Abstract
One main challenge for medical investigators is the early diagnosis of Alzheimer’s disease (AD) because it provides greater opportunities for patients to be eligible for more clinical trials. In this study, higher order spectra of human speech signals during AD were explored to analyze and compare the quadratic phase coupling of spontaneous speech signals for healthy and AD subjects using bispectrum and bicoherence. The results showed that the quadratic phase couplings of spontaneous speech signal of persons with Alzheimer’s were reduced compared to healthy subject. However, the speech phase coupled harmonics shifted to the higher frequencies in Alzheimer’s than healthy subjects. In addition, it was shown not only are there significant differences between Alzheimer’s and control subjects in parameters estimated, but also the speech patterns appeared to have fluctuated in both types of spontaneous speech.
Keywords: Alzheimer’s disease, Spontaneous speech signal, Bispectrum estimation, Bicoherence estimation, Phase coupling
Introduction
Alzheimer’s disease (AD) is a common progressive neurodegenerative mental disorder among older people, causing a gradual loss of memory, understanding, judgment, thinking, language and so on (McKhann et al. 2011; Jack et al. 2011; Dauwels et al. 2010; Buiza 2010; Martinez et al. 2012). The Lack of verbal communication due to Alzheimer depends on the stage of disease. These speech deficits can be divided into three stages. In the first phase, subjects face problems finding the correct words in spontaneous speech which is often not found. In the intermediate stage, the language and vocabulary in daily use become week. Finally, in the advanced stage, subjects provide limited answers confined to a very few words. Several techniques have been devised for AD diagnose including: electroencephalogram (EEG) signals (Dauwels et al. 2010), magnetic resonance imaging (MRI) (Zhang et al. 2015a, b), computed tomography (CT) imaging (Reynolds 2013), functional magnetic resonance imaging (fMRI) (Zhang et al. 2015a), positron emission tomography (PET) (Kippenhan et al. 1994), single-photon emission computed tomography (SPECT) (Salas-Gonzalez et al. 2010). However, these methods are expensive, inaccurate, difficult and time-consuming to use. In recent years, medical investigators have aimed for the early diagnosis of AD patients in that it can provide greater opportunities for more clinical trials, with enough time to plan for medical and financial decisions. Moreover, an early diagnosis along with the right medicines will be beneficial to postpone the symptoms (Hu et al. 2010). In this context, spontaneous speech analysis is a potentially useful inexpensive non-invasive technique for AD diagnosis. López et al. (2013) used emotional analysis of spontaneous speech and Fractal Dimension with neural network classifier for AD diagnosis, obtaining a classification accuracy of 97.7 %. In our previous work (Nasrolahzadeh et al. 2015a), acoustic features with Adaptive Neuro-Fuzzy Inference Systems (ANFIS) classifier was used to discriminate healthy and AD subjects, with a similar accuracy of 97.96 %. It was Nasrolahzadeh et al. (2014, 2015b) shown that nonlinear features of spontaneous speech signals such as Lyapunov exponent and Correlation dimension have potential in AD diagnosis.
The human speech is an example of complex physiologic fluctuations. The neural control of the glottal pulse system exhibits the complex nonlinear behavior, with nonlinear interactions r because physiological conditions would most likely involve autonomic nervous system regulation based on a dynamic simultaneous activity of the human speech responses to physical environmental stresses (Smith et al. 2013).
Recently, dynamic methods of speech analysis have been proposed to reveal nonlinear characteristics of speech signals. In addition, the speech signal can be analyzed using higher order spectra (HOS) (also known as polyspectra), that is, spectral representations of higher order moments or cumulants of a signal (Nikias and Raghuveer 1987). Currently, most feature extraction methods are based on the autoregressive (AR) models (Ganapathy et al. 2014; Mesot and Barber 2007; Han et al. 2009). However, these schemes are assumed to be linear, Gaussian and minimum phase, i.e., the speech signals are normally distributed, their frequency components are uncorrelated and their statistical properties do not change over time (Oveisgharan and Shamsollahi 2004; Shekofteh and Almasganj 2013). Thus, these can be used to estimate power and intensity, ignoring phase information. However, in practice, the speech signal may be non-Gaussian, non-linear and non-stationary. The speech signal is produced from a unique non-linear system, having many sinusoidal components at different frequencies. They interact nonlinearly to produce one or more sinusoidal components at difference and sum frequencies, which cannot be fully determined by the autocorrelation functions. Higher order spectra analysis has several additional features useful for speech processing relating to frequency interacting by coupling that.
This paper proposed a set of features based on higher order spectra (HOS) of the spontaneous speech signal for discrimination of early stage of AD and healthy groups.
HOS, a non-linear method, captures subtle signal changes, used as features for the automated classification. It contains information not provided by conventional spectral analysis. It performs better even for weak and noisy signals. HOS can reveal information about nonlinear signal generation mechanism, in particular, mechanisms with quadratic nonlinearities and deviation from Gaussianity (Chua et al. 2010).
Bispectrum and bicoherence are frequently used in research studies because they can efficiently quantify any nonlinear interactions among the harmonic peaks (such as phase coupling). Furthermore, some research studies have shown that the non-zero bispectrum represents non-linear interactions.
Several authors have used HOS techniques for speech signal analysis. Toddler et al. (2013) used bispectral analysis of inter-word time intervals in psychotic speech to classify psychotic and healthy subjects. Their results showed a higher level of phase coupling for psychotic group, biocoherence. Azarbarzin and Moussavi (2011) estimated the bispectrum and bicoherence of each sound segment for discriminating snoring segments, showing that all the recorded fragments were non-Gaussian.
Current research is directed toward developing a novel method for early diagnosis of AD based on the nonlinearity evaluation of speech signals using HOS. The present study was designed to further characterize speech dynamics for two groups, Alzheimer’s and control subjects, comparing their spontaneous speech using higher order spectra. The purpose of this study is to decide whether HOS features of the spontaneous speech signal can provide practical parameters for early diagnosis of AD?
The outline of this study is as follows: in the next section, the datasets used for this study is presented. Then, the methods and quantification analysis applied are presented. Moreover, the simulation results and performance of analysis on data sets are presented. Finally, a discussion and conclusions are dealt with.
Materials
Data collection
The study was approved by the Institutional Review Board of all participants and therefore was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. After the patients were briefed about the objectives of the study, they gave their informed consent prior to their inclusion in the study.
Some 60 subjects were purposefully selected for the present study: a group of 30 healthy control subjects (HCS), (52–98 years old, 15 women, 15 men) and a group of 30 AD patients (52–98 years old, 14 women, 16 men), with three levels of AD: first stage (FS), second stage (SS) and third stage (TS), (FS = 6, SS = 15, TS = 9).
They were specifically asked to tell graceful personal stories, express their feelings, and converse in a friendly way.
The speech signals were recorded at the OLD NURSING HOME in Sabzevar using an audio recorder. The audio was extracted in WAV format. The sampling frequency and bit per second were 16 kHz and 16 Kbits, respectively. The speech signals were recorded about 15–17 h for the control and AD groups, respectively. The recording atmosphere was relaxed and friendly.
The recording time was shorter for the subjects with Alzheimer’s in that Alzheimer’s patients spent more time than the healthy individuals to find words. They spoke more slowly, less clearly, with longer pauses. Their message was interrupted or remained incomplete, with more time needed for them to find the words. As a result, they became tired early and wanted the researcher to stop recording. And he did so. The database was intentionally multicultural (Mashhad, Sabzevar, Bojnord, Tehran, Fariman, Ghochan, Kordestan, Neyshabour) so that a new methodology could be developed which was independent of the cultural, social and linguistic factors.
Methodology
Signal preprocessing
Non-analyzable events such as laughing, coughing, short hard noises and segments during which speakers overlapped, were eliminated after speech signals were recorded. Next, background noise was removed using the method described in Mohammadpoory and Haddadnia (2014). After that, there remained 4 h for the AD group and 12 h for the control subjects for further analysis. Finally, the speech was divided into some consecutive segments of 60 s in order to obtain comparable segments for all speakers. Therefore, the database of about 960 segments of spontaneous speech (70 segments for FS, 110 segments for SS, 60 segments for TS and 720 segments for HCS group) were created.
Bispectrum estimation
Higher order spectra analysis can provide additional information to the power spectrum. They are the spectral representations of higher order statistics such as cumulants or moments of higher orders (Nikias and Raghuveer 1987). A special case of higher order spectra is the third-order spectrum also known as the bispectrum. The prefix bi refers to the two frequencies of a signal. The third order cumulants is defined by Eq. (1):
| 1 |
where x(n) is the zero mean stationary process, x* is the complex conjugate of x and are two different time increments.
The bispectrum is defined by Eq. (2):
| 2 |
The bispectrum shows the cross correlation between frequency components in a two-dimensional frequency plot (Nikias and Raghuveer 1987). Let us assume that a long signal length is divided into K frame with length N. Number the frame’s j = 1, 2, …k. Let X1,…, XN denote the samples of a generic frame. Bispectrum is then the average over the K bispectra:
| 3 |
Bispectrum can be estimated by various methods (Ning and Bronzino 1990). In this paper both the direct (FFT-based) and parametric method were used.
Direct (FFT-based) method
In order to estimate the bispectrum estimation using the direct (FFT-based) method (Xianda 1995; Mendal 1991), the recorded signal are sampled and segmented into several overlapping frames. The mean of each record is removed and the FFT is calculated, the bispectrum of the Nth record is computed by the relationship between the moment and the cumulant spectrum (Li et al. 2005). It is defined as follows:
| 4 |
where denotes the FFT of the Nth record. The data are windowed and an optional frequency domain smoother is applied. So that the estimation is variance reduces (Li et al. 2005). In this paper, the Rao–Gabr window is used (SubbaRao and Gabr 1984).
Parametric method
In the parametric approach, the AR model was chosen. Because the coefficient of the AR model can be easily estimated by solving several linear equations, when new data are available, the coefficients can be updated efficiently by the Kalman filter equation (Ning and Bronzino 1990; Childers 1978).
Bicoherence estimation
The bicoherence gives information about the phase coupling between the frequency components at , and . It defines by Eq. (5) (Ning and Bronzino 1990):
| 5 |
where P(f) denotes the power spectrum. Since it takes values from the range of [0, 1], it is known as the normalized bispectrum or skewness.
A bicohersnce index of close to unity at a frequency pair where phase coupling has occurred indicates an almost 100 % degree of phase coherence. On the other hand, a near zero value of Bic () at a certain pair of frequencies will suggest an absence of any phase coupling. In fact, the bicoherence measures the portion of the signal energy in bifrequency that is quadratically phase coupled (Sigl and Chamoun 1994).
Statistical analysis
In the present study, a t test was used to compare significant changes in maximum magnitudes of bispectrum between the two groups of healthy and Alzheimer’s subjects. The Mann–Whitney U-test was used to compare the bicoherence values between the two groups of subjects. A P value <0.05 was considered significant.
Results
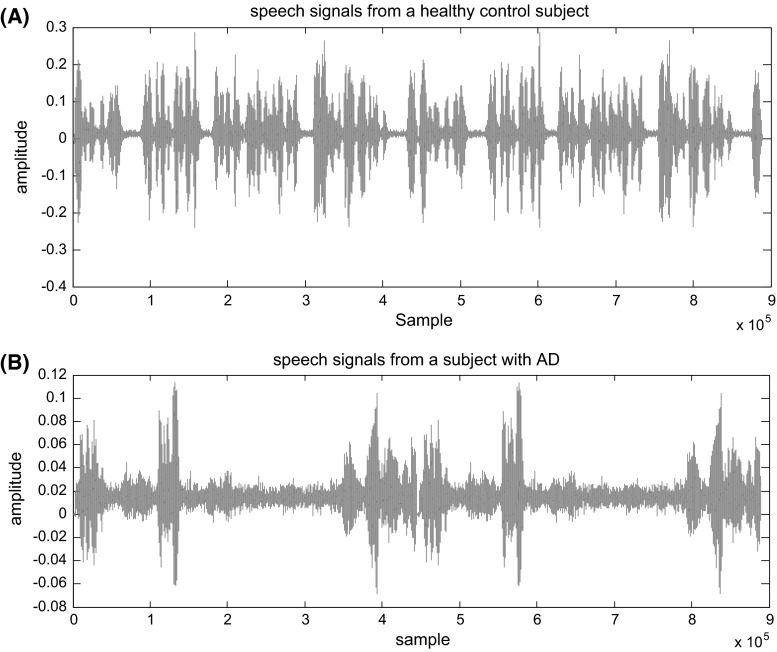
To implement the proposed method, the data base described in before section was used. Since the purpose of this study was early detection of AD, the data of FS and HCS groups just were used for experimental results (60 segments were selected for each group from the database). Two spontaneous speech signals of a HCS and an AD patient are shown in Fig. 1a, b, respectively. Due to the loss of language skills reflected in talking, understanding and relationship with the natural environment, the poverty of the speech signals for the patient with Alzheimer is clearly observable.
Fig. 1.
Spontaneous speech signals of a control subject (a) and an Alzheimer’s patient (b)
Bispectrum of each speech segment is estimated via both direct (FFT) and parametric methods, using the MATLAB toolbox. The FFT length is set to 128 and the percentage overlap between segments is set to zero for both methods. In parametric method, we used an AR order of ten. The results of bispectrum analysis of speech signals of Alzheimer’s were compared to those in the healthy control stages (HCS).
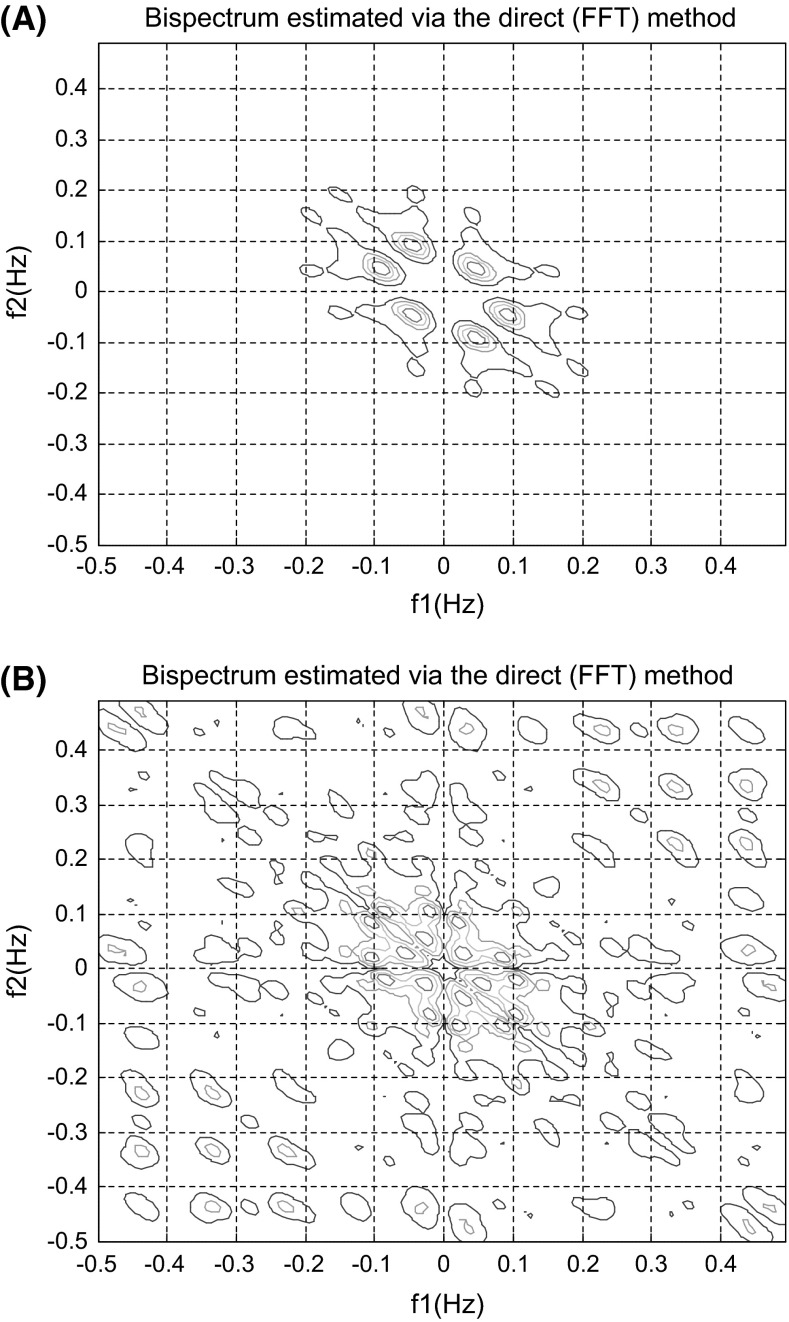
The bispectrum estimated via direct (FFT) method for spontaneous speech of a HCS and an AD patient are presented in Fig. 2a, b, respectively. The colors indicate the relative changes in amplitude of bispectrum. Blue and red represent the highest decrease and increase, respectively.
Fig. 2.
Bispectrum of spontaneous speech estimated via the directed (FFT) method for a healthy subject (a) and (b) for an Alzheimer’s patient
According to Fig. 2, two bispectrums are significantly different. Analysis of 120 bispectrums indicated that on average the phase coupled harmonics were less than 0.2 Hz for the healthy individuals and below 0.5 Hz for the patient with Alzheimer’s. The bispectrum showed sharp peak placed around (0.05, 0.05) Hz for healthy subjects while they were roughly between 0.1 and 0.5 Hz for the Alzheimer’s patients. They were symmetrically distributed.
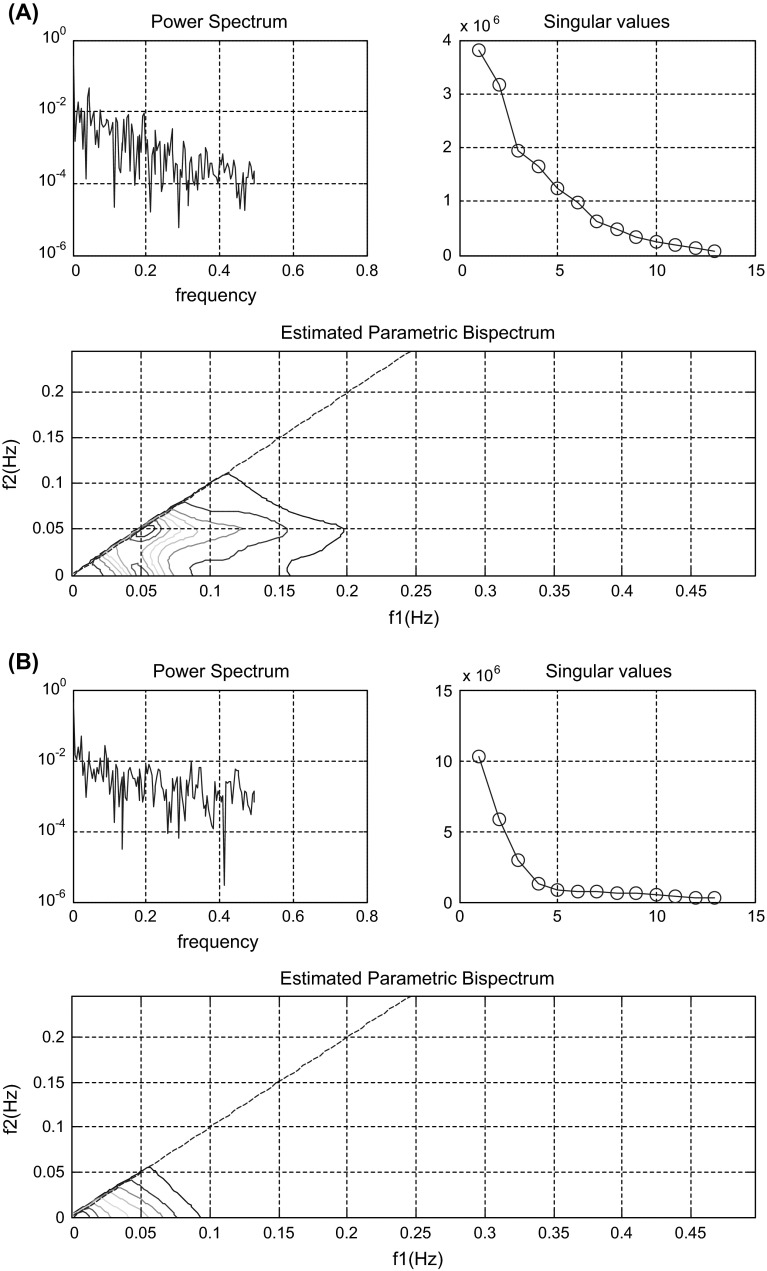
Figure 3a, b demonstrates power spectrums, singular values and bispectrum estimated via parametric method for spontaneous speech of a HCS subject and an Alzheimer’s patient, respectively. As can be seen, these figures are significantly different for two groups. The analysis of the power spectrums, singular values and bispectrums estimated via parametric method for 120 segments indicated that on average the maximum value of the power spectrum was about for healthy subjects but it was about for the group with Alzheimer’s. Hence, the power spectrum of signal decreased for Alzheimer’s subjects.
Fig. 3.
Power spectrum, singular values and estimated parametric bispectrum of spontaneous speech signals. a For a healthy subject and b for an Alzheimer’s patient
It also depicts, there are 11 significant singular values for control group and 13 significant singular values for AD group, corresponding to one quadratically coupled triplet. For healthy individuals, the dominant peak was at (f 1, f 2) = (0.05078, 0.05078) Hz and it was at (0, 0) Hz for AD patients respectively. The dominant peaks exhibiting the presence of a second harmonic were about 0.05 Hz for HCS groups and 0.1 Hz for AD patients. According to bispectrum estimated via parametric model, the phase-coupled harmonics were below 0.15 Hz for HCS groups and they were approximately 0.05 Hz for AD groups.
The average values of maximum magnitudes of bispectrums obtained via parametric method for two groups are shown in Table 1. According this Table, AD had an effect on the maximum magnitude of bispectrum of the spontaneous speech signal. In comparison with healthy control subjects, the maximum magnitude of bispectrum of the spontaneous speech signal increased for AD group. The results suggest that different forms of AD appear to alter differentially specific components of speech signals. P value <0.05 indicates that this feature values were significantly different between two groups and this feature had the ability of discriminating two groups.
Table 1.
Average values of maximum magnitudes of bispectrum estimated via parametric method between the two groups of control and AD subjects
| During spontaneous speech | Control subjects | Subjects with AD |
|---|---|---|
| Maximum bispectrum | ||
| P value <0.05, h = 1 | 0.0046 | 0.0092 |
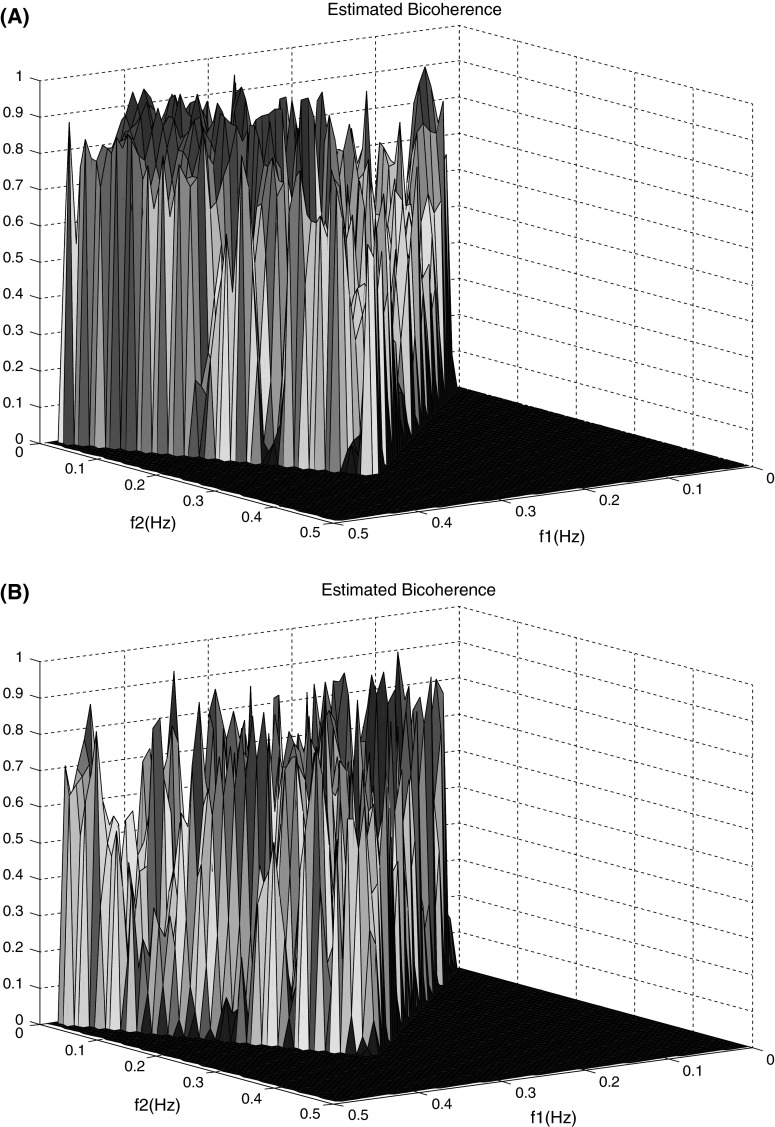
Since the peak is observed was the estimated bispectrum, the bicoherence index was evaluated. Figure 4 presents bicoherence of spontaneous speech for a healthy control and an AD patient. The color represents the relative change in amplitude of bicoherence estimated. For HCS groups the colors tend to warm colors implying the greatest rise in bicoherence amplitude and quadratic phase coupling, while for Alzheimer’s patients the cool colors are dominant suggesting the greatest decrease in bicoherence amplitude and quadratic phase coupling.
Fig. 4.
Bicoherence of the spontaneous speech signals. a For the healthy subjects and b for the Alzheimer’s patients
In addition, for control subjects during spontaneous speech, phase coupling tends to lower frequencies than AD group. The average value of frequency components of bicoherence estimation are shown in Table 2.
Table 2.
Average values of frequency components of bicoherence estimation between the two groups of control and AD subjects
| During Spontaneous speech | Control subjects | Subjects with AD |
|---|---|---|
| BIC estimation | ||
| P value <0.05, h = 1 | 0.0013 | 0.0035 |
Discussion
The main goal of this study was to examine the phase coupling of spontaneous speech signals in the specific psychophysiological state. To this end, the researchers estimated the bispectrum of human speech signals for two groups; people with early stage of Alzheimer’s disease and healthy subjects.
The results showed that the maximum value of the power spectrum decreased during Alzheimer’s disease Fig. 3. That is reasonable, because of poverty existing in the spontaneous speech signals of AD patients. The average values of maximum magnitudes of bispectrum of spontaneous speech signals increased during Alzheimer’s disease (P < 0.05). The decrees of phase coupling were more obvious in the AD than the healthy group. Besides that phase-coupled harmonics shifted to the higher frequencies in Alzheimer’s than healthy subjects.
It is known that speech signals are produced through the air pressure transmission in the vocal tract which is a system with highly nonlinear dynamics, but there is no evidence indicating that the activation of human speech is Gaussian. We believe that more of the information available in the speech signals must be exploited, such as the information of nonlinearity and non-Gaussianality. The proposed feature extraction method has several key advantages. First, the proposed feature set includes high-order statistics information, while the widely used conventional features with only second-order measures (such as the power spectrum and autocorrelation functions) do not. As a consequence, non-minimum-phase signals, such as speech signals, cannot be accurately characterized by the second-order measures. Moreover, some types of phase coupling in speech signals associated with nonlinearities cannot be accurately recognized by the second-order measures. Finally, the proposed feature set is less affected by Gaussian background noise than the conventional features with only second-order measures due to the property of bispectrum: the bispectrum of Gaussian signal is zero.
Recent studies have shown that bispectrum techniques can suggest beneficial information for the study of the cardiac function. Zgallai (2012) has shown that the ECG bispectral contour template matching technique is effectual in detecting the occurrence of the fetal heartbeats in the frequency domain even when it is completely buried in noise.
In addition, Zhang et al. (2010) the results of the bispectrum amplitude investigated that the strength of the frequency coupling was not as strong in the normal subjects as in the congestive heart failure cases.
The results demonstrate that the bispectral analysis of the spontaneous speech signals can expose extra information non acquirable from the power spectrum and may provide insights into the formation of speech signals in different psychological sates (subjects with Alzheimer’s and healthy subjects).
Furthermore, this paper presents the phase coupling of speech signals using bicoherence. Results showed that the speech bispectra of Alzheimer’s during spontaneous speech had quadratic phase couplings frequencies approximately between 0.1 and 0.3 Hz. These results indicate that the higher frequency components of the Alzheimer’s speech during spontaneous speech are partially contributed by the quadratic phase coupling of lower frequency components.
Through applying the bispectral estimation, it was inferred that the quadratic phase coupling of spontaneous speech signal of persons with Alzheimer’s and therefore nonlinear interactions were reduced. These results confirm results of previous studies such as (Nasrolahzadeh et al. 2015a, b, 2016; Nasrolahzadeh and Haddadnia 2016). In these studies, it has been shown using different nonlinear measures, that the spontaneous speech signals of AD patients are less chaotic and nonlinear than healthy subjects. This can be due to brain damages causing the typical symptoms of frontotemporal dementia including changes in personality and behavior and difficulties with language (Rohrer 2012).
Conclusion
A novel method based on HOS was presented in this paper for extracting discriminative information from human spontaneous speech with the aim of the early diagnosis of AD. The results suggested that quadratic phase coupling indices may serve as a quantitative measure for detection of the early stage of Alzheimer’s disease. The results indicated that the proposed method could provide a good choice for the early diagnosis of AD. Therefore this method could be employed as a very easy cheap test for pre-clinical evaluation of AD diagnosis.
Contributor Information
Mahda Nasrolahzadeh, Email: ms.nasrolahzadeh@yahoo.com.
Zeynab Mohammadpoory, Email: z.mohammadpoory@gmail.com.
Javad Haddadnia, Email: haddadnia@hsu.ac.ir.
References
- Azarbarzin A, Moussavi Z (2011) Nonlinear properties of snoring sounds. In: 2011 IEEE international conference on acoustics, speech and signal processing (ICASSP), pp 4316–4319
- Buiza C. Evaluación y tratamiento de los trastornosdellenguaje. Donostia: Matia Fundazioa; 2010. [Google Scholar]
- Childers DG, editor. Modern spectrum analysis. New York: IEEE Press; 1978. [Google Scholar]
- Chua KC, Chandran V, Acharya UR, Lim CM. Application of higher order statistics/spectra in biomedical signals—a review. Med Eng Phys. 2010;32:679–689. doi: 10.1016/j.medengphy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Cichocki A. Diagnosis of Alzheimer’s disease from EEG signals: where are we standing? Curr Alzheimer Res. 2010;7:487–505. doi: 10.2174/156720510792231720. [DOI] [PubMed] [Google Scholar]
- Ganapathy S, Mallidi SH, Hermansky H. Robust feature extraction using modulation filtering of autoregressive models. IEEE/ACM Trans Audio Speech Lang Process. 2014;22:1285–1295. doi: 10.1109/TASLP.2014.2329190. [DOI] [Google Scholar]
- Han Y, Ma Z, Zhou P (2009) A study of gaits in Parkinson’s patients using autoregressive model. In: IEEE Conference Publications, bio-inspired computing, 2009. BIC-TA ‘09. Fourth international conference, pp 1–4
- Hu WT, McMillan C, Libon D, Leight S, Forman M, Lee VMY, Trojanowski JQ, Grossman M. Multimodal predictors for Alzheimer’s disease in non fluent primary progressive aphasia. Neurology. 2010;75:595–602. doi: 10.1212/WNL.0b013e3181ed9c52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippenhan JS, Barker WW, Nagel J, Grady C, Duara R. Neural-network classification of normal and Alzheimer’s disease subjects using high-resolution and low-resolution PET cameras. J Nucl Med. 1994;35:7–15. [PubMed] [Google Scholar]
- Li Z, Wu Z, He Y, Fulei C. Hidden Markov model-based fault diagnostics method in speed-up and speed-down process for rotating machinery. Mech Syst Signal Process. 2005;19:329–339. doi: 10.1016/j.ymssp.2004.01.001. [DOI] [Google Scholar]
- López de Ipiña K et al (2013) Automatic analysis of emotional response based on non-linear speech modeling oriented to Alzheimer disease diagnosis. In: IEEE 17th international conference on intelligent engineering systems, 19–21 June 2013
- Martinez F, Garcia J, Perez E, Carro J, Anara JM. Patrones de Prosodiaexpresiva en pacientes con enfermedadde Alzheimer. Psicothema. 2012;24:16–21. [PubMed] [Google Scholar]
- McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendal JM. Tutorial on higher-order statistics (spectra) in signal processing and system theory: theoretical results and some application. Proc IEEE. 1991;79:278–305. doi: 10.1109/5.75086. [DOI] [Google Scholar]
- Mesot B, Barber DA (2007) Bayesian alternative to gain adaptation in autoregressive hidden markov models, acoustics, speech and signal processing. In: ICASSP 2007. IEEE international conference, vol 2, pp II-437–II-440
- Mohammadpoory Z, Haddadnia J. Speech enhancement using laplacian mixture model under signal presence uncertainty. IJE Trans C Asp. 2014;27:1367–1376. [Google Scholar]
- Nasrolahzadeh M, Haddadnia J. Poincaré plots of Spontaneous Speech Signals during Alzheimer’s disease. MitteilungenSaechsischerEntomologen. 2016;119:358–365. [Google Scholar]
- Nasrolahzadeh M, Mohhamadpoori Z, Haddadnia J. Optimal way to find the frame length of the speech signal for diagnosis of Alzheimer’s disease with PSO. Asian J Math Comput Res. 2014;2:33–41. [Google Scholar]
- Nasrolahzadeh M, Mohhamadpoori Z, Haddadnia J. Adaptive neuro-fuzzy inference system for classification of speech signals in Alzheimer’s disease using acoustic and non-linear characteristics. Asian J Math Comput Res. 2015;3:122–131. [Google Scholar]
- Nasrolahzadeh M, Mohhamadpoori Z, Haddadnia J. Alzheimer’s disease diagnosis using spontaneous speech signals and hybrid features. Asian J Math Comput Res. 2015;7:322–331. [Google Scholar]
- Nasrolahzadeh M, Mohhamadpoori Z, Haddadnia J. Analysis of mean square error surface and its corresponding contour plots of spontaneous speech signals in Alzheimer’s disease with adaptive wiener filter. Comput Hum Behav. 2016;61:364–371. doi: 10.1016/j.chb.2016.03.031. [DOI] [Google Scholar]
- Nikias CL, Raghuveer MR. Bispectrum estimation: a digital signal processing framework. Proc IEEE. 1987;75:869–891. doi: 10.1109/PROC.1987.13824. [DOI] [Google Scholar]
- Ning T, Bronzino JD. Autoregressive and bispectral analysis techniques: EEG applications. IEEE Eng Med Biol. 1990;9:47–50. doi: 10.1109/51.62905. [DOI] [PubMed] [Google Scholar]
- Oveisgharan S, Shamsollahi MB (2004) Speech modeling and voiced/unvoiced/mixed/silence speech segmentation with fractionally Gaussian noise based models. In: Acoustics, speech, and signal processing, 2004. Proceedings. (ICASSP ‘04). IEEE International Conference, vol 1, pp I-613–I-616
- Reynolds A. Alzheimer disease: focus on computed tomography. Radiol Technol. 2013;2085:187CT–211CT. [PubMed] [Google Scholar]
- Rohrer JD. Structural brain imaging in frontotemporal dementia. Biochim Biophys Acta (BBA) Mol Basis Dis. 2012;1822:325–332. doi: 10.1016/j.bbadis.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Salas-Gonzalez D, Gorriz JM, Ramírez J, López M, Alvarez I, Segovia F, Chaves R, Puntonet CG. Computer-aided diagnosis of Alzheimer’s disease using support vector machines and classification trees. Phys Med Biol. 2010;5:2807–2817. doi: 10.1088/0031-9155/55/10/002. [DOI] [PubMed] [Google Scholar]
- Shekofteh Y, Almasganj F. Autoregressive modeling of speech trajectory transformed to the reconstructed phase space for ASR purposes. Digit Signal Process. 2013;23:1923–1932. doi: 10.1016/j.dsp.2013.06.011. [DOI] [Google Scholar]
- Sigl JC, Chamoun NG. An introduction to bispectral analysis for the electroencephalogram. J Clin Monit. 1994;10:392–404. doi: 10.1007/BF01618421. [DOI] [PubMed] [Google Scholar]
- Smith BL, Nemcek SP, Swinarski KA, Jiang JJ. Nonlinear source-filter coupling due to the addition of a simplified vocal tract model for excised larynx experiments. J Voice. 2013;27:261–266. doi: 10.1016/j.jvoice.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SubbaRao T, Gabr MM (1984) An introduction to bispectral analysis and bilinear time series models (Lecture notes in statistics), vol 24. Springer, New York. ISBN 0-387-96039-2
- Todder D, Avissar S, Schreiber G (2013) Non-linear dynamic analysis of inter-word time intervals in psychotic speech. IEEE J Transl Eng Health Med 1:2200107. doi:10.1109/JTEHM.2013.2268850 [DOI] [PMC free article] [PubMed]
- Xianda Z. Modern signal processing. Beijing: Tsinghua University Press; 1995. pp. 373–433. [Google Scholar]
- Zgallai WA (2012) Non-invasive fetal heartbeat detection using bispectral contour matching. In: ICEBEA 2012, Dubai
- Zhang Q, Yang J, Li L, Li B, Liu C (2010) Study of pulse rate variability signals using bispectrum analysis. In: 4th International conference on bioinformatics and biomedical engineering (ICBBE), Chengdu
- Zhang Z, Zheng H, Liang K, Wang H, Kong S, Hu J, Wu F, Sun G. Functional degeneration in dorsal and ventral attention systems in amnestic mild cognitive impairment and Alzheimer’s disease: an fMRI study. Neurosci Lett. 2015;585:160–165. doi: 10.1016/j.neulet.2014.11.050. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang S, Phillips P, Dong Z, Ji G, Yang J. Detection of Alzheimer’s disease and mild cognitive impairment based on structural volumetric MR images using 3D-DWT and WTA-KSVM trained by PSOTVAC. Biomed Signal Process Control. 2015;21:58–73. doi: 10.1016/j.bspc.2015.05.014. [DOI] [Google Scholar]