Summary
The development of human erythroid cells has been mostly examined in models of adult hematopoiesis, while their early derivation during embryonic and fetal stages is largely unknown. We observed the development and maturation of erythroblasts derived from human pluripotent stem cells (hPSCs) by an efficient co-culture system. These hPSC-derived early erythroblasts initially showed definitive characteristics with a glycophorin A+ (GPA+) CD34lowCD36− phenotype and were distinct from adult CD34+ cell-derived ones. After losing CD34 expression, early GPA+CD36− erythroblasts matured into GPA+CD36low/+ stage as the latter expressed higher levels of β-globin along with a gradual loss of mesodermal and endothelial properties, and terminally suppressed CD36. We establish a unique in vitro model to trace the early development of hPSC-derived erythroblasts by serial expression of CD34, GPA, and CD36. Our findings may provide insight into the understanding of human early erythropoiesis and, ultimately, therapeutic potential.
Keywords: hESC, hiPSC, hematopoiesis, development, erythroblasts, erythropoiesis, endothelial cells, GPA, CD36, CD34
Highlights
-
•
The hPSC/AGM-S3 co-culture system generates considerable definitive erythroblasts
-
•
hPSC-derived erythroblasts initiate from a unique GPA+CD34lowCD36− fraction
-
•
Human early erythropoiesis can be traced by serial expression of CD34, GPA, and CD36
In this article, Ma and colleagues show that considerable definitive erythroblasts could be generated from hPSCs by co-culturing with AGM-S3 cells. Tracing through serial expression of CD34, GPA, and CD36 on hPSC-derived erythroblasts revealed that they arose from a unique GPA+CD34lowCD36− cell fraction sharing both erythroid and mesodermal endothelium characteristics.
Introduction
Erythroblasts are one of the first blood cells to be generated during embryonic hematopoiesis (Palis and Yoder, 2001). Investigating the early erythropoiesis is helpful in comprehending the occurrence and regulation of early embryonic hematopoiesis. Research in early erythropoiesis is largely performed on murine models (Keller et al., 1999, Palis et al., 1999). The lack of proper in vitro and in vivo systems hampers the observation of this process during human embryonic/fetal development. The isolation of human embryonic stem cells (hESCs) (Thomson et al., 1998) and the establishment of human induced pluripotent stem cells (hiPSCs) (Takahashi et al., 2007) have provided excellent tools to investigate early events in human hematopoiesis. Large-scale production of hPSC-derived erythroblasts can be achieved in vitro by mimicking in vivo erythropoiesis (Lu et al., 2008, Ma et al., 2008). Previously, we found that erythroblasts were derived from hESC/mouse fetal liver stromal cell (mFLSC) co-culture and matured continuously (Ma et al., 2008). In this study, we have modified this method by co-culturing hPSCs with aorta-gonad-mesonephros (AGM)-S3 cells (Xu et al., 1998), which were obtained from the earliest definitive hematopoiesis-supporting region (Matsuoka et al., 2001), to study human early erythropoiesis.
The maturation of human erythroblasts from adult CD34+ hematopoietic stem/progenitor cells (HSPCs) can be assessed by serially tracing the expression of erythroid lineage markers. These markers for adult-type erythroblasts' developmental process are widely used to recognize hPSC-derived erythroblasts, along with analysis of their functional properties. However, there is no detailed information about how erythroid cells originate from hPSC-derived progenitors and which phenotypes they share during the early developmental stages, hindering the elucidation of step-by-step mechanisms that control human early erythropoiesis from hPSCs.
Phenotypically, adult-type hematopoietic stem cell (HSC)-derived erythroblasts mature by losing CD34 and then gaining glycophorin A (GPA; also known as CD235a) on their surface. CD36 co-expresses with GPA when cells are committed to erythroid lineage. Expression of CD36 is then gradually downregulated during the terminal maturation stage on enucleated red blood cells (RBCs) (Okumura et al., 1992, Neildez-Nguyen et al., 2002, Fajtova et al., 2013, Li et al., 2014). However, in this study we demonstrated that the expression process of CD34, GPA, and CD36 on hPSC/AGM-S3 co-culture-derived early erythroblasts was distinct. These hPSC-derived early erythroblasts were generated from endothelium precursors, underwent maturation by losing mesodermal and endothelial properties, and gradually gained definitive erythropoietic potential. Our results suggest that a unique pathway for early definitive erythropoiesis from hPSCs is phenotypically different from that of adult HSPC-derived definitive erythropoiesis.
Results
Generation of Erythroblasts from hPSC/AGM-S3 Co-culture
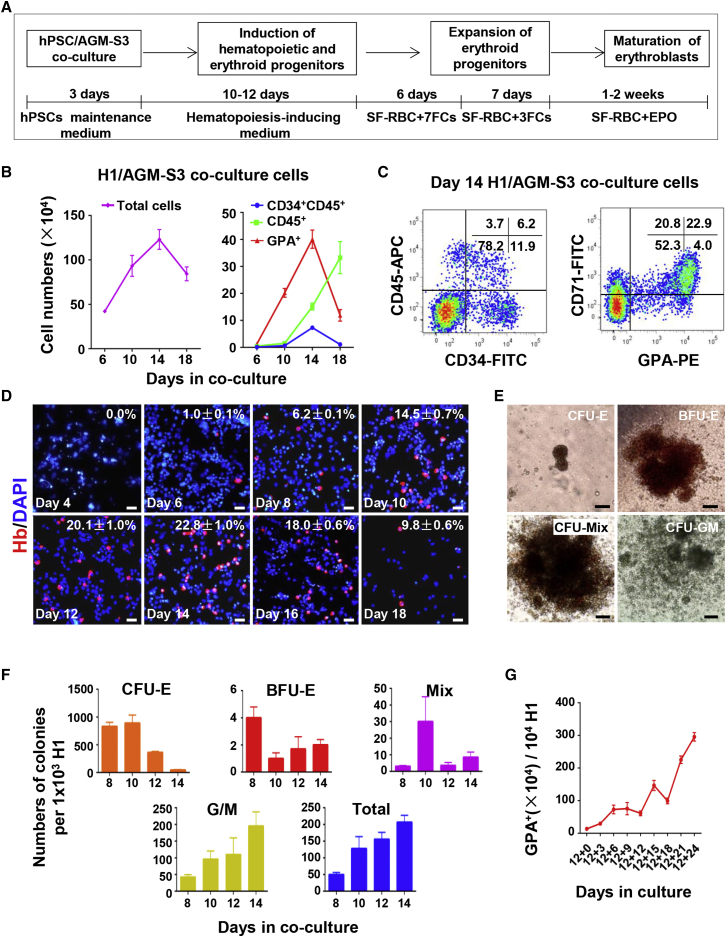
We have established an efficient culture system to generate considerable numbers of erythroblasts from hPSCs by co-culture with AGM-S3 cells (Figure 1A). Hematopoiesis in hPSC/AGM-S3 co-culture was similar to that in hESC/mFLSC co-culture, as we reported previously (Ma et al., 2008). In hPSC/AGM-S3 co-culture the total cell number gradually increased, peaked at day 14, and decreased thereafter (Figure 1B). Fluorescence-activated cell sorting (FACS) analysis at various time points revealed that the hematopoietic cells had been generated from hESC/AGM-S3 co-culture. About 7.3 ± 0.58 × 104 CD34+CD45+ cells, 1.5 ± 0.13 × 105 CD45+ cells, and 4.01 ± 0.34 × 105 GPA+ cells were generated on day 14 from 1 × 104 H1 cells (Figures 1B and 1C). GPA is an accepted surface maker for adult erythroid cells. We examined further the expression of human hemoglobin in co-culture over time by immunofluorescence (IF) staining analysis. Human hemoglobin-expressing cells were firstly observed in day-6 co-cultures. The number of these cells peaked on day 14 and decreased thereafter (Figure 1D).
Figure 1.
Generation of Erythroid Cells Derived from hPSC/AGM-S3 Co-culture
(A) Schematic showing the strategy for generation of erythroid cells from hPSCs via co-culture with AGM-S3 cells.
(B) Cells were digested with 0.25% trypsin/EDTA. The figure shows numbers of total cells, HSPCs (CD34+CD45+), hematopoietic cells (CD45+), and GPA+ cells derived from H1 cells in co-culture over time (independent experiments, n = 3; mean ± SD). There were 1 × 104 H1 cells initially. The total cell numbers exclude the numbers of AGM-S3 cells in co-culture.
(C) Representative flow cytometry data showing co-expression of CD34/CD45 and GPA/CD71 in day-14 co-culture. CD34 and CD45 are important hematopoietic markers while GPA and CD71 are markers of erythroid cells.
(D) Immunofluorescence (IF) analysis showing human hemoglobin expression in H1/AGM-S3 co-cultured cells obtained every other day from day 4 to day 18 (independent experiments, n = 3; mean ± SD). Scale bars, 50 μm.
(E) Typical morphology of hematopoietic colonies was presented. Total day-12 H1/AGM-S3 co-cultured cells were recultured in semi-solid culture. Scale bars, 100 μm.
(F) Total H1/AGM-S3 co-cultured cells from day 8 to day 14 were recultured in semi-solid culture. Numbers of colonies derived from 1 × 103 H1 cells initially (independent experiments, n = 3; mean ± SD). Total colony numbers include the numbers of all colonies except CFU-E colonies.
(G) Proliferation of GPA+ cells in suspension culture. There were 1 × 104 H1 cells initially. Total day-12 H1/AGM-S3 co-cultured cells were resuspended in RBC differentiation medium for further expansion and maturation of erythroid cells (independent experiments, n = 3; mean ± SD).
See also Figure S1.
To investigate the efficiency of erythroblasts generated in co-culture at different time points, we performed a hematopoietic colony-forming cell assay. Considerable numbers of erythroid colonies (CFU-E [colony-forming unit-erythroid], BFU-E [burst-forming unit-erythroid], and CFU-Mix [CFU mixed]) along with myeloid colonies (CFU-GM [CFU- granulocyte/macrophage]) were generated from co-cultured cells. Most erythroid progenitors and CFU-GM progenitors were detected in co-cultures at days 8–10 and day 14, respectively (Figures 1E and 1F). This is in accordance with the fact that erythroblasts originate earlier than other myeloid cells in human ontogenesis (Palis and Yoder, 2001).
The proliferation of erythroblasts determined by IF staining analysis was consistent with flow cytometry data (Figures 1B and 1D). The data also suggested that the co-culture system cannot support the development of erythroblasts continuously. For further expansion and maturation of erythroblasts, co-cultures at days 10–12 were resuspended in optimized erythroblast differentiation medium. After 24 days of suspension culture, initial 1 × 104 H1 cells yielded more than 3 × 106 GPA+ erythroblasts (purity >95%) (Figure 1G). A high percentage of these GPA+ erythroblasts at day 24 in suspension culture expressed adult-type β-globin (84.9% ± 3.1%), proving that they were of definitive origin, most of which co-expressed γ-globin (98.5% ± 1.8%) and ɛ-globin (77.6% ± 5.5%), showing that they still retained embryonic/fetal erythroblast features (Figure S1).
Thus, hPSC/AGM-S3 co-culture system for the efficient production of hPSC-derived erythroblasts has been established, which clearly originated via a definitive pathway of early hematopoiesis (with a high percentage of cells expressing β-globin). Notably, except for a low concentration of vascular endothelial growth factor (VEGF), no additional factor specifically for erythroid development was added into co-culture, suggesting that the generation of erythroblasts from hPSCs is a natural event in the co-culture. This method provides a feasible tool with which to conduct subtle investigations of early erythropoiesis from hPSCs.
Expression of CD36 on hPSC-Derived GPA+ Cells
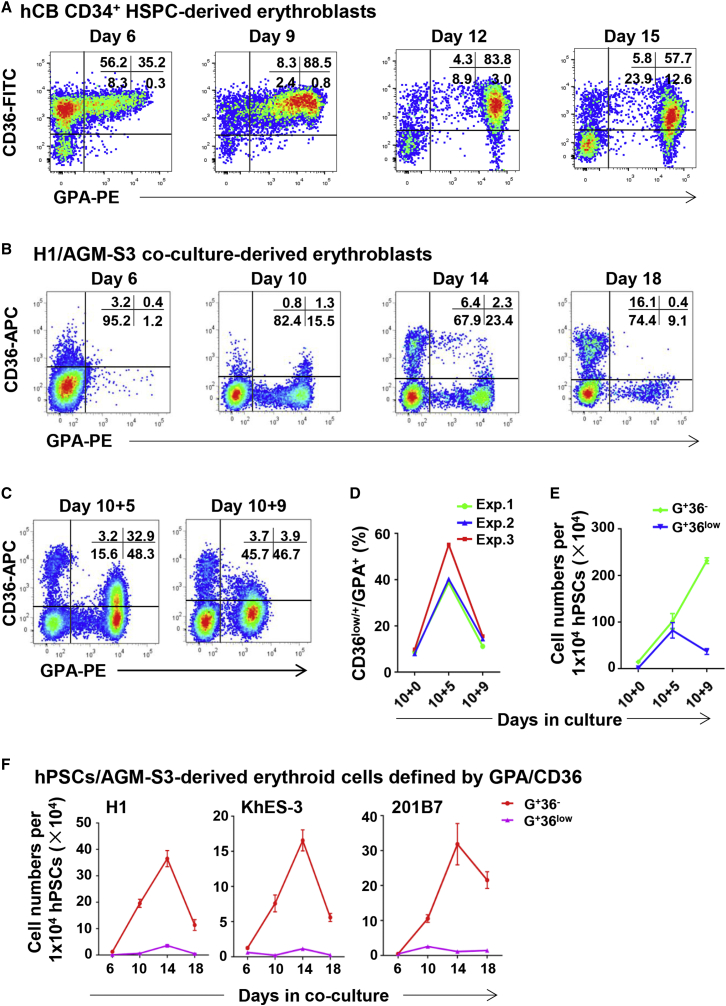
The surface markers of adult HSPC-derived erythroblasts have been well studied (Chen et al., 2009). Our flow cytometry data were consistent with previous reports that as human CD34+ HSPCs differentiate toward erythroblasts, the expression of CD34 disappeared exclusively before gaining expression of erythroid lineage markers such as GPA, CD71, CD36, and CD47 (Figures 2A and S2A). To elucidate the expression process of surface markers on hPSC-derived erythroblasts in detail, we detected erythroid-lineage-related markers established for adult-type erythroblasts, including GPA, CD71, CD47, CD81, EPO-R, CD117 (c-Kit), CD45, and CD34 (Figure S2B). Among these, the expression of CD36 on hESC-derived erythroblasts was obviously different from that on human cord blood (hCB)-CD34+ HSPC-derived erythroblasts (Figures 2A and 2B). Throughout the co-culture period, CD36 was only expressed at a low level on a small proportion of hPSC-derived GPA+ cells. In humans, CD36 is a determinant marker of committed erythroid progenitors that is expressed continuously on normal immature erythroblasts, and its expression decreases gradually with their maturation (Okumura et al., 1992). In our study, when the erythroblasts were expanded by replating day-10 co-cultured cells, the cell number of the GPA+CD36low (abbreviated as G+36low) cell fraction gradually increased and decreased again, similar to the CD36 expression process on hCB-CD34+ HSPC-derived erythroblasts (Figures 2C–2E).
Figure 2.
Expression Process of CD36 on Erythroid Cells of Different Origins
(A–C) Representative flow cytometry profiles showing expression of CD36 on GPA+ cells derived from hCB-CD34+ HSPCs (A), H1/AGM-S3 co-culture (B), and day-10 + 5 and 10 + 9 suspension cultures (C).
(D) The percentage of CD36low/+ cells among GPA+ cells at day 10, day 10 + 5, and day 10 + 9 cultures. Data are representative of three independent experiments.
(E) Proliferation of GPA+CD36low (G+36low) and GPA+CD36− (G+36−) cell fractions at day 10, 10 + 5, and 10 + 9 derived from 1 × 104 H1 cells (independent experiments, n = 3; mean ± SD).
(F) Proliferation of G+36low and G+36− cell fractions in co-culture derived from 1 × 104 H1, KhES-3, and 201B7 cells on different days (independent experiments, n = 3; mean ± SD).
See also Figure S2.
To further investigate the expression of CD36 during maturation of hPSC-derived erythroblasts, we recultured day-12 H1/AGM-S3 co-cultured cells were in semi-solid medium, which favors optimal colony formation (with the addition of six cytokines). After 14 days, seven BFU-E colonies (1–5 × 105 cells per colony) were randomly chosen and subjected to flow cytometry analysis individually. In each colony, the majority of GPA+ cells were CD36+ (90.3% ± 8.2%) (Figure S2C). Day-10 co-cultured cells were replated in suspension culture. The percentage and absolute number of G+36low/+ cells increased after 5 days (day 10 + 5) and gradually decreased after an additional 4 days (day 10 + 9) (Figures 2C–2E). These data imply that early erythroid progenitors derived from hPSC/AGM-S3 co-culture seldom expressed CD36. However, the CD36 expression was upregulated when co-culture-derived HSPCs developed to a mature stage, and was gradually downregulated when they matured further, similar to hCB-CD34+ HSPCs.
With the same efficiency as they were generated from the H1 cell line, erythroblasts could also be generated from other hPSC lines by co-culture with AGM-S3 cells (Figure 2F). CD36 was expressed at an extremely low level on KhES-3- and 201B7-derived GPA+ cells in co-culture, similar to its expression on H1/AGM-S3 co-culture-derived erythroblasts (Figure 2F). This result clearly demonstrates that low or negative expression of CD36 on early erythroblasts is a common phenomenon in early erythropoiesis from hPSCs.
H1/AGM-S3 Co-culture-Derived GPA+CD36low Erythroblasts Are More Mature than Early GPA+CD36− Erythroblasts
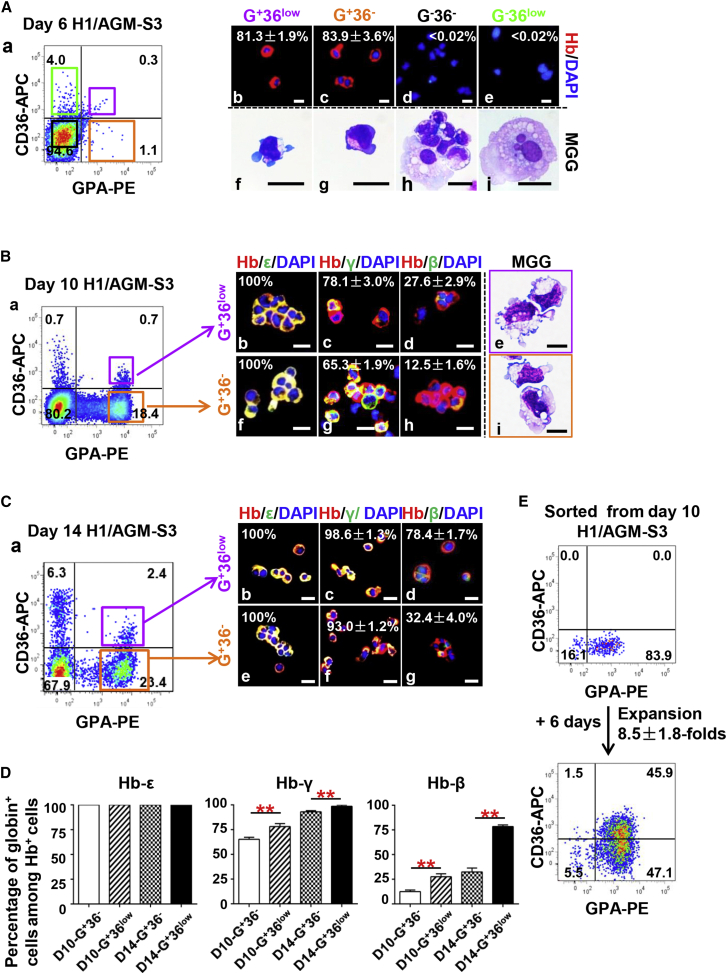
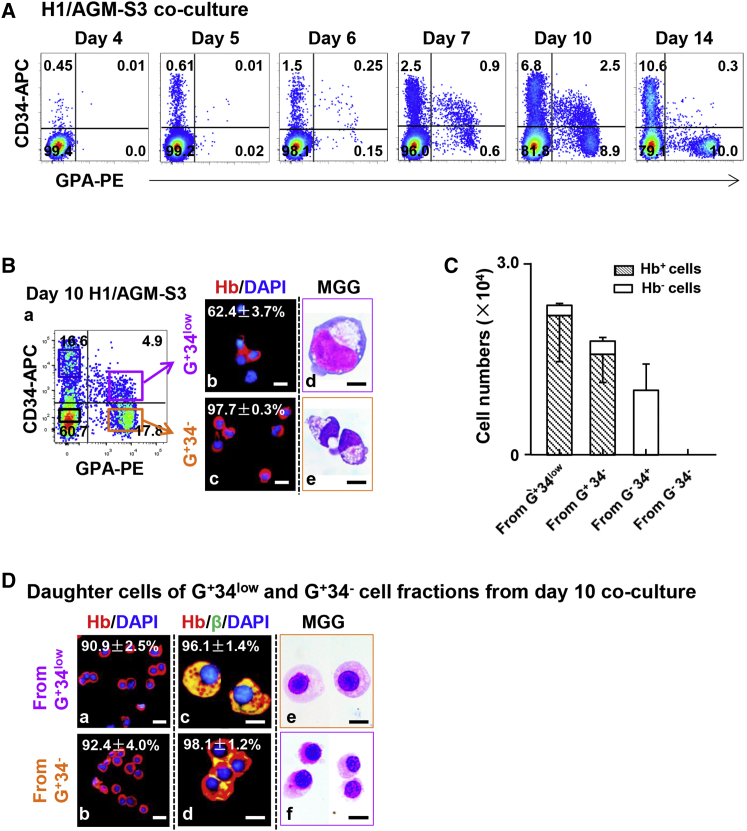
To clarify the developmental process of erythroblasts defined by their expression of GPA and CD36, we conducted further examination by cell sorting. As already noted, human hemoglobin-expressing cells were first observed in day-6 co-culture (Figure 1D). Thus, cells in day-6 co-culture were divided into four fractions by FACS according to their expression of GPA and CD36. May-Grünwald-Giemsa (MGG) and IF staining showed that more than 80% of GPA+CD36− cells (abbreviated as G+36− cells) and G+36low cells were erythroblasts with expression of human hemoglobin, while GPA− cells rarely expressed human hemoglobin (Figure 3A).
Figure 3.
Characteristics of GPA+CD36low and GPA+CD36− Erythroid Cell Fractions Sorted from H1/AGM-S3 Co-culture
(A) (a) Four cell fractions defined by expression of GPA and CD36 were sorted by FACS from day-6 H1/AGM-S3 co-culture. (b–e) IF analysis showing human hemoglobin expression in each cell fraction (independent experiments, n = 3; mean ± SD). Scale bars, 20 μm. (f–i) MGG staining showing typical morphology of each cell fraction. Scale bars, 25 μm.
(B) (a) G+36low and G+36− cell fractions were sorted by FACS from day-10 H1/AGM-S3 co-culture. IF analysis showing co-expression of human hemoglobin and ɛ-, γ-, and β-globins in G+36low (b–d) and G+36− (f–h) cell fractions (independent experiments, n = 3; mean ± SD). Scale bars, 20 μm. (e and i) MGG staining showing typical morphology of G+36low and G+36− cell fractions. Scale bars, 10 μm.
(C) (a) G+36low and G+36− cell fractions were sorted by FACS from day-14 H1/AGM-S3 co-culture. IF analysis showing co-expression of human hemoglobin and ɛ-, γ-, and β-globins in G+36low (b–d) and G+36− (e–g) cell fractions (independent experiments, n = 3; mean ± SD). Scale bars, 20 μm.
(D) The percentages of cells co-expressing hemoglobin and ɛ-, γ-, and β-globins among erythroid cell fractions (independent experiments, n = 3; mean ± SD; ∗∗p < 0.01).
(E) Flow cytometry profiles showing the purity of sorted G+36− cells from day-10 H1/AGM-S3 and expression of CD36 on their progeny after they were recultured in SF-RBC+7FCs medium for an additional 6 days. The total number of cells expanded 8.5 ± 1.8-fold (independent experiments, n = 3; mean ± SD).
The G+36− and G+36low cell fractions at day-10 and day-14 co-culture were sorted and examined. IF staining showed that 100% of G+36− and G+36low cells from day-10 and day-14 co-cultures expressed human hemoglobin, confirming that they were all erythroblasts (Figures 3B and 3C). From days 10 to 14, both G+36− and G+36low erythroblasts exhibited a significant increase in γ- and β-globin expression. Furthermore, the ratios of G+36low cells expressing γ- and β-globins were considerably higher than those of the G+36− fraction in both day-10 and day-14 co-culture (Figures 3B–3D). The hemoglobin composition assay showed that cells underwent further maturation gradually. Taken together, these data strongly indicated that hESC-derived erythroblasts matured over time and that the maturity of GPA+ cells could be associated with their expression of CD36.
Thus, we speculated that G+36low cells developed from G+36− cells. To verify this, we separated G+36− cells in day-10 H1/AGM-S3 co-culture by FACS and replated them in SF-RBC+7FCs medium (see Experimental Procedures). 6 days later (day 10 + 6), the cell number increased by 8.5 ± 1.8-fold, and almost half of these daughter cells expressed CD36 (Figure 3E). Furthermore, the sorted G+36+ cells from day 10 + 5 were recultured for a further 4 days (day 10 + 5+4) and then matured into G+36− cells (Figure S3A). This provides concrete evidence that hPSC/AGM-S3 co-culture-derived erythroblasts were initially G+36−, developed into a more mature CD36low/+ stage, and finally reached a stage at which all cells were an upregulation of β-globin along with downregulated expression of CD36.
Early GPA+CD36− Erythroblasts Co-express Endothelial and Early Hematopoiesis-Related Markers at High Levels
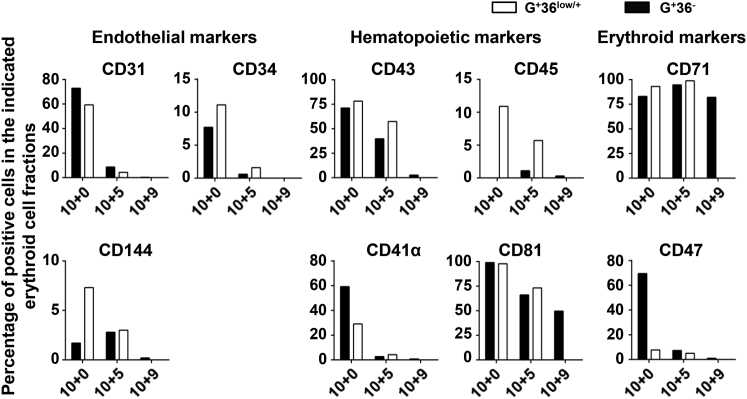
We also detected other endothelial/hematopoietic markers that defined early hematopoiesis from hPSCs, such as CD31, CD144, CD34, CD43, CD41α, CD45, and CD81, as reported previously (Nakayama et al., 2000, Vodyanik et al., 2006). These markers were detected on different erythroid populations classified according to CD36 expression from co-cultures of day 10, and suspension cultures of day 10 + 5 and day 10 + 9 (Figure 4). The majority of hPSC-derived erythroblasts of day-10 co-cultures co-expressed endothelial marker CD31 at a high level (72.9% for G+36− cells and 59.6% for G+36low cells), more than half of which co-expressed definitive hematopoiesis markers CD43 and CD41α. Along with progressive maturation, these erythroblasts exhibited downregulation of CD43 and CD41α associated with the loss of endothelial markers. These findings indicate that early hPSC-derived erythroblasts experienced a unique pathway that was generated from precursors sharing endothelial differentiation potential.
Figure 4.
Phenotypic Expressions of Indicated Erythroid Cell Fractions Defined by GPA and CD36
Flow cytometry analysis showing representative phenotypic expression of erythroblast fractions G+36low/+ and G+36− at day 10 and day 10 + 5 as well as G+ 36− cells at day 10 + 9 during a single culture.
Gene Expression Profiles of hESC-Derived Erythroblasts Defined by Expression of CD36 and GPA
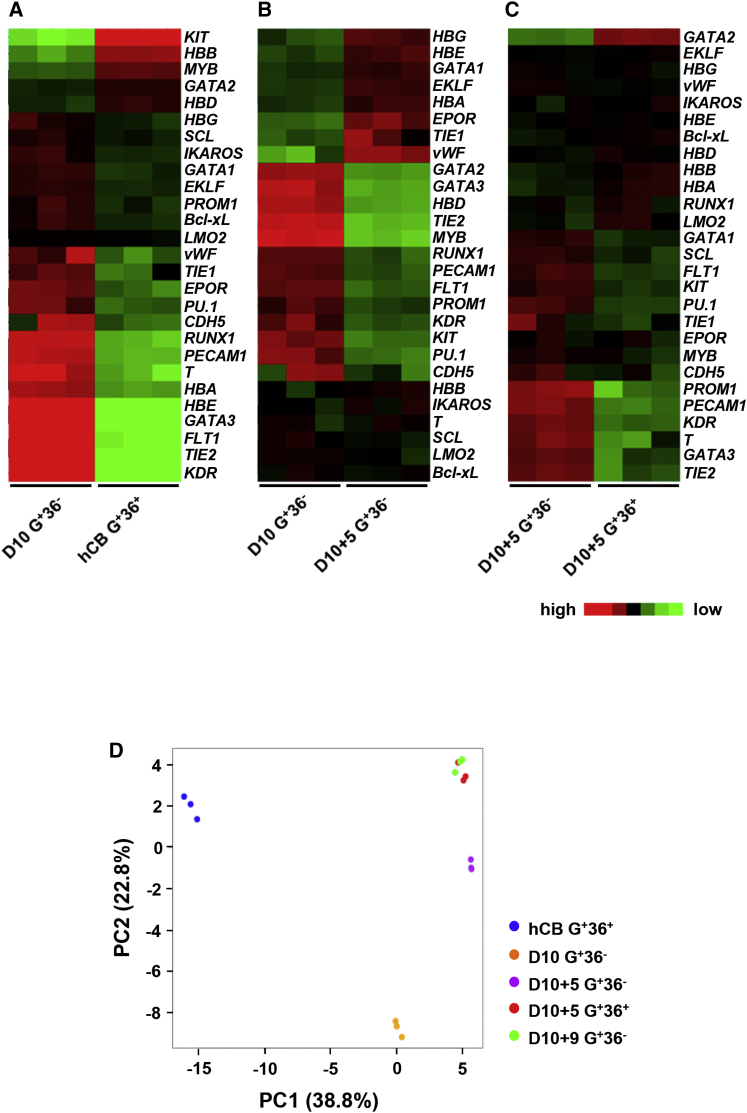
To elucidate the developmental pathway of hPSC-derived early erythroblasts, we further investigated G+36− cells from day-10 co-cultures, and G+36− and G+36+ cell fractions from day-10 + 5 cultures. MGG and IF staining showed that all sorted cell fractions were erythroblasts (Figures S3B and S3C). The gene expression level of these fractions was analyzed by qRT-PCR and compared with those of hCB-CD34+ HSPC-derived erythroblasts (G+36+) (Figure S3D). To further analyze the differences in transcriptional profiles among erythroid cell fractions, we performed pairwise comparisons of genes of interest (Figures 5A–5C).
Figure 5.
Heatmaps and PCA for qRT-PCR Results of hESC-Derived Erythroid Cell Fractions Defined by Expression of GPA and CD36
(A–C) Pairwise comparison of transcription levels for selected genes in the erythroid cell fractions. Heatmaps of selected genes associated with mesodermal, endothelial, hematopoietic, and erythroid cells. In each row, red, black, and green reflect high, medium, and low expression of the given gene, respectively (independent experiments, n = 3).
(D) PCA plots of three biological replicates of the five erythroid cell fractions.
See also Figure S3.
The transcript levels of the typical mesodermal-related gene T and endothelial-related genes (KDR, FLT1, PECAM1, TIE2, and CDH5) were higher in early G+36− erythroblasts at day 10 of co-culture. In hESC-derived erythroblasts, expression of the genes (GATA3, PU.1, and PROM1) which were related to the lymphoid development (Wei et al., 2011, Zhang et al., 2000, Gorgens et al., 2013) obviously decreased along with their maturation. These data show that progressive maturation of hESC-derived erythroblasts is associated with downregulated mesodermal/endothelial features and reduction of lymphoid/myeloid potentials.
SCL and LMO2 are involved in both primitive and definitive hematopoiesis (Porcher et al., 1996, Warren et al., 1994), and RUNX1 is a key hematopoietic transcription factor required for definitive hematopoiesis (Okuda et al., 1996). SCL and LMO2 expression levels in H1/AGM-S3 co-culture-derived erythroblasts were comparable with those in hCB-CD34+ HSPC-derived erythroblasts, while RUNX1 expression was higher in hESC-derived erythroblasts. These data suggest that hESC-derived erythroblasts in our system have a tendency to form definitive hematopoiesis.
GATA switch is a key regulation pathway for erythropoiesis in mice (Suzuki et al., 2003, Tsai and Orkin, 1997) and also from human adult-type HSPCs (Li et al., 2014). GATA1 expression was higher than GATA2 in hESC-derived erythroblasts. During maturation, GATA1 expression in hPSC-derived G+36− cells from day-10 + 5 suspension culture was higher than that from day-10 co-culture, then decreased when cells reached the G+36+ stage at day 10 + 5 of suspension culture. Expression of GATA2 was opposite to that of GATA1. Our findings demonstrate that decisive switching of GATA factors also occurs during the early erythropoiesis from hESCs.
hESC-derived erythroblasts expressed higher levels of embryonic and fetal (ɛ- and γ-) globins and lower levels of adult-type (δ- and β-) globins than hCB-CD34+ HSPC-derived erythroblasts. Meanwhile, HBB expression gradually increased following the progressive maturation of hESC-derived erythroblasts. Similar to previous reports, we found increases in IKAROS and EKLF expression and a decrease in MYB expression, which confirmed that the γ-/β-globin switch occurred in erythropoiesis from hESC (Bottardi et al., 2009, Dijon et al., 2008, Jiang et al., 2006).
In principal component analysis (PCA) (Figure 5D), three biological replicates of different erythroid cell fractions were tightly clustered, demonstrating that the cell fractions provided reproducible transcription profiles. G+36+ erythroblasts derived from hCB-CD34+ HSPCs were separated from all hESC-derived erythroid cell fractions according to PC1, which was primarily associated with differences in expression of HBE, KDR, and KIT. hESC-derived erythroblasts expressed relatively high levels of HBE and KDR and a low level of KIT, suggesting that they had mesodermal and embryonic properties during their development and were less reliant on stem cell factor (SCF) than hCB-CD34+ HSPC-derived erythroblasts. hESC-derived erythroblasts were clearly separated according to PC2, which was mainly associated with downregulation of TIE2, GATA3, and KDR. In complete agreement with our previous results, hESC-derived erythroblasts lost their mesodermal/endothelial features and lymphoid/myeloid potentials continuously during their progressive maturation.
GPA+CD34low Cells Define a Unique Pathway for Early Development of Definitive Erythroblasts
From flow cytometry data and gene expression profiles, hESC-derived G+36− erythroblasts at day 10 have obviously mesodermal and endothelial features. To explore their precursors, we tracked the expression of mesodermal and endothelial surface markers on GPA+ cells in early H1/AGM-S3 co-culture. We found that there exists a transient specific cell fraction GPA+CD34low (abbreviated as G+34low), which was generated from early H1/AGM-S3 co-culture by day5, burst out on day 7, peaked on day 10, and then disappeared gradually (Figure 6A).
Figure 6.
Characteristics of GPA+CD34low and GPA+CD34− Cell Fractions in H1/AGM-S3 Co-culture
(A) Representative flow cytometry data showing co-expression of CD34 and GPA on co-cultured cells over time. The co-cultured cells were digested by 0.1% trypsin/EDTA.
(B) G+34low and G+34− cell fractions in day-10 H1/AGM-S3 co-culture. (a) Cell fractions defined by expression of GPA and CD34 were sorted by FACS from day-10 H1/AGM-S3 co-culture. (b and c) IF analysis showing co-expression of human hemoglobin in G+34low and G+34− cell fractions (independent experiments, n = 3; mean ± SD). Scale bars, 20 μm. (d and e) MGG staining showing represent morphology of G+34low and G+34− cell fractions, respectively. Scale bars, 10 μm.
(C) Cell fractions defined by GPA and CD34 were sorted from day-10 H1/AGM-S3 co-culture and recultured in myeloid cells supporting medium. Original 1 × 104 cells were replated in one 24-plate well. After 8 days, hemoglobin+ and hemoglobin− cell numbers derived from G+34low, G+34−, G−34+, and G−34− cell fractions were presented, respectively (independent experiments, n = 3; mean ± SD).
(D) Daughter cells of G+34low and G+34− cells sorted from day-10 H1/AGM-S3 co-culture. (a and b) IF analysis showing expression of human hemoglobin in the daughter cells (independent experiments, n = 3; mean ± SD). Scale bars, 20 μm. (c and d) IF analysis showing co-expression of human hemoglobin and β-globin in the daughter cells (independent experiments, n = 3; mean ± SD). Scale bars, 10 μm. (e and f) MGG staining showing typical morphology of the daughter cells. Scale bars, 10 μm.
See also Figure S4.
Expression of surface markers including endothelial, hematopoietic, and erythroid lineage was investigated on G+34low and G+34− cells at day 7 of co-culture. The G+34low cells expressed a high level of endothelial marker CD31 and a low level of definitive hematopoietic markers (CD43 and CD41α). They also expressed a low level of CD71 and little CD36, both serving as erythroid lineage markers (Figure S4A). Fewer than 10% of day-7 G+34low cells expressed human hemoglobin (data not shown), while adult-type β-globin was detectable in both G+34low and G+34− cells in day-7 co-culture, with a positive ratio of 1.7% ± 0.2% and 13.8% ± 1.4%, respectively (Figure S4B). These data showed that the G+34low cells in day-7 co-culture were composed of a majority of endothelium and a small portion of definitive erythroblasts, while loss of expression of CD34 on GPA+ cells led to further erythroid maturation.
We further analyzed the properties of GPA+CD34low/− cell fraction in day-10 co-culture. Compared with G+34low/− cells at day 7, they expressed a lower level of CD31 but higher CD144. The expression levels of CD43, CD41α, and CD71 also increased remarkably (Figure S4A). We found that 62.4% ± 3.7% of G+34low cells in day-10 co-culture expressed human hemoglobin, showing their erythroid features, while lost CD34 expression gave rise to almost solely erythroid cells (97.7% ± 0.3% of G+34− cells expressing human hemoglobin) (Figure 6B).
For the detection of differentiation potential, four cell fractions defined by expression of GPA and CD34 were sorted from day-10 H1/AGM-S3 co-culture and recultured in medium, fully supporting myeloid cell differentiation. After 8 days, daughter cell numbers of G+34low, G+34−, and G−34+ cell fractions expanded 2.38 ± 0.48-, 1.75 ± 0.5-, and 1 ± 0.41-fold, respectively. While G−34− cells were not maintained in the culture (Figure 6C), most of the daughter cells from sorted G+34low and G+34− cells were erythroid cells expressing adult-type β-globin (Figure 6D) and few other myeloid cells were found. 2.15 ± 0.71 × 104 and 1.55 ± 0.44 × 104 hemoglobin+ cells were generated from 1 × 104 original G+34low and G+34− cells, respectively. Obviously the proliferation potential of G+34low cells was higher than that of G+34− cells, and both terminally gave rise to β-globin+ erythroblasts. On the other hand, daughter cells from G−34+ cells generated myeloid progenitors, mast cells, and macrophages but never erythroid cells (Figure S4C).
Discussion
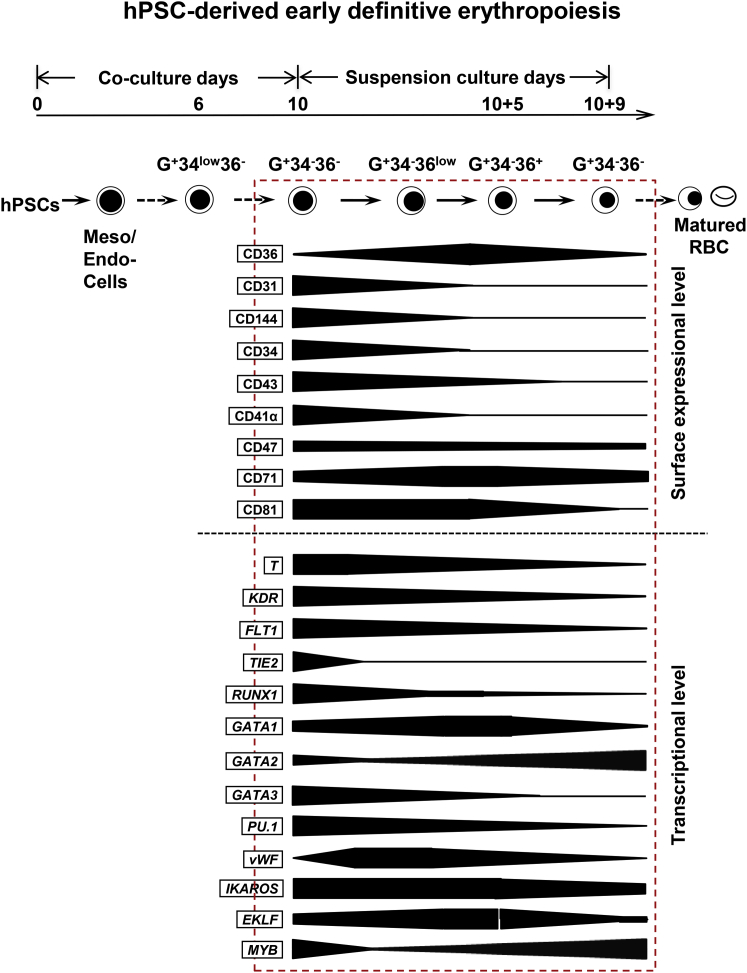
Human early erythropoiesis has been primarily studied using adult models with definitive hematopoiesis. Through subtle investigations, several laboratories reported that the regulatory pathway of definitive erythropoiesis development from adult HSPCs can be serially tracked by the expression of erythroid lineage surface markers, particularly CD34, GPA, CD71, and CD36 (Freyssinier et al., 1999, Gregory and Eaves, 1978). However, this may not be applicable to definitive erythropoiesis of hPSCs (Giarratana et al., 2011, Lu et al., 2008). In the present study, we investigated hPSC-derived early definitive erythroblasts during their development defined by expression of GPA, CD34, and CD36 (Figure 7). A unique cell population of GPA+CD34lowCD36− is firstly generated by day 5 of H1/AGM-S3 co-culture. Then, by losing CD34 expression, they developed and matured sequentially to G+34−36−, G+34−36low/+ and then G+34−36− erythroblasts with a gradual upregulation of β-globin. It is reported that before CD34+cells occur, GPA is firstly expressed on KDR+CD34−CD144− (abbreviated as G+K+34−144−) mesodermal cells (Sturgeon et al., 2014, Vodyanik et al., 2006), which could generate exclusively primitive hematopoiesis. However, the GPA+CD34low36− cells generated in H1/AGM-S3 co-culture are different from those early mesodermal cells. Since CD34 is known as an important marker of endothelial/hematopoietic cells, according to our data GPA+CD34low36− cells in day-7 co-culture are endowed with endothelial features while a small portion of them have already shared definitive erythroid potential. More than one-quarter of them express CD71 and even 1.7% ± 0.2% have already expressed adult-type β-globin. Consequently, the GPA+CD34low36− (G+34low36−) cells in day-10 co-culture showed an enhanced generation of erythroblasts (hemoglobin-positive cells >60%). When purified by cell sorting, these day-10 co-culture-derived GPA+CD34low cells can further proliferate and generate definitive erythroblasts with very high levels of β-globin (>95%), proving their definitive erythroid potential.
Figure 7.
Model of Early Definitive Erythroblasts Derived from hPSCs Defined by Expression of CD34, GPA, and CD36
There is a specific development stage during erythropoiesis from hPSCs/AGM-S3 co-culture. Early definitive erythroblasts are derived from G+34low36−, which occurred by day 6. They develop to G+34−36−, G+34−36low/+, and G+34−36− cells in sequence. During G+34−36low/+ cells mature into G+34−36− cells, flow cytometry data and gene expression profiles show hPSC-derived early definitive erythroblasts mature in association with decreases in endothelial and lymphoid potentials.
Accumulated data in our laboratory suggest that, by co-culture with AGM-S3, the hPSC-derived early erythroblasts may originate from definitive endothelium precursors that differ from adult-type HSC-derived erythroblasts. Human adult-type HSPCs enriched in hCB or peripheral blood (hPB) are phenotypically CD34+CD45+, while committed erythroid cells expressing GPA appear only after CD34 disappears. There is no co-expression of GPA and CD34 stage throughout the developmental process of adult-type HSC-derived erythroblasts. Clearly, the G+34low36− early precursors derived from our H1/AGM-S3 co-culture are CD45 negative, showing their lack of HSPC or myeloid source.
The Douay group reported that when adult HSC-derived mature erythroblasts were peripherally injected into the donor, at least half could survive in circulation for as long as 26 days (Giarratana et al., 2011). However, these in vitro expanded mature erythroblasts did not accumulate in a specific organ when injected into NOD/SCID mice (Neildez-Nguyen et al., 2002), suggesting that they are not a suitable long-term transplantable cell source for curing erythroblast deficiencies in patients. hPSC-derived early G+34low36− cells highly co-expressed endothelial markers and generated more erythroblasts than G+34−36− cells. These G+34low36− cells may be more efficient in homing to the hematopoietic niche and thus in helping to cure disorders with early erythropoiesis. Some practical mouse models have been established to track and qualify human erythroblasts in vivo (Getman et al., 2014, Ghinassi et al., 2011, Hu et al., 2011). In future, we will transplant hPSC-derived early G+34low36− cells into immunodeficient mice on a large scale to study their behavior in vivo.
Upon precise sorting and reculture, hPSC-derived early immature G+34−36− erythroblasts in our co-culture system intrinsically developed into a more mature G+34−36low stage with a high proliferation potential. This demonstrates that the early development and maturation of hPSC-derived erythroblasts can be traced by the co-expression of GPA and CD36. Similar to the maturation pathway of hCB-CD34+ HSPC-derived erythroblasts, expression of CD36 on hPSC-derived erythroblasts was downregulated at the terminal differentiation stage. Our observations provide evidence of a poorly characterized early erythroid developmental stage during hPSC differentiation, typically characterized as G+34−36− cells.
CD36 is an integral transmembrane protein with a high affinity for a variety of antibodies. CD36 gene deficiency is much more prevalent in Asian populations than in Caucasians (0%–0.3%) (Tanaka et al., 2001). This deficiency causes platelet refractoriness, post-transfusion thrombocytopenic purpura, neonatal alloimmune thrombocytopenia, and other disorders (Hirano et al., 2003, Neculai et al., 2013, Rac et al., 2007). However, deficiency of CD36 has no effect on development of erythroblasts (Toba et al., 2001). CD36, in our present study, is only an effective co-marker for tracing the developing erythroid lineage cells, a method that has been widely used (Wong et al., 2010, Okumura et al., 1992).
The finding that early erythroblasts derived from hPSC/AGM-S3 (H1, KhES-3, and 201B7) were G+36− suggests that a CD36− stage is common in early erythropoiesis from hPSCs. hPSC-derived erythroblasts generated from erythroid bodies (EBs) in vitro seldom express CD36 (Giarratana et al., 2011, Lu et al., 2008). It is unclear whether these hPSC-derived CD36− erythroblasts share immature properties with early erythroblasts in our system or have undergone terminal maturation into a CD36− stage.
Similar to hCB-CD34+ HSPC-derived erythroblasts, BFU-Es derived from day-12 co-culture mostly gave rise to CD36+ daughter cells in suspension culture and reached 100% expression of β-globin (data not shown). These findings demonstrate that our co-culture method favors progression to definitive hematopoiesis. On the other hand, EB-derived hematopoietic progenitors do not directly develop to the mature stage. Whether hPSC-derived hematopoietic cells follow a definitive pathway largely depends on their culture environment. The system of EBs co-culture with OP9 is widely used to generate definitive hematopoietic cells from hPSCs. However, because of the unnatural property of OP9 and the artificially sectioned culture steps, this model is not appropriate for the study of the natural progression of human erythropoiesis.
In the current co-culture system, undifferentiated hPSCs are cultured with definitive hematopoietic niche-derived AGM-S3 cells. Thus, this approach provides a unique and ideal tool for the analysis of the serial progression and maturation of hPSC-derived erythroblasts. This enables us to further elucidate the mechanisms underlying diseases with early erythropoiesis, particularly by establishing iPSCs from patients such as those with Diamond-Blackfan anemia (Vlachos et al., 2014). Many efforts have also been made for the efficient large-scale manufacture of hPSC-derived erythrocytes (Slukvin, 2010, Olivier et al., 2012, Perugini et al., 2009, Smith et al., 2013, Kurita et al., 2013). Meanwhile, practical solutions for enucleation (Fujimi et al., 2008) and hemoglobin switching (Xu et al., 2012) of erythrocytes developed from hPSCs are required. Our co-culture system developed here may benefit the breaking down of these barriers to generating mature erythrocytes in future clinical applications.
Experimental Procedures
Maintenance of hPSC Lines
Two hESC lines (H1 and KhES-3) and one hiPSC line (201B7) were used in this study. All hPSC lines were maintained in an undifferentiated state and passaged weekly on irradiated mouse embryonic fibroblasts as described previously (Thomson et al., 1998). The study was approved by the Institutional Ethic Committee of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences & Peking Union Medical College.
Differentiation of hPSCs into Erythroblasts
Approximately 1–2 × 104 undifferentiated hPSCs were seeded together with 2–3 × 105 irradiated AGM-S3 cells in each well of a 6-well plate and cultured in hPSC maintenance medium. Three days later, the medium was replaced by Iscove’s modified Dulbecco’s medium (IMDM) +10% fetal bovine serum (FBS) (Hyclone), 1% non-essential amino acids, 50 mg/mL ascorbic acid, and 20 ng/mL VEGF, representing day 0 of differentiation. Co-culture at days 10–12 was harvested and transferred to an ultra-low attachment plate with serum-free expansion medium supplemented with 100 ng/mL SCF, 100 ng/mL interleukin-6 (IL-6), 5 ng/mL IL-3, 10 ng/mL fetal liver, 10 ng/mL thrombopoietin (TPO), 4 IU/mL erythropoietin (EPO), and 20 ng/mL VEGF for 6 days (named SF-RBC+7FCs). Thereafter, cells were cultured as described previously (Giarratana et al., 2005). Cultures were referred to days of co-culture plus days of suspension culture.
Erythroblasts Derived from Human Cord Blood CD34+ HSPCs
To generate adult-type erythroblasts, we isolated hCB-CD34+ HSPCs using CD34+ Dynabeads (Invitrogen; purity >95%) and cultured them in serum-free medium as described previously (Giarratana et al., 2005).
Sorted Cells Recultured in Myeloid Supporting Medium
Cell fractions defined by GPA and CD34 were sorted from day-10 H1/AGM-S3 and were recultured in IMDM added with 20% FBS (Hyclone), containing cytokines cocktails (100 ng/mL SCF, 10 ng/mL IL-3, 50 ng/mL IL-6, 10 ng/mL Flt3 ligand, 8 IU/mL EPO, 10 ng/mL granulocyte colony-stimulating factor, and 10 ng/mL TPO).
Hematopoietic Colony Assay
CFU-E, BFU-E, CFU-Mix, and CFU-GM progenitors in H1/AGM-S3 co-culture were characterized as previously described (Ma et al., 2008).
Flow Cytometric Analysis and Cell Sorting
In briefly, the aforementioned cells were prepared as previously described (Ma et al., 2008). The antibodies used are presented in Table S1. Cells were then acquired using a flow cytometer (BD FACS Canto II) or sorted using a FACS Cytometer System (MoFlo Astrios, Beckman Coulter). Data were analyzed using FlowJo software (Version 7.6.5).
hCB-CD34+ HSPC-derived erythroblasts strongly expressed CD36, as determined by staining with a fluorescein isothiocyanate (FITC)-conjugated anti-CD36 antibody. On H1/AGM-S3-derived GPA+ cells in co-culture, CD36 was almost undetectable with an FITC-conjugated anti-CD36 antibody but detectable with an APC-conjugated anti-CD36 antibody (data not shown). Hence, the APC-conjugated anti-CD36 antibody was used to subsequently examine hPSC-derived erythroblasts.
Morphological Observation and Immunofluorescence Staining Analysis
Harvested cells were centrifuged onto glass slides using a Cell Cytospin machine (Cytospin 4, Thermo Fisher Scientific). For morphological observation, MGG staining was performed. The hemoglobin composition of erythroblasts was examined by IF staining analysis as described previously (Ma et al., 2008). The antibodies used are presented in Table S2.
qRT-PCR Analysis
The gene expression profiles of the various erythroblast fractions were analyzed by qRT-PCR. Total RNA was extracted from cells using the PureLink RNA Micro Kit (Invitrogen). cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). The primers used are listed in Table S3. The reactions were performed in a thermal cycler (iQ5, Bio-Rad). Gene expression levels were calculated using the minimal cycle threshold (Ct) values normalized to the expression of the housekeeping gene GAPDH in each sample. All reactions were performed in triplicate.
Heatmaps and Principal Component Analysis
qRT-PCR data were analyzed to generate heatmaps. Cluster analysis was performed using Cluster and visualized using Java Treeview. PCA was performed using Cluster and visualized using R package (ggplot2).
Statistical Analysis
The mean and SE of three independent experiments were calculated. Data are shown as the mean ± SD. Statistical significance was evaluated using the Student's t test. p < 0.05 was considered significant.
Author Contributions
Conception and design: F.M., B.M., J.Z., and T.N. Performed research: B.M., S.H., X.L., W.S., Y.Z., X.P., J.Y., M.L., B.C., and G.B. Collection and assembly of data: B.M., S.H., and Y.Z. Data analysis and interpretation: B.M., F.M., S.H., and S.M. Manuscript writing: B.M. and F.M. Final approval of manuscript: all authors.
Acknowledgments
We thank Professor Tao Cheng at the State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, CAMS & PUMC for generously providing the H1 line; Professor H. Suemori at the Laboratory of Embryonic Stem Cell Research Institute for Frontier Medical Sciences, Kyoto University for providing the KhES-3 cell line; and Professor S. Yamanaka at CiRA, Kyoto University for providing the 201B7 line. We thank Professor Min Wu at the University of North Dakota for his critical comments and polishing up our manuscript. This work was supported by the National Basic Research Program (973 Program: 2015CB964902) and the National Natural Science Foundation of China (H81170466, H81370597) awarded to F.M., and the Union Youth Fund of the Chinese Academy of Medical Sciences (3332013018) awarded to B.M.
Published: October 6, 2016
Footnotes
Supplemental Information includes four figures and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.09.002.
Supplemental Information
References
- Bottardi S., Ross J., Bourgoin V., Fotouhi-Ardakani N., Affar el B., Trudel M., Milot E. Ikaros and GATA-1 combinatorial effect is required for silencing of human gamma-globin genes. Mol. Cell. Biol. 2009;29:1526–1537. doi: 10.1128/MCB.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Liu J., Heck S., Chasis J.A., An X., Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijon M., Bardin F., Murati A., Batoz M., Chabannon C., Tonnelle C. The role of Ikaros in human erythroid differentiation. Blood. 2008;111:1138–1146. doi: 10.1182/blood-2007-07-098202. [DOI] [PubMed] [Google Scholar]
- Fajtova M., Kovarikova A., Svec P., Kankuri E., Sedlak J. Immunophenotypic profile of nucleated erythroid progenitors during maturation in regenerating bone marrow. Leuk. Lymphoma. 2013;54:2523–2530. doi: 10.3109/10428194.2013.781167. [DOI] [PubMed] [Google Scholar]
- Fujimi A., Matsunaga T., Kobune M., Kawano Y., Nagaya T., Tanaka I., Iyama S., Hayashi T., Sato T., Miyanishi K. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int. J. Hematol. 2008;87:339–350. doi: 10.1007/s12185-008-0062-y. [DOI] [PubMed] [Google Scholar]
- Freyssinier J.M., Lecoq-Lafon C., Amsellem S., Picard F., Ducrocq R., Mayeux P., Lacombe C., Fichelson S. Purification, amplification and characterization of a population of human erythroid progenitors. Br. J. Haematol. 1999;106:912–922. doi: 10.1046/j.1365-2141.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- Getman M., England S.J., Malik J., Peterson K., Palis J., Steiner L.A. Extensively self-renewing erythroblasts derived from transgenic beta-yac mice is a novel model system for studying globin switching and erythroid maturation. Exp. Hematol. 2014;42:536–546.e538. doi: 10.1016/j.exphem.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinassi B., Ferro L., Masiello F., Tirelli V., Sanchez M., Migliaccio G., Whitsett C., Kachala S., Riviere I., Sadelain M. Recovery and biodistribution of ex vivo expanded human erythroblasts injected into NOD/SCID/IL2Rgamma mice. Stem Cell Int. 2011;2011:673752. doi: 10.4061/2011/673752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarratana M.C., Kobari L., Lapillonne H., Chalmers D., Kiger L., Cynober T., Marden M.C., Wajcman H., Douay L. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat. Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- Giarratana M.C., Rouard H., Dumont A., Kiger L., Safeukui I., Le Pennec P.Y., Francois S., Trugnan G., Peyrard T., Marie T. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgens A., Radtke S., Horn P.A., Giebel B. New relationships of human hematopoietic lineages facilitate detection of multipotent hematopoietic stem and progenitor cells. Cell Cycle. 2013;12:3478–3482. doi: 10.4161/cc.26900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C.J., Eaves A.C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978;51:527–537. [PubMed] [Google Scholar]
- Hirano K., Kuwasako T., Nakagawa-Toyama Y., Janabi M., Yamashita S., Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc. Med. 2003;13:136–141. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- Hu Z., Van Rooijen N., Yang Y.G. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Best S., Menzel S., Silver N., Lai M.I., Surdulescu G.L., Spector T.D., Thein S.L. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood. 2006;108:1077–1083. doi: 10.1182/blood-2006-01-008912. [DOI] [PubMed] [Google Scholar]
- Keller G., Lacaud G., Robertson S. Development of the hematopoietic system in the mouse. Exp. Hematol. 1999;27:777–787. doi: 10.1016/s0301-472x(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Kurita R., Suda N., Sudo K., Miharada K., Hiroyama T., Miyoshi H., Tani K., Nakamura Y. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS One. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hale J., Bhagia P., Xue F., Chen L., Jaffray J., Yan H., Lane J., Gallagher P.G., Mohandas N. Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood. 2014;124:3636–3645. doi: 10.1182/blood-2014-07-588806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.J., Feng Q., Park J.S., Vida L., Lee B.S., Strausbauch M., Wettstein P.J., Honig G.R., Lanza R. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F., Ebihara Y., Umeda K., Sakai H., Hanada S., Zhang H., Zaike Y., Tsuchida E., Nakahata T., Nakauchi H. Generation of functional erythroblasts from human embryonic stem cell-derived definitive hematopoiesis. Proc. Natl. Acad. Sci. USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Tsuji K., Hisakawa H., Xu M., Ebihara Y., Ishii T., Sugiyama D., Manabe A., Tanaka R., Ikeda Y. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood. 2001;98:6–12. doi: 10.1182/blood.v98.1.6. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Lee J., Chiu L. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 2000;95:2275–2283. [PubMed] [Google Scholar]
- Neculai D., Schwake M., Ravichandran M., Zunke F., Collins R.F., Peters J., Neculai M., Plumb J., Loppnau P., Pizarro J.C. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 2013;504:172–176. doi: 10.1038/nature12684. [DOI] [PubMed] [Google Scholar]
- Neildez-Nguyen T.M., Wajcman H., Marden M.C., Bensidhoum M., Moncollin V., Giarratana M.C., Kobari L., Thierry D., Douay L. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat. Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S.W., Grosveld G., Downing J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Okumura N., Tsuji K., Nakahata T. Changes in cell surface antigen expressions during proliferation and differentiation of human erythroid progenitors. Blood. 1992;80:642–650. [PubMed] [Google Scholar]
- Olivier E., Qiu C., Bouhassira E.E. Novel, high-yield red blood cell production methods from CD34-positive cells derived from human embryonic stem, yolk sac, fetal liver, cord blood, and peripheral blood. Stem Cells Transl. Med. 2012;1:604–614. doi: 10.5966/sctm.2012-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J., Yoder M.C. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- Palis J., Robertson S., Kennedy M., Wall C., Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Perugini M., Varelias A., Sadlon T., D'Andrea R.J. Hematopoietic growth factor mimetics: from concept to clinic. Cytokine Growth Factor Rev. 2009;20:87–94. doi: 10.1016/j.cytogfr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Porcher C., Swat W., Rockwell K., Fujiwara Y., Alt F.W., Orkin S.H. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Rac M.E., Safranow K., Poncyljusz W. Molecular basis of human CD36 gene mutations. Mol. Med. 2007;13:288–296. doi: 10.2119/2006-00088.Rac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin I.I. Generation of mature blood cells from pluripotent stem cells. Haematologica. 2010;95:1621–1623. doi: 10.3324/haematol.2010.029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.W., Rozelle S.S., Leung A., Ubellacker J., Parks A., Nah S.K., French D., Gadue P., Monti S., Chui D.H. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood. 2013;122:376–385. doi: 10.1182/blood-2012-11-466722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon C.M., Ditadi A., Awong G., Kennedy M., Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Suwabe N., Ohneda O., Obara N., Imagawa S., Pan X., Motohashi H., Yamamoto M. Identification and characterization of 2 types of erythroid progenitors that express GATA-1 at distinct levels. Blood. 2003;102:3575–3583. doi: 10.1182/blood-2003-04-1154. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Nakata T., Oka T., Ogawa T., Okamoto F., Kusaka Y., Sohmiya K., Shimamoto K., Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001;42:751–759. [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Toba K., Hanawa H., Watanabe K., Fuse I., Masuko M., Miyajima S., Takahashi M., Sakaue M., Abo T., Aizawa Y. Erythroid involvement in CD36 deficiency. Exp. Hematol. 2001;29:1194–1200. doi: 10.1016/s0301-472x(01)00691-9. [DOI] [PubMed] [Google Scholar]
- Tsai F.Y., Orkin S.H. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- Vlachos A., Blanc L., Lipton J.M. Diamond Blackfan anemia: a model for the translational approach to understanding human disease. Expert Rev. Hematol. 2014;7:359–372. doi: 10.1586/17474086.2014.897923. [DOI] [PubMed] [Google Scholar]
- Vodyanik M.A., Thomson J.A., Slukvin I.I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A.J., Colledge W.H., Carlton M.B., Evans M.J., Smith A.J., Rabbitts T.H. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Wei G., Abraham B.J., Yagi R., Jothi R., Cui K., Sharma S., Narlikar L., Northrup D.L., Tang Q., Paul W.E. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S., Keyvanfar K., Wan Z., Kajigaya S., Young N.S., Zhi N. Establishment of an erythroid cell line from primary CD36+ erythroid progenitor cells. Exp. Hematol. 2010;38:994–1005. doi: 10.1016/j.exphem.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.J., Tsuji K., Ueda T., Mukouyama Y.S., Hara T., Yang F.C., Ebihara Y., Matsuoka S., Manabe A., Kikuchi A. Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta-gonad-mesonephros-derived stromal cell lines. Blood. 1998;92:2032–2040. [PubMed] [Google Scholar]
- Xu J., Shao Z., Glass K., Bauer D.E., Pinello L., Van Handel B., Hou S., Stamatoyannopoulos J.A., Mikkola H.K., Yuan G.C., Orkin S.H. Combinatorial assembly of developmental stage-specific enhancers controls gene expression programs during human erythropoiesis. Dev. Cell. 2012;23:796–811. doi: 10.1016/j.devcel.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhang X., Iwama A., Yu C., Smith K.A., Mueller B.U., Narravula S., Torbett B.E., Orkin S.H., Tenen D.G. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood. 2000;96:2641–2648. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.