Abstract
Hemorrhagic shock (HS) is the major cause of death during trauma. Mortality due to HS is about 50%. Dysfunction of hematopoietic progenitor cells (HPCs) has been observed during severe trauma and HS. HS induces the elevation of cytokines, granulocyte-colony stimulating factor (G-CSF), peripheral blood HPCs, and circulating catecholamines, and decreases the expression of erythropoietin receptor connected with suppression of HPCs. Impaired HPCs may lead to persistent anemia and risk of susceptibility to infection, sepsis, and MOF. There is a need to reactivate impaired HPCs during trauma hemorrhagic shock.
Keywords: Hematopoietic progenitor cells, Trauma hemorrhagic shock, Cytokine, Granulocyte-colony stimulating factor, Erythropoietin
1. Introduction
Trauma is the third leading cause of death and disability in all age groups. Globally, per year, 6 million people die due to trauma. Trauma accounts for 12% global burden of disease, of which 90% occur in low- and middle-income countries. Trauma accounts for 20% of global burden of death.1, 2 1,69,000 people die as a result of injury each year. 50% of deaths that are due to trauma occur in the first 24 h of admission.3
After trauma, hemorrhagic shock (HS) is the next leading cause of death. Mortality due to HS is approximately 50%. Concerns related to fluid, blood component, and control of hemorrhage has been the cornerstone of HS management since many decades. Resuscitation with fluids and blood products induces reperfusion injury due to the production of reactive oxygen species and activation of immune cells.4
HS induces excessive production of inflammatory cytokines that leads to systemic inflammatory response syndrome (SIRS), sepsis, and multiorgan failure (MOF) following severe trauma and HS. It contributes to tissue damage. Elevated cytokines, which also causes hematopoietic progenitor cells (HPCs) apoptosis, leads to multiorgan failure (MOF), following severe injuries and HS in human and animal models.5, 6
HPCs apoptosis may lead to persistent anemia and risk of susceptibility to infection and sepsis.5, 6 Dysfunction of HPCs is a multifactorial process. Elevated inflammatory cytokines, G-CSF, circulating catecholamines, and peripheral blood HPCs are associated with HPCs dysfunction in T/HS.7, 8, 9 Therefore, the purpose of this review was to understand the mechanism behind the hematopoietic dysfunction following T/HS.
2. Cytokine and impaired hematopoietic progenitor cells
HS induces inflammation that leads to changes in active cytokine milieu. Pro- and anti-inflammatory cytokines (TNF-α, IL-6, IL-10, IL-8) and MCP-1 are thought to play important roles in immune dysfunction resulting in multiorgan failure (MOF) and death.7 Cytokines are proteins that are important in cell signaling. It is produced by innate and adaptive immune systems and they act as effectors or immune modulators of inflammatory response, which in turn play prominent roles in the development of sepsis or MOF. An imbalance between the early SIRS and the later compensatory anti-inflammatory response (CARS) may be responsible for increased susceptibility to infections, sepsis, and MOF.7, 10
Elevated cytokines also causes hematopoietic progenitor cells apoptosis. A recent study reported that elevation of TNF-α and IL-6 were directly associated with suppression of HPCs in patients with T/HS.11 Excessive production of cytokines leads to an imbalance in erythropoiesis following severe trauma. Elevated levels of TNF-α result in its binding to the receptor on bone marrow, which activates caspase-8 leading to apoptosis during severe trauma (Fig. 1),6 but there are more pathways leading to impaired erythropoiesis.12, 13 Maturation of erythroid progenitor cells was inhibited by IL-1, IL-6, IL-8, and transforming growth factor (TGF)-β during severe trauma.5, 6 Selleri et al. showed that TNF-α and IFN-γ cytokines are associated with HPCs apoptosis. Suppressive effects were observed in cultures supplemented with the combination of both cytokines than in cultures treated with IFN-γ or TNF-α alone.14
Fig. 1.
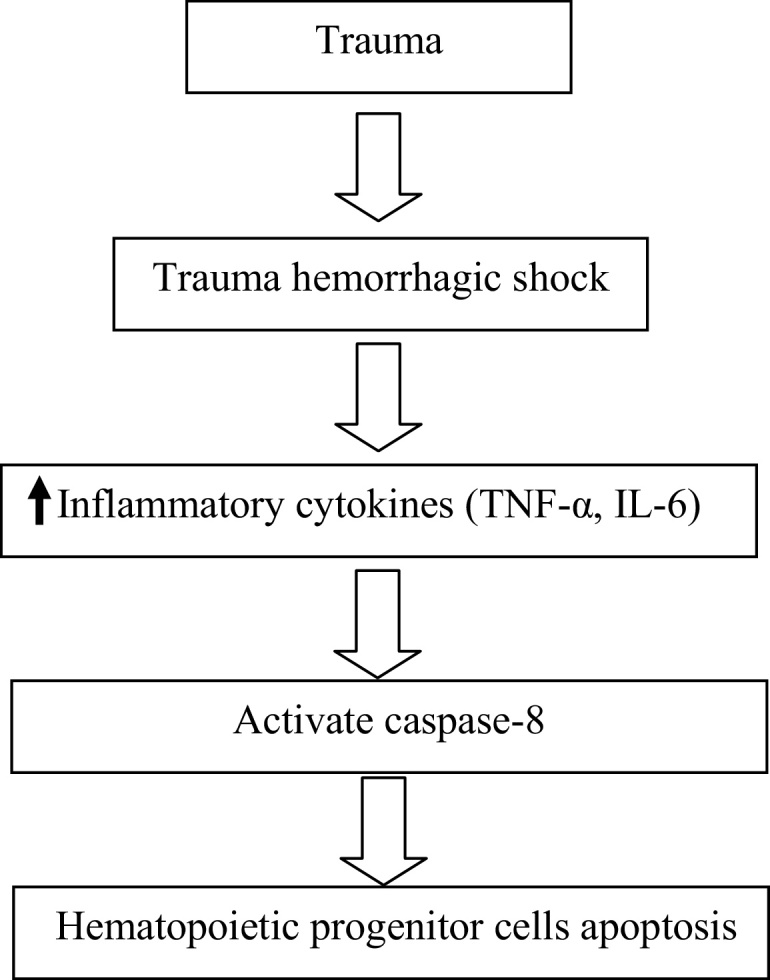
Overview of impaired hematopoietic progenitor cells by inflammatory cytokines.
3. Mobilization of hematopoietic progenitor cells
Peripheral blood HPCs were increased in T/HS when compared with the healthy volunteer. In subgroup analysis of T/HS patients, peripheral blood HPCs were elevated among nonsurvivors when compared to survivors.8 Badami et al. reported that in severe injury and HS, mobilization of HPCs from BM to peripheral blood is associated with BM dysfunction.15 Livingston et al. showed that when peripheral blood HPCs were grown in methylcellulose media, their number increased in severely injured patients in comparison to normal volunteers (15 ± 26 vs. 3 ± 1, <0.05).5 This study only recruited patients of the severe injury group and did not correlate with the outcomes. Previous studies indicated that mobilization of HPCs from BM to peripheral blood and then to injured or inflammatory tissue is beneficial for wound healing and maintaining immune response.16 Elevated peripheral blood HPCs lead to BM dysfunction following T/HS. It is also associated with poor outcomes.8
4. G-CSF and mobilization of hematopoietic progenitor cells
Recent studies showed that granulocyte-colony stimulating factor (G-CSF) was elevated in T/HS when compared with the healthy control.9 A similar result was observed in severe trauma and neutropenic patients and is the potent stimulator of hematopoietic mobilization.17, 18 Baranski et al. showed that peripheral blood HPCs and plasma levels of G-CSF were increased in both HS and lung contusion hemorrhagic shock (LC/HS) patients.19 Elevated levels of G-CSF were reported in trauma, burns, and sepsis patients.20, 21, 22
5. Norepinephrine and hematopoietic progenitor cells
T/HS-induced stress condition increased circulatory levels of norepinephrine, promoting mobilization of HPCs from bone marrow.23 Excessive release of inflammatory cytokines leads to sustained elevation of catecholamine concentrations.23, 24 Therefore, trauma-induced hypercathecolamine that alters the regulation of CXCR4 and stromal cell-derived factor-1 (SDF-1) cause suppression of bone marrow HPCs and continued mobilization of HPCs into peripheral blood leading to persistent anemia (Fig. 2).23 A recent study reported that SDF-1 maintains the HPCs connection between the bone marrow and the periphery.19 The role of SDF-1 is to provide HPCs from bone marrow to injured tissue. HPCs help in healing of injured tissues by differentiating within the healing tissues.6, 19, 23
Fig. 2.
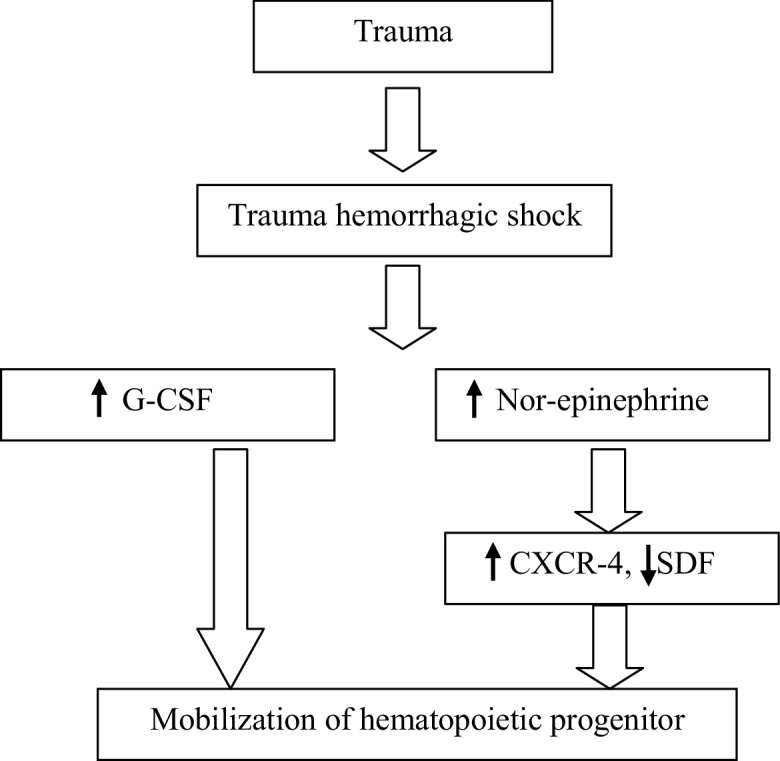
Overview of mobilization of hematopoietic progenitor cells.
6. Decreased expression of erythropoietin receptor
In our recent study, we observed elevated serum levels of EPO in T/HS patients when compared with control group. Elevated levels of EPO were not affected on reactivation of BM dysfunction. However, we measured the expression of BM-EpoR and found that EpoR expression was decreased in T/HS in comparison to control group. Modulation of BM-EpoR might result in bone marrow failure.25
Erythropoietin receptor (EpoR) may be one of the parameters of BM erythropoiesis. A previous study showed that blunted EPO leads to low hemoglobin concentrations, increase in inflammatory mediators, and a hypoferremic state, resulting in anemia during severe trauma.26 Recent evidence suggested that blunted EPO contributes to anemia in specific subsets of critically ill patients.27
EpoR is a protein that preexists as dimers.28 Upon binding by EPO (a 34 kDa ligand), EpoR changes its conformation to monomer state. EPO binds to an EpoR to regulate bone marrow erythroid cell proliferation, differentiation, and survival.28 Leist et al. suggested that EPO mediates tissue protection through a receptor (tissue-protective receptor) that is pharmacologically distinct from the classic EPO receptor that is known to mediate erythropoiesis.28 EpoR signaling prevents neuronal and erythroid progenitor cells apoptosis.29, 30
7. Hematopoietic stem cells in general
Hematopoietic stem cells (HSCs) are the blood cells derived from mesoderm. Previous studies have demonstrated that HSCs have regeneration capacities and are committed to multipotent, oligopotent, and unipotent progenitors. HSCs self-renewal is thought to occur in the stem cell niche. HPCs microenvironment is controlled by a complex interplay between intrinsic signals surrounded by BM microenvironment. Dysregulation of this balance can leads to BM dysfunction, leading to such hematopoietic and myeloproliferative disorders.31
8. Therapeutic strategies that may be used for reactivate bone marrow dysfunction
8.1. Propranolol
Bible et al. reported that administration of propranolol following lung contusion and lung contusion/hemorrhagic shock decreased mobilization of HPCs from the bone marrow and restored plasma G-CSF levels. Propranolol may be the future therapeutic target to reduce the bone marrow dysfunction following T/HS.32
8.2. EPO and growth factors
Studies have shown that erythropoietin (EPO) acts as an anti-apoptosis, neuroprotective, anti-inflammatory, and angiogenetic hormone. It helps proliferation and differentiation of erythroid progenitor cells. A recent study indicated that pretreatment with EPO, 3 days prior to induction of HS, protects against renal dysfunction, and liver and neuromuscular injuries, when compared with pretreatment with vehicle (placebo). In humans, tibiofibular fractures treatment with EPO helps to accelerate healing. EPO receptor is found on early burst forming unit-erythroid (BFU-E), as well as late erythroid progenitor cells [colony forming unit-erythroid (CFU-E)]; the first cells are recognizable as committed to erythroid differentiation and nonhematopoietic tissue, including central nervous system, endothelium, cardiac myocytes, kidney, and some solid cancer line. Previous studies have indicated that IL-3 and GM-CSF also promote proliferation and differentiation of HPCs.31
8.3. Mesenchymal stem cells (MSCs)
The recent studies demonstrated that treatment with MSCs after polytrauma reduces inflammation and promotes tissue regeneration. MSCs have therapeutic promises in numerous preclinical and clinical models of diseases, such as graft-versus-host disease, sepsis, hepatic failure, and acute renal failure. In previously published reports, BM-MSCs have been shown in preclinical and clinical use for tissue regeneration. Recently, more than 283 clinical trials are registered with MSCs (www.clinicaltrials.gov) and 45 studies for MSCs were done in December 2012, of which two were related to lung injury. Previous studies demonstrated that human MSCs maintain immunosuppressive microenvironment by secreting the cytokines and avoid allorecognition, and interfere with dendritic cell and T-cell function. Administration of a single dose of MSCs impaired wound healing via Treg cells after trauma in a rat model. Intravenously administered MSCs reduced pulmonary endothelial cell permeability and inflammation, and impaired wound healing in a rat model of HS and trauma. MSCs may have the therapeutic effect not only for the reactivation of BM dysfunction but also for acute lung injury and acute respiratory distress syndrome caused by T/HS in humans. Differentiation capacity of MSCs may be applied for the treatment of T/HS.33
8.4. Bone marrow mononuclear cells
Previous studies reported that bone marrow mononuclear cells (BMCs) have the capacity of self-renewal and tissue regeneration in nervous system and spinal cord injuries. Intravenous administration of BMCs protects against organ injury/dysfunction caused by severe HS via activation of signaling pathway and promotes immune reconstitution in the presence of acute graft-versus-host disease. BMCs modulate immune system, increase the growth of HPCs, reduce mobilization of HPCs into peripheral blood, and preserve bone marrow cellularity in injured tissue. Previous studies have shown that BMCs are immunologically safe. There is no ethical issue and they are easily isolated.34
8.5. Hematopoietic stem cells
HSCs transplantation has been used as an adjunct treatment of bone marrow failure, hemoglobinopathies, and immune system disorder. Li et al. showed that human hematopoietic stem/progenitor cells (HSPCs) promoted kidney repair and regeneration using an established ischemia reperfusion injury model in mice. Human CD34+ cells and HSPCs promoted vasculogenesis and osteogenesis in stroke and bone injury. HSCs transplantation may be explored as a therapeutic option for the hematopoietic failure in patients with trauma hemorrhagic shock.35
9. Conclusion
Hemorrhagic shock induces elevated inflammatory cytokines (TNF-α and IL-6), G-CSF, and mobilization of HPCs from bone marrow to peripheral blood leading to impairment of HPCs.
Impaired HPCs may be reversed with the treatment of propranolol, EPO, BM-MSCs, HSCs, and BMCs. Clinical trial should be designed with EPO, BM-MSCs, HSCs, and BMCs on patients with trauma hemorrhagic shock.
Conflicts of interest
The authors have none to declare.
References
- 1.Rastogi D., Meena S., Sharma V., Singh G.K. Causality of injury and outcome in patients admitted in a major trauma center in North India. Int J Crit Illn Inj Sci. 2014;4(4):298–302. doi: 10.4103/2229-5151.147523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsiao M., Malhotra A., Thakur J.S. Road traffic injury mortality and its mechanisms in India: nationally representative mortality survey of 1.1 million homes. BMJ Open. 2013;3(8):e002621. doi: 10.1136/bmjopen-2013-002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haagsma J.A., Graetz N., Bolliger I. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev. 2015 doi: 10.1136/injuryprev-2015-041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhoi S., Tiwari S., Kumar M. Estrogen: is it a new therapeutic paradigm for trauma hemorrhagic shock? Int J Crit Illn Inj Sci. 2016;6:53. doi: 10.4103/2229-5151.183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston D.H., Anjaria D., Wu J. Bone marrow failure following severe injury in human. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson Y., Hostmann A., Matenov A., Ertel W., Oberholzer A. Erythropoiesis in multiple injured patients. J Trauma. 2006;61:1285–1291. doi: 10.1097/01.ta.0000240969.13891.9b. [DOI] [PubMed] [Google Scholar]
- 7.Kumar M., Rao D.N., Mohanty S., Selvi A., Bhoi S. Interleukin (IL)-8 is an early predictor of mortality following trauma hemorrhagic shock. Int J Adv Res Biol Sci. 2015;2(7):12–20. [Google Scholar]
- 8.Kumar M., Bhoi S., Selvi A., Kamal V.K., Mohanty S., Rao D.N. Evaluation of circulating hematopoietic progenitor cells in patients with trauma hemorrhagic shock and its correlation with clinical outcome. Int J Crit Illn Inj Sci. 2016;6:56–60. doi: 10.4103/2229-5151.183016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar M., Bhoi S., Selvi A., Kamal V.K., Mohanty S., Rao D.N. Evaluation of serum granulocyte colony stimulating factor in patients admitted with trauma hemorrhagic shock. Int J Adv Res Biol Sci. 2015;2(7):107–114. [Google Scholar]
- 10.Roumen R.M., Hendriks T., Wevers R.A., Goris R.J.A. Intestinal permeability after severe trauma and hemorrhagic shock is increased, without relation to septic complications. Arch Surg. 1993;128:453–457. doi: 10.1001/archsurg.1993.01420160095016. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M., Rao D.N., Bhoi S. Tumour necrosis-α and interleukin-6 suppressed hematopoietic stem cell growth in trauma hemorrhagic shock. Shock. 2015;44(October (suppl 2)):20. [Google Scholar]
- 12.Trey J.E., Kushner I. The acute phase response and the hematopoietic system: the role of cytokines. Crit Rev Oncol Hematol. 1995;21:1–18. doi: 10.1016/1040-8428(94)00141-3. [DOI] [PubMed] [Google Scholar]
- 13.Schubert T., Echtenacher B., Hofstädter F., Männel D.N. TNF-independent development of transient anemia of chronic disease in a mouse model of protracted septic peritonitis. Lab Investig. 2003;83(12):1743–1750. doi: 10.1097/01.lab.0000101693.12149.2c. [DOI] [PubMed] [Google Scholar]
- 14.Selleri C., Sato T., Anderson S., Young N.S., Maciejewski J.P. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165(3):538–546. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 15.Badami C.D., Livingston D.H., Sifri Z.C. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 16.Malone D.L., Dunne J., Tracy J.K., Putnam A.T., Scalea T.M., Napolitano L.M. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 17.Thomas J., Liu F., Link D.C. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr Opin Hematol. 2002;9:183–189. doi: 10.1097/00062752-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Cook K.M., Sifri Z.C., Baranski G.M., Mohr A.M., Livingston D.H. The role of plasma granulocyte colony stimulating factor and bone marrow dysfunction after severe trauma. J Am Coll Surg. 2013;216(1):57–64. doi: 10.1016/j.jamcollsurg.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baranski G.M., Offin M.D., Sifri Z.C. β-Blockade protection of bone marrow following trauma: the role of G-CSF. J Surg Res. 2011;170:325–331. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka H., Ishikawa K., Nishino M., Shimazu T., Yoshioka T. Changes in granulocyte colony stimulating factor concentration in patients with trauma and sepsis. J Trauma. 1996;40:718–725. doi: 10.1097/00005373-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Presneill J.J., Waring P.M., Layton J.E. Plasma granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor levels in critical illness including sepsis and septic shock: relation to disease severity, multiple organ dysfunction, and mortality. Crit Care Med. 2000;28:2344–2354. doi: 10.1097/00003246-200007000-00028. [DOI] [PubMed] [Google Scholar]
- 22.Finnerty C.C., Herndon D.N., Chinkes D.L., Jeschke M.G. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca R.B., Mohr A.M., Wang L., Sifri Z.C., Rameshwar P., Livingston D.H. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–890. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 24.Robinson Y., Matenov A., Tschöke S.K. Impaired erythropoiesis after haemorrhagic shock in mice is associated with erythroid progenitor apoptosis in vivo. Acta Anaesthesiol Scand. 2008;52(5):605–613. doi: 10.1111/j.1399-6576.2008.01656.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar M., Bhoi S., Kamal V.K., Mohanty S., Rao D.N., Galwankar S. Evaluation of bone marrow erythropoietin receptor in trauma hemorrhagic shock. Int J Adv Res Biol Sci. 2015;2(8):43–49. [Google Scholar]
- 26.Hobisch-Hagen P., Wiedermann F., Mayr A. Blunted erythropoietic response to anemia in multiply traumatized patients. Crit Care Med. 2001;29:743–747. doi: 10.1097/00003246-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 27.DeAngelo A.J., Bell D.G., Quinn M.W., Long D.E., Ouellette D.R. Erythropoietin response in critically ill mechanically ventilated patients: a prospective observational study. Crit Care. 2005;9:R172–R176. doi: 10.1186/cc3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leist M., Ghezzi P., Grasso G. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 29.Morishita E., Masuda S., Nagao M., Yasuda Y., Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 30.Koury M.J., Bondurant M.C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 31.Kumar M., Bhoi S. Does erythropoietin reactivate bone marrow dysfunction in trauma hemorrhagic shock? Int J Crit Illn Inj Sci. 2015;5:230–231. doi: 10.4103/2229-5151.170848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bible L.E., Pasupuleti L.V., Gore A.V., Sifri Z.C., Kannan K.B., Mohr A.M. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2015;158(3):595–601. doi: 10.1016/j.surg.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar M., Bhoi S. Mesenchymal stem cells: can it be used for the treatment of trauma hemorrhagic shock? Int J Stud Res. 2015;5:15–16. [Google Scholar]
- 34.Kumar M., Bhoi S. Do bone marrow mononuclear cells can be used as a therapeutic target for trauma hemorrhagic shock? Int J Med Sci Res Pract. 2015;2(3):1. [Google Scholar]
- 35.Kumar M., Bhoi S. Hematopoietic stem cells: can it be therapeutic option for the hematopoietic failure in patients with trauma hemorrhagic shock? J Emerg Med Trauma Shock. 2016;9:51–52. doi: 10.4103/0974-2700.179458. [DOI] [PMC free article] [PubMed] [Google Scholar]


