF response was eliminated. Observations made from 9 ON–OFF RGCs with oscillation-like activities (3 retinas, 6 RGCs with both ON- and OFF-oscillation, 3 RGCs with OFF-oscillation) presented that the ON response and the oscillation-like pattern of the OFF response were blocked by l-AP4. Disruption of the ON pathway with l-AP4 caused the elimination of the oscillation-like pattern of the OFF response. These results indicate that the oscillation-like activity of the OFF response may be dependent on the ON pathway.
Fig. 6.
Effects of l-AP4 on the oscillation-like activity of the OFF response. A In control, the oscillatory frequency was 40 and 35 Hz for the ON and OFF responses, respectively. B After application of l-AP4 (100 μM), the ON response was totally blocked and the oscillation-like activity pattern of the OFF response was eliminated (N = 6 neurons from 3 retinas)
Discussion
In the present study, oscillation-like activities in ON–OFF RGC of bullfrog retina were observed, and its properties were investigated. Pharmacological experiments revealed that GABAergic inhibition was involved in generating the oscillation-like activities in the ON and OFF pathways in ON–OFF RGCs (Fig. 3). The oscillation-like pattern of the OFF response was eliminated by glycine receptor antagonist or gap junction blocker, while the oscillation-like pattern of the ON response was not affected (Figs. 4, 5). Moreover, blockade of the ON pathway with l-AP4 abolished the oscillation-like pattern of the OFF response (Fig. 6). This suggests that the oscillation-like activities in the OFF pathway might be modulated by the ON pathway through a glycinergic inhibitory cross circuit.
Oscillation-like activity in bullfrog ON–OFF RGC
In our results, 100 out of 313 ON–OFF RGCs’ autocorrelograms had large side peaks, and the corresponding power spectrum showed a peak in γ range (20–80 Hz). This was because the spike trains of these ON–OFF RGCs were composed of a few recurrent spike events with regular interval, and the intervals were similar in ON–OFF RGCs (ON: 22–31 ms, OFF: 27–40 ms, N = 100). This means that the latter spiking events were rhythmic recurrent activities rather than irregular spike events. Such properties of this activity pattern in ON–OFF RGCs were similar with the oscillation in dimming detector which was also composed of recurrent spike events with regular interval (around 30 ms) (Ishikane et al. 1999, 2005; Arai et al. 2004). However, as there are only two or three regular events in ON–OFF RGCs, it is weak to define such activity pattern in ON–OFF RGCs as oscillation. Thus we call it oscillation-like activity.
Modulation of the OFF pathway by the ON pathway
The group III metabotropic glutamate receptors (mGluRs) agonist l-AP4, which hyperpolarizes ON bipolar cell, was used to suppress the ON response of RGC (Shiells et al. 1981; Slaughter and Miller 1981). Our results showed that after adding l-AP4 (100 μM), the ON response was blocked as expected while the oscillation-like pattern of the OFF response was also suppressed. Higher concentration of l-AP4 (200 μM) also suppressed the oscillation-like pattern of the OFF response, while lower concentration (50 μM) had little effect on that (data not shown). It is most likely that blockade of the ON pathway leads to the elimination of the oscillation-like pattern in the OFF pathway. Another possibility is that l-AP4 has direct effect on the oscillation-like activity in the OFF pathway because group III mGluRs are also located on axon of OFF bipolar cell (Brandstatter et al. 1996), and the glutamate release from OFF bipolar cell axon terminals was suppressed by l-AP4, with the modulation being light intensity-dependent (Awatramani and Slaughter 2001; Higgs et al. 2002). However, the excitatory input mediated by glutamate could not account for the elimination of oscillation-like pattern because it is inhibitory rather than excitatory input that is essential in generating oscillatory activities (Ritz and Sejnowski 1997). Thus, we believe that the suppression of the oscillation-like activity pattern in the OFF pathway is caused by blockade of the ON pathway, which indicates that the ON pathway contributes to the oscillation-like activity in the OFF pathway.
Here comes another question: how the ON pathway affects the OFF pathway at light-offset? It was reported that ON cone bipolar cells displayed depolarization after light offset (Euler and Masland 2000). In addition, bipolar cells express Ih, a hyperpolarization-activated depolarizing current (Ma et al. 2003). These two mechanisms might account for the activation of the ON pathway at light-offset, which in turn modulates the OFF pathway during light-off stimulation.
Our results also showed that strychnine (2 μM) eliminated the oscillation-like activity pattern in the OFF pathway while leaving the oscillation-like activity in the ON pathway undisturbed. Higher concentration of strychnine (5, 10 μM) had similar effects (data not shown). Thus, it would be reasonable to suppose that the ON pathway modulates the oscillation-like activity in the OFF pathway through glycinergic inhibition. In retina, there is a cross circuit between ON and OFF pathways, which is mediated by glycinergic amacrine cell, and many recent studies reported some imbalance of ON and OFF responses results from this inhibitory cross circuit (Popova et al. 2000; Molnar and Werblin 2007; Pang et al. 2007b; Manookin et al. 2008; Petrides and Trexler 2008; Liang and Freed 2010; Oesch et al. 2011). Therefore, via this glycinergic cross circuit, the ON pathway could modulate the oscillation-like activity in the OFF pathway.
The oscillation-like pattern in the OFF pathway was eliminated by MFA (100 μM). Higher concentration of MFA (200 μM) also suppressed the oscillation-like pattern of the OFF response, while lower concentration (50 μM) had little effect on that (data not shown). The most reasonable explanation of these pharmacological results is that there exists gap junction in the inhibitory cross circuit between ON and OFF pathways. After adding gap junction blocker, the modulation from the ON pathway on the OFF pathway via this cross circuit was eliminated, and the oscillation-like activity pattern in the OFF pathway was abolished. One possible explanation is that there exists amacrine-to-bipolar cell gap junction, although such connection has yet rarely been reported in frog retina,and further researches are required to explore how gap junction is involved in generating oscillation-like activity in the OFF pathway.
In conclusion, the ON pathway modulates the oscillation-like activity in the OFF pathway through a cross circuit which might be mediated by glycinergic amacrine cell and gap junction.
Contribution of lateral inhibition to the oscillation-like activities in the ON and OFF pathways
Our results showed that the oscillation-like patterns in ON and OFF pathways were eliminated by picrotoxin (100 μM). Higher concentration of picrotoxin (150 μM) also suppressed oscillation-like patterns, while lower concentration (50 μM) had little effect on that (data not shown). These results indicated that GABAergic inhibition is essential for generating the oscillation-like activities in ON and OFF pathways of ON–OFF RGCs. In addition, the inhibitory cross circuit between ON and OFF pathways contributes to the oscillation-like activity in OFF pathway. Combining the two factors together, we proposed two different mechanisms underling the oscillation-like activities in the ON and OFF pathways of ON–OFF RGCs. The oscillation-like activity in the ON pathway is likely caused by GABAergic amacrine cell network, while the oscillation-like activity in the OFF pathway needs the combined effects of GABAergic amacrine cell network and glycinergic amacrine cell which mediated the inhibition from ON pathway to OFF pathway. The model is consistent with our experimental results: GABA blockade abolished the oscillation-like patterns of both ON and OFF responses; cutting off the cross circuit by blocking the glycine receptor eliminated the oscillation-like pattern in the OFF pathway while leaving the oscillation-like activity in the ON pathway undisturbed; blocking the ON pathway totally with l-AP4 eliminated oscillation-like pattern in the OFF pathway.
The multifunction of the cross circuit mediated by amacrine cell
A key component of the different oscillation-like activities in the ON and OFF pathways is the cross circuit mediated by amacrine cell. The cross circuit is widespread in the vertebrate retina and is multifunctional. In mammals, under scotopic illumination, the function of this cross circuit is to transmit rod signals to cone pathway; under photopic illumination, with the same cross circuit, the ON pathway could inhibit the OFF pathway (Liang and Freed 2010; Oesch et al. 2011). The inhibition from the ON pathway enables the OFF pathway a larger response range, and this is consistent with the natural world which contains more negative contrast than positive (Ratliff et al. 2010). On the other hand, oscillatory activity in frog RGC was proved to carry essential information for the animal (Ishikane et al. 2005). Our results suggest a new role of the inhibitory cross circuit as its involvement in generating oscillation-like activities of ON–OFF RGCs. The multifunction of the cross circuit may imply the high efficiency of retina in processing signals.
In conclusion, the present study focused on the oscillation-like activities in the ON and OFF pathways in ON–OFF RGCs. We found differences between the oscillation-like activities in the ON and OFF pathways, and investigated some properties of the oscillation-like activities in the two pathways. This study suggests a new role of the inhibitory cross circuit between the ON and OFF pathways, and might provide us a good insight into the underlying mechanism of oscillation.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 31471054, P.J.L; No. 61375114, P.M.Z).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All experimental procedures were performed according to the humane treatment and use of animals as prescribed by the Association for Research in Vision and Ophthalmology, and were approved by the Ethic Committee, School of Biomedical Engineering, Shanghai Jiao Tong University.
References
- Ackert JM, Farajian R, Volgyi B, Bloomfield SA. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J Physiol. 2009;587:4481–4495. doi: 10.1113/jphysiol.2009.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. doi: 10.1038/35018568. [DOI] [PubMed] [Google Scholar]
- Arai I, Yamada Y, Asaka T, Tachibana M. Light-evoked oscillatory discharges in retinal ganglion cells are generated by rhythmic synaptic inputs. J Neurophysiol. 2004;92:715–725. doi: 10.1152/jn.00159.2004. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci. 2001;21:741–749. doi: 10.1523/JNEUROSCI.21-02-00741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowska J, Trenholm S, Awatramani GB. An intrinsic neural oscillator in the degenerating mouse retina. J Neurosci. 2011;31:5000–5012. doi: 10.1523/JNEUROSCI.5800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter JH, Koulen P, Kuhn R, van der Putten H, Wassle H. Compartmental localization of a metabotropic glutamate receptor (mGluR7): two different active sites at a retinal synapse. J Neurosci. 1996;16:4749–4756. doi: 10.1523/JNEUROSCI.16-15-04749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Chen EY, Chou J, Park J, Schwartz G, Berry MJ., 2nd The neural circuit mechanisms underlying the retinal response to motion reversal. J Neurosci. 2014;34:15557–15575. doi: 10.1523/JNEUROSCI.1460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Eguiluz VM, Ospeck M, Choe Y, Hudspeth AJ, Magnasco MO. Essential nonlinearities in hearing. Phys Rev Lett. 2000;84:5232–5235. doi: 10.1103/PhysRevLett.84.5232. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Farajian R, Pan F, Akopian A, Volgyi B, Bloomfield SA. Masked excitatory crosstalk between the ON and OFF visual pathways in the mammalian retina. J Physiol. 2011;589:4473–4489. doi: 10.1113/jphysiol.2011.213371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs MH, Romano C, Lukasiewicz PD. Presynaptic effects of group III metabotropic glutamate receptors on excitatory synaptic transmission in the retina. Neuroscience. 2002;115:163–172. doi: 10.1016/S0306-4522(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Ishikane H, Kawana A, Tachibana M. Short- and long-range synchronous activities in dimming detectors of the frog retina. Vis Neurosci. 1999;16:1001–1014. doi: 10.1017/S0952523899166033. [DOI] [PubMed] [Google Scholar]
- Ishikane H, Gangi M, Honda S, Tachibana M. Synchronized retinal oscillations encode essential information for escape behavior in frogs. Nat Neurosci. 2005;8:1087–1095. doi: 10.1038/nn1497. [DOI] [PubMed] [Google Scholar]
- Jing W, Liu WZ, Gong XW, Gong HQ, Liang PJ. Visual pattern recognition based on spatio-temporal patterns of retinal ganglion cells’ activities. Cogn Neurodyn. 2010;4:179–188. doi: 10.1007/s11571-010-9119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell K, Wang X, Hirsch JA, Sommer FT. Exploring the function of neural oscillations in early sensory systems. Front Neurosci. 2010;4:53. doi: 10.3389/neuro.01.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- LeBeau FE, Towers SK, Traub RD, Whittington MA, Buhl EH. Fast network oscillations induced by potassium transients in the rat hippocampus in vitro. J Physiol. 2002;542:167–179. doi: 10.1113/jphysiol.2002.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettvin JY, Maturana HR, McCulloch WS, Pitts WH. What the frog’s eye tells the frog’s brain. Proc IRE. 1959;47:1940–1951. doi: 10.1109/JRPROC.1959.287207. [DOI] [Google Scholar]
- Li X, Morita K, Robinson HP, Small M. Impact of gamma-oscillatory inhibition on the signal transmission of a cortical pyramidal neuron. Cogn Neurodyn. 2011;5:241–251. doi: 10.1007/s11571-011-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci. 2010;30:5533–5543. doi: 10.1523/JNEUROSCI.4733-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YP, Cui J, Hu HJ, Pan ZH. Mammalian retinal bipolar cells express inwardly rectifying K+ currents (IKir) with a different distribution than that of Ih. J Neurophysiol. 2003;90:3479–3489. doi: 10.1152/jn.00426.2003. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Singer JH, Detwiler PB. Network oscillations drive correlated spiking of ON and OFF ganglion cells in the rd1 mouse model of retinal degeneration. PLoS One. 2014;9:e86253. doi: 10.1371/journal.pone.0086253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol. 2007;98:3423–3435. doi: 10.1152/jn.00838.2007. [DOI] [PubMed] [Google Scholar]
- Neuenschwander S, Singer W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996;379:728–732. doi: 10.1038/379728a0. [DOI] [PubMed] [Google Scholar]
- Neuenschwander S, Castelo-Branco M, Singer W. Synchronous oscillations in the cat retina. Vis Res. 1999;39:2485–2497. doi: 10.1016/S0042-6989(99)00042-5. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Kothmann WW, Diamond JS. Illuminating synapses and circuitry in the retina. Curr Opin Neurobiol. 2011;21:238–244. doi: 10.1016/j.conb.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci. 2007;24:609–618. doi: 10.1017/S0952523807070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol. 2007;580:397–410. doi: 10.1113/jphysiol.2006.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Cross-talk between ON and OFF channels in the salamander retina: indirect bipolar cell inputs to ON–OFF ganglion cells. Vis Res. 2007;47:384–392. doi: 10.1016/j.visres.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Petrides A, Trexler EB. Differential output of the high-sensitivity rod photoreceptor: AII amacrine pathway. J Comp Neurol. 2008;507:1653–1662. doi: 10.1002/cne.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova E, Mitova L, Vitanova L, Kupenova P. Effect of 2-amino-4-phosphonobutyrate on the OFF responses of frog retinal ganglion cells and local ERG after glycinergic blockade. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126:139–151. doi: 10.1016/s0742-8413(00)00107-9. [DOI] [PubMed] [Google Scholar]
- Poria D, Dhingra NK. Spontaneous oscillatory activity in rd1 mouse retina is transferred from ON pathway to OFF pathway via glycinergic synapse. J Neurophysiol. 2015;113:420–425. doi: 10.1152/jn.00702.2014. [DOI] [PubMed] [Google Scholar]
- Ratliff CP, Borghuis BG, Kao YH, Sterling P, Balasubramanian V. Retina is structured to process an excess of darkness in natural scenes. Proc Natl Acad Sci USA. 2010;107:17368–17373. doi: 10.1073/pnas.1005846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria RC, Tian N, Cang JH, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz R, Sejnowski TJ. Synchronous oscillatory activity in sensory systems: new vistas on mechanisms. Curr Opin Neurobiol. 1997;7:536–546. doi: 10.1016/S0959-4388(97)80034-7. [DOI] [PubMed] [Google Scholar]
- Roberts WM, Rutherford MA. Linear and nonlinear processing in hair cells. J Exp Biol. 2008;211:1775–1780. doi: 10.1242/jeb.017616. [DOI] [PubMed] [Google Scholar]
- Rojas-Libano D, Kay LM. Olfactory system gamma oscillations: the physiological dissection of a cognitive neural system. Cogn Neurodyn. 2008;2:179–194. doi: 10.1007/s11571-008-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G, Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294:592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, et al. Intrinsic oscillatory activity arising within the electrically coupled AII amacrine-ON cone bipolar cell network is driven by voltage-gated Na+ channels. J Physiol. 2012;590:2501–2517. doi: 10.1113/jphysiol.2011.225060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Meclofenamic acid blocks electrical synapses of retinal AII amacrine and on-cone bipolar cells. J Neurophysiol. 2009;101:2339–2347. doi: 10.1152/jn.00112.2009. [DOI] [PubMed] [Google Scholar]
- Wassle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vis Res. 1998;38:1411–1430. doi: 10.1016/S0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Xie J, Wang Z. Effect of inhibitory feedback on correlated firing of spiking neural network. Cogn Neurodyn. 2013;7:325–331. doi: 10.1007/s11571-013-9241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]