Abstract
Introduction
Open tibial fractures are associated with a high incidence of mainly osteomyelitis. Negative pressure wound therapy (NPWT) is a novel form of treatment that uses subatmospheric pressure to effect early wound healing.
Objectives and study design
To determine the effect of NPWT on incidence of deep infections/osteomyelitis after open tibial fractures using a prospective randomized study design.
Materials and methods
Ninety-three open tibial fractures were randomized into two groups receiving NPWT and the second group undergoing periodic irrigation, cleaning and debridement respectively. The wounds were closed or covered on shrinkage in size and sufficient granulation. Evidence of infection was sought during the course of treatment and follow up. Also serial cultures were sent every time the wound was cleaned.
Results and conclusions
Patients in the control group developed a total of 11 infections (22%) as opposed to only 2 (4.6%) in the NPWT group (p < 0.05). The relative risk was 5.5 (95% confidence interval) suggesting patients who received NPWT were 5.5 times less likely to develop infection. Twenty patients developed positive growth when samples were sent for culture with 3 (6.9%) in the NPWT group and 17 (34%) in the control group (p < 0.05). Only 5 patients (25%) went on the develop osteomyelitis, all being a part of the control group. Thus negative pressure wound therapy is indeed beneficial for preventing the incidence of both acute infections and osteomyelitis in open fractures. However a significant difference was not seen in the time required for the wound to be ready for delayed primary closure or coverage.
Keywords: Negative pressure wound therapy, Osteomyelitis, Vacuum assisted closure, Open fracture, Infection
1. Introduction
Open fractures are a commonly encountered problem in orthopaedic practice. They are associated with a high rate of deep infections and osteomyelitis. Many studies have reported an infection rate in the tune of 16–66% in open fractures.1, 2, 3, 4, 5, 6, 7, 8 With modern treatment protocols and availability of better antibiotics, this percentage has decreased significantly but still amounts to a sizeable chunk. This scenario is worse for open diaphyseal tibial fractures as they are associated with a higher contamination, soft tissue damage and skin loss.
Negative pressure wound therapy (NPWT) is a novel form of treatment that uses subatmospheric pressure (vacuum) to effect early wound healing.9, 10, 11, 12, 13, 14, 15, 16 Its efficacy in reducing infections in open fractures has not been conclusively established. We aim to elucidate the effect of this therapy with respect to incidence of deep infections in open diaphyseal tibial fracture in this prospective randomized study.
2. Materials and methods
This study evaluated the role of negative pressure therapy on the incidence of deep infections in open tibial diaphyseal fractures. This study was commenced after clearance of the departmental review board and institutional ethics committee of our centre. Patients were included in the study only after their due consent. Adult patients (greater than 18 years) suffering from an open tibial fracture who were willing to be a part of the trial were included. Patients whose wounds could be closed at the index surgery, patients not needing repeated debridements and dressing, patients less than 18 years of age and those not willing to give consent were excluded from the study. Also, patients with periarticular tibial fractures, those needing amputations and those with wounds on which it would not be possible to use negative pressure therapy were also excluded.
All fractures were classified using the Gustilo Anderson classification system used for open fractures.1 All Grade I and most of Grade II fractures had to be excluded from the study as the wounds could be closed after debridement during the primary surgery itself. The majority of the fractures that were included were Grade II and Grade IIIA fractures with heavy contamination and severe soft tissue and bony injury along with all Grade IIIB and Grade IIIC fractures. All patients underwent a thorough intraoperative debridement with stabilization of the fractures commonly with an external fixator. All patients received perioperative antibiotic coverage as per the institutional protocol which included a third generation cephalosporin, an aminoglycoside and clindamycin. These antibiotics were continued post-operatively.
The patients were randomized into two groups: the first group receiving VAC (vacuum assisted closure) dressing and the second group receiving daily cleaning, dressing and debridement. The VAC dressing consisted of a custom cut open cell foam and gauze that was put over the wound under an adhesive occlusive dressing. As per our institutional protocol, a negative pressure of about 125 mmHg was applied intermittently. Several studies have shown intermittent pressure mode to be more efficacious compared to continuous negative pressure.20 The wound was opened every fourth day for reapplication of dressing and swab was sent for aerobic and anaerobic culture every time the wound was opened. Once the wound had sufficient granulation tissue such that it could undergo skin grafting or the wound had contracted to such a size that it could surgically closed, it was either closed or covered with skin graft. Serial irrigation and debridement was continued till the wounds were ready for closure or coverage.
Confounding factors such as nutrition and mobilization protocol were standardized as per the institutional guidelines and kept same. Basic demographic data of the patients was collected and tabulated. Also noted was the presence of comorbidities like Diabetes mellitus, chronic kidney disease and any immunosuppressive medications that the patient was receiving. History of smoking was also elicited as smoking has a clear negative impact on wound healing. The serum albumin levels of patients in both groups were noted. The distribution of these factors in the two groups was analyzed to remove bias. All patients were followed up regularly to look for presence of any delayed infection. Any patient who developed signs of acute wound infection like pyrexia, raised total leucocyte count and local signs like pus discharge from the wound with erythema of skin edges within 1 week of primary debridement was considered to have an acute infection. Deep infections included cases developing features of chronic osteomyelitis like a discharging sinus, fixed puckered overlying soft tissue and radiological changes consistent with chronic osteomyelitis. A case was considered to be culture positive if even a single culture out of the serial analysis showed quantitative bacterial growth.
Continuous variables were analyzed and tabulated using arithmetic mean, standard deviations and range. An unpaired t test was used to determine if the occurrence of various confounding factors in the two groups was significant. The relative risk was calculated using 95% confidence intervals. The differences in the incidence of infection in the two groups were calculated using Fischer exact test and probability (p value) less than 0.05 was considered significant.
3. Results
A total of 95 patients were enrolled in the study of which two required amputation as primary mode of treatment on the operating surgeon's discretion. These two patients were excluded. Using a random number generator, 50 patients were allotted to the control group and 43 to the group receiving negative pressure therapy. A total of 60 males and 33 females were randomly distributed in the two groups. The mean age of the patients receiving negative pressure therapy was 34.8 years while that of the ones in the control group was 37.4 (p > 0.1). Most of the patients presented to us within 48 h of trauma; however, there were some patients having delayed presentation. The mean interval between the time of injury and presentation to our institute was 4.6 days in the trial group compared to 4.3 days in the control group. A total of 8 patients were known cases of diabetes mellitus on medical treatment. They included 3 (6.9%) patients in the trial group and 5 (10%) patients in the control group. This difference was not statistically significant. A total of 25 patients were smokers being distributed as 25.5% and 28% in the trial and control groups respectively (p > 0.05). Only 2 patients, both in the trial group, had chronic renal disease while one patient in the trial group was on immunosuppressive medications in view of rheumatoid arthritis. Serum albumin is an important factor in wound healing especially in countries where malnourishment is rampant. The mean serum albumin was 2.4 and 2.52 in trial and control groups respectively highlighting the effective randomization and nutrition depleted status of these patients (Table 1).
Table 1.
Patient data and demographics.
| NPWT | Control group | |
|---|---|---|
| Total | 43 | 50 |
| Male | 28 | 32 |
| Female | 15 | 18 |
| Age (mean) | 34.8 | 37.4 |
| Diabetes mellitus | 3 (6.9%) | 5 (10%) |
| Serum albumin levels | 2.4 ± 0.4 | 2.52 ± 0.4 |
| Chronic kidney disease | 2 (4.6%) | – |
| Immunosuppressive therapy | – | 1 (2%) |
| Smoking | 11 (25.5%) | 14 (28%) |
The wound characteristics were also studied in detail. Majority of the fractures in the study belonged to Gustilo Anderson Grade IIIA and IIIB open fractures. 15 (34.8%) fractures and 14 (28%) fractures were Grade IIIA and 22 (51.1%) fractures and 27 (54%) fractures were Grade IIIB in the NPWT and control groups respectively. No Grade I fractures and very few Grade II and IIIA fractures were included as most underwent internal fixation with primary closure of the wound after debridement. Hence, only heavily contaminated wounds in these groups that required further debridements were possible to be included (Table 2). The average time required for the wound to be ready for grafting or delayed closure was 8.3 days and 9.8 days in the two groups respectively. This difference was not statistically significant (p > 0.05).
Table 2.
Wound characteristics.
| NPWT | Control group | |
|---|---|---|
| Days until wound ready for closure/coverage | 8.3 | 9.8 |
| Wound dimensions (cm) | ||
| Length | 10.4 ± 9.2 | 12.1 ± 8.6 |
| Breadth | 6.1 ± 5.4 | 6.2 ± 5.2 |
| Depth (in deepest zone) | 1.6 | 1.6 |
| Method of wound closure | ||
| Delayed closure | 34 | 40 |
| Skin graft | 8 | 8 |
| Flap (free/rotational) | 1 | 2 |
Wound dimensions were measured with the help of a ruler and were similar in both groups. The mean dimension in the group receiving NPWT was 10.4 cm × 6.1 cm × 1.6 cm. The average dimension of the wound in the group undergoing cleaning, debridement and dressing was 12.1 cm × 6.2 cm × 1.6 cm. Most of the wound in both groups underwent delayed closure while a total of 16 patients (eight in each group) needed skin grafting. Only three required a flap procedure for coverage (Table 3). One patient required a delayed amputation in view of unsalvageable soft tissue damage distal to the fracture. However, data from this patient was retained in view of the index wound being proximal to the amputation site. All patients were followed up at regular intervals with the mean follow-up being around 23 weeks ± 6 weeks.
Table 3.
Distribution of fractures by grading of open fractures.
| NPWT | Control group | |
|---|---|---|
| Grade I | 0 | 0 |
| Grade II | 5 (11%) | 8 (16%) |
| Grade IIIA | 15 (34.8%) | 14 (28%) |
| Grade IIIB | 22 (51.1%) | 27 (54%) |
| Grade IIIC | 1 (2.3%) | 1 (2%) |
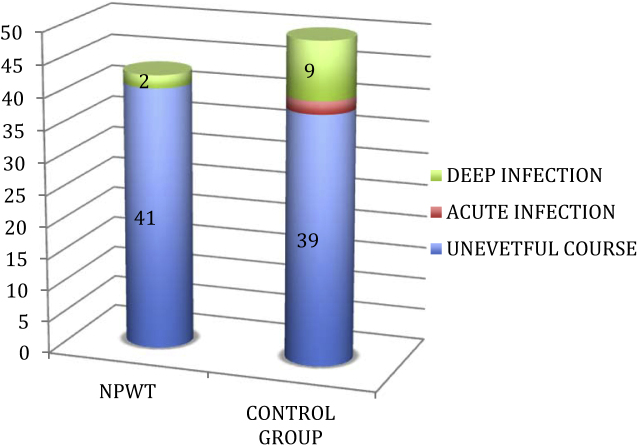
Patients in the control group developed a total of 11 infections (22%) as opposed to only 2 (4.6%) in the group who received NPWT. The relative risk was 5.5 (95% confidence interval) suggesting patients who received NPWT were 5.5 times less likely to develop infection as compared to patients in the trial group. This difference was statistically significant (p < 0.05) (Table 4) (Fig. 1). Patients in the control group developed 2 (4%) acute infections characterized by wound discharge and clinical signs of infection as compared to zero infections in the trial group. This number increased to 9 (18%) which was the rate of deep infections including osteomyelitis in the control group. The mean time of presentation of patients with deep infections was 7 weeks. Only 2 patients in the trial group developed osteomyelitis with a mean presentation at 9.5 weeks. A total of 11 patients developed osteomyelitis/deep infection (2 in the trial group and 9 in the control group). Of these, 3 patients were known diabetics while seven were smokers. All patients who developed osteomyelitis/deep infection were Grade III open fractures. Of the eleven patients who sustained chronic infection, 4 were Grade IIIA and seven were Grade IIIB.
Table 4.
Incidence of acute and deep infections in the two groups.
| NPWT | Control group | |
|---|---|---|
| Acute wound infection | 0 | 2 (4%) |
| Delayed deep infection (osteomyelitis) | 2 (4.6%) | 9 (18%) |
| Total | 2 (4.6%) | 11 (22%) |
Fig. 1.
Incidence of acute and deep infections in the NPWT and control groups.
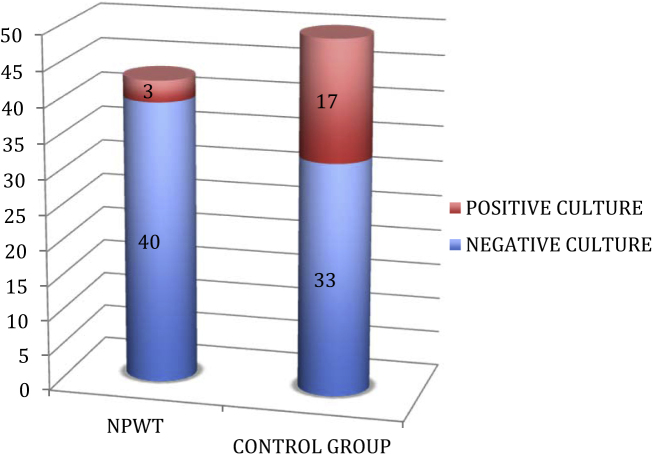
A total of 20 patients developed positive growth when samples were sent for culture with 3 (6.9%) in the NPWT group and 17 (34%) in the control group (p < 0.05). Only one (from control group) of the above patients had successive positive cultures while others had only a single quantitative positive culture. Only 5 patients (25%) went on the develop osteomyelitis (all five in the control group) (Fig. 2). This underlines the importance of repeated debridements in elimination of infection and also highlights the poor correlation between a wound swab culture and clinical infection. The organisms isolated in these 5 patients included 3 cases growing methicillin-resistant Staphylococcus aureus (MRSA), one case growing Pseudomonas aeruginosa and one showing a mixed growth of Escherichia coli and Acinetobacter.
Fig. 2.
Distribution of positive culture in the two groups.
4. Discussion
Open tibial fracture is a common problem faced by any centre in view of high chance of infections and other complications like non-union.8 The incidence of infection has been reported to be significantly higher than closed fractures in the tune of 16–66%.3, 17 The prognosis is especially worse in Grade III fractures.8
Negative pressure wound therapy is a novel advance in management of various wounds including those associated with open fractures. The basic principle of working of this device is use of subatmospheric pressure (i.e. pressure below the ambient level) in the tune of 125 mmHg in intermittent or continuous manner.18, 19, 20, 30 This is associated with increased angiogenesis, decreased oedema and rapid formation of granulation tissue.9, 21, 22, 20 This is due to narrowing of endothelial spaces with restoration of integrity of capillary basement membranes and increasing number and diameter of the capillaries that are formed.23 However, the effect of vacuum-assisted closure on reduction and prevention of infection has not been established conclusively.20 A study undertaken by Moues et al. showed contrasting results with bacterial loads of gram-negative bacilli being lower and gram-positive cocci being higher in wounds treated with NPWT.24 However, our study showed that up to 34% of control wounds showed bacterial colonization while only 6.9% of wounds on NPWT gave a positive culture. This difference being statistically significant (p < 0.05) underlines the fact that NPWT indeed has a role in reducing bacterial load in a wound. Various studies and series have evaluated the use of NPWT on infected wounds although infection being a relative contraindication for the use of NPWT.13, 16, 25
Open tibial fractures present with a common and vexing problem in view of large wounds and poor ability of the wounds to heal.26 This study is the first prospective study that attempts to investigate the role of negative pressure therapy in reducing infection and rate of osteomyelitis in acute open tibial fractures. Contrary to our assumption, the time required for the wounds to be ready for closure or coverage was similar both groups (8.3 days versus 9.8 days) (p > 0.05). However, the rate of infection was significantly lower (4.6%) in the group receiving NPWT compared to the control group (22%). This difference is significant and establishes the role of NPWT in reducing the incidence of infection. A small prospective study involving 59 patients with open fractures found a deep infection rate of 28% in control group and 5.4% in the group receiving NPWT and serial debridements.27 A retrospective study by Dedmond et al. showed contrasting results with 20% deep infection rate. The only benefit according to this study was reduced time before a flap/grafting procedure could be carried out.28 There are no other studies evaluating NPWT in open diaphyseal tibial fractures.
Of the 11 patients who developed osteomyelitis, seven were smokers whilst three were known diabetics. This underlines the role of smoking and comorbidities like diabetes in delayed healing. Seven patients had Grade IIIB open fractures of which 3 presented more than 48 h after injury without receiving any prophylactic antibiotic in the interim period. This also could be a cause of infection in these cases. We also believe that severity of the initial trauma with extensive communition and periosteal stripping (Grade IIIB) predisposes to infection. Only 5 patients out of 11 showed a positive culture during the course of treatment. However, a total 20 patients had positive quantitative cultures. This highlights poor correlation between a negative culture and lack of infection, thus endorsing poor sensitivity and specificity of this investigation.31, 32
Being a prospective randomized study, various confounding factors associated with wound healing like the age of patients, smoking, diabetes and poor levels of serum albumin were equitably distributed in both groups. We presume other confounding factors not accounted for were equally distributed considering the fact that randomization was done. This definitely confers additional credibility to the study. A possible drawback of the study was non-inclusion of wounds that dehisced after primary closure. Also, another pitfall is the surgeon's judgement to deem a wound for primary closure or subject it to further debridements. We have not studied the effect of NPWT on wound after it was primarily closed. A recent study showed increased blood flow under intact skin after application of NPWT.29
5. Conclusions
We conclude that negative pressure wound therapy is indeed beneficial for preventing the incidence of both acute and deep infections or osteomyelitis in open fractures (4.6%) against a control group (22%) (p < 0.05). It is also associated with lesser bacterial colonization (6.9% against 34%) of wounds compared to control (p < 0.05). The probability of an infection with NPWT for a wound with open fracture was 5.5 times less. However, a significant difference was not seen in the time required for the wound to be ready for delayed primary closure or coverage.
Conflicts of interest
The authors have none to declare.
Contributor Information
Siddharth R. Virani, Email: siddharthvirani@gmail.com.
Aditya A. Dahapute, Email: adityahapute1986@gmail.com.
References
- 1.Gustilo R.B., Anderson J.T. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Jt Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 2.Turen C.H., DiStasio A.J. Treatment of grade IIIB and grade IIIC open tibial fractures. Orthop Clin N Am. 1994;25:561–571. [PubMed] [Google Scholar]
- 3.Jacob E., Erpelding J.M. A retrospective analysis of open fractures sustained by U.S. military personnel during operation just cause. Mil Med. 1992;157:552–556. [PubMed] [Google Scholar]
- 4.Court-Brown C.M. Reamed intramedullary tibial nailing. An overview and analysis of 1106 cases. J Orthop Trauma. 2004;18:96–101. doi: 10.1097/00005131-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Harley B.J., Beaupre L.A., Jones C.A. The effect of time to definitive treatment on the rate of nonunion and infection in open fractures. J Orthop Trauma. 2002;16:484–490. doi: 10.1097/00005131-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Khatod M., Botte M.J., Hoyt D.B. Outcomes in open tibia fractures: relationship between delay in treatment and infection. J Trauma. 2003;55:949–954. doi: 10.1097/01.TA.0000092685.80435.63. [DOI] [PubMed] [Google Scholar]
- 7.Schandelmaier P., Krettek C., Rudolf J. Superior results of tibial rodding versus external fixation in grade 3B fractures. Clin Orthop Relat Res. 1997;342:164–172. [PubMed] [Google Scholar]
- 8.Hoogendoorn J.M., van der Werken C. Grade III open tibial fractures. Functional outcome with quality of life in amputees versus patients with successful reconstruction. Injury. 2001;32:329–334. doi: 10.1016/s0020-1383(00)00250-3. [DOI] [PubMed] [Google Scholar]
- 9.Argenta L.C., Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–577. [PubMed] [Google Scholar]
- 10.DeFranzo A.J., Argenta L.C., Marks M.W. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg. 2001;108:1184–1191. doi: 10.1097/00006534-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 11.deLange M.Y., Schasfoort R.A., Obdeijn M.C. Vacuum-assisted closure: indications and clinical experience. Eur J Plast Surg. 2000;23:178–182. [Google Scholar]
- 12.Deva A.K., Buckland G.H., Fisher E. Topical negative pressure in wound management. Med J Aust. 2000;173:128–131. doi: 10.5694/j.1326-5377.2000.tb125564.x. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson R., Johnsson P., Algotsson L. Vacuum-assisted closure therapy guided by C-reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg. 2002;123:210–215. doi: 10.1067/mtc.2002.121306. [DOI] [PubMed] [Google Scholar]
- 14.Joseph E., Hamori C.A., Bergman S. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic nonhealing wounds. Wounds. 2000;12:60–67. [Google Scholar]
- 15.Meara J.G., Guo L., Smith J.D. Vacuum-assisted closure in the treatment of degloving injuries. Ann Plast Surg. 1999;42:589–594. doi: 10.1097/00000637-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Wongworawat M.D., Schnall S.B., Holtom P.D. Negative pressure dressings as an alternative technique for the treatment of infected wounds. Clin Orthop Relat Res. 2003;414:45–48. doi: 10.1097/01.blo.0000084400.53464.02. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama K., Itoman M., Shindo M. Contributing factors influencing type III open tibial fractures. J Trauma. 1995;38:788–793. doi: 10.1097/00005373-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Fabian T.S., Kaufman H.J., Lett E.D. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg. 2000;66:1136–1143. [PubMed] [Google Scholar]
- 19.Genecov D.G., Schneider A.M., Morykwas M.J. A controlled sub-atmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40:219–225. doi: 10.1097/00000637-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Morykwas M.J., Faler B.J., Pearce D.J. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547–551. doi: 10.1097/00000637-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Webb L.X. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10:303–311. doi: 10.5435/00124635-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Morykwas M.J., Argenta L.C., Shelton-Brown E.I., McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Chen S.Z., Li J., Li X.Y. Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg. 2005;28:211–217. doi: 10.1016/S1015-9584(09)60346-8. [DOI] [PubMed] [Google Scholar]
- 24.Moues C.M., Vos M.C., van den Bemd G.J. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 25.Mehbod A.A., Ogilvie J.W., Pinto M.R. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14–17. doi: 10.1097/01.bsd.0000133493.32503.d3. [DOI] [PubMed] [Google Scholar]
- 26.Prodromidis A.D., Charalambous C.P. The 6-hour rule for surgical debridement of open tibial fractures. A systematic review and meta-analysis of infection and non-union rates. J Orthop Trauma. 2016;(March) doi: 10.1097/BOT.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 27.Stannard J.P., Volgas D.A., Stewart R., McGwin G., Jr., Alonso J.E. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23(September (8)):552–557. doi: 10.1097/BOT.0b013e3181a2e2b6. [DOI] [PubMed] [Google Scholar]
- 28.Dedmond B.T., Kortesis B., Punger K. The use of negative-pressure wound therapy (NPWT) in the temporary treatment of soft-tissue injuries associated with high-energy open tibial shaft fractures. J Orthop Trauma. 2007;21:11–17. doi: 10.1097/BOT.0b013e31802cbc54. [DOI] [PubMed] [Google Scholar]
- 29.Timmers M.S., Le Cessie S., Banwell P. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665–671. doi: 10.1097/01.sap.0000187182.90907.3d. [DOI] [PubMed] [Google Scholar]
- 30.Greer S., Kasabian A., Thorne C. The use of a subatmospheric pressure dressing to salvage a Gustilo grade IIIB open tibial fracture with concomitant osteomyelitis to avert a free flap. Ann Plast Surg. 1998;41:687. doi: 10.1097/00000637-199812000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Zuluaga A.F., Galvis W., Jaimes F., Vesga O. Lack of microbiological concordance between bone and non-bone specimens in chronic osteomyelitis: an observational study. BMC Infect Dis. 2002;2(May):8. doi: 10.1186/1471-2334-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard L., Uçkay I., Vuagnat A. Two consecutive deep sinus tract cultures predict the pathogen of osteomyelitis. Int J Infect Dis. 2010;14(May (5)):e390–e393. doi: 10.1016/j.ijid.2009.06.019. [DOI] [PubMed] [Google Scholar]