Abstract
Purpose
Veno-arterial extracorporeal life support (ECLS) is increasingly used in patients during cardiac arrest and cardiogenic shock, to support both cardiac and pulmonary function. We performed a systematic review and meta-analysis of cohort studies comparing mortality in patients treated with and without ECLS support in the setting of refractory cardiac arrest and cardiogenic shock complicating acute myocardial infarction.
Methods
We systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials and the publisher subset of PubMed updated to December 2015. Thirteen studies were included of which nine included cardiac arrest patients (n = 3098) and four included patients with cardiogenic shock after acute myocardial infarction (n = 235). Data were pooled by a Mantel-Haenzel random effects model and heterogeneity was examined by the I 2 statistic.
Results
In cardiac arrest, the use of ECLS was associated with an absolute increase of 30 days survival of 13 % compared with patients in which ECLS was not used [95 % CI 6–20 %; p < 0.001; number needed to treat (NNT) 7.7] and a higher rate of favourable neurological outcome at 30 days (absolute risk difference 14 %; 95 % CI 7–20 %; p < 0.0001; NNT 7.1). Propensity matched analysis, including 5 studies and 438 patients (219 in both groups), showed similar results. In cardiogenic shock, ECLS showed a 33 % higher 30-day survival compared with IABP (95 % CI, 14–52 %; p < 0.001; NNT 13) but no difference when compared with TandemHeart/Impella (−3 %; 95 % CI −21 to 14 %; p = 0.70; NNH 33).
Conclusions
In cardiac arrest, the use of ECLS was associated with an increased survival rate as well as an increase in favourable neurological outcome. In the setting of cardiogenic shock there was an increased survival with ECLS compared with IABP.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4536-8) contains supplementary material, which is available to authorized users.
Keywords: Extracorporeal membrane oxygenation, Extracorporeal life support, Acute myocardial infarction, Cardiac arrest, Cardiogenic shock, Cardiopulmonary resuscitation, Systematic review
Introduction
Veno-arterial extracorporeal life support (ECLS), also called extracorporeal membrane oxygenation (ECMO), is a modified form of cardiopulmonary bypass to support both cardiac and pulmonary function. Technological improvements and miniaturisation have made this technique more accessible and its use has increased over the past years, especially in patients with refractory cardiogenic shock or circulatory arrest [1, 2].
Cardiogenic shock (CS) remains the leading cause of death in patients hospitalised for ST-segment elevation myocardial infarction (STEMI), as it may lead to multi-organ failure due to insufficient organ perfusion [3, 4]. In addition to pharmacological measures, treatment with mechanical circulatory support can be considered, especially in more severe forms of circulatory failure.
The aim of mechanical circulatory support in general is to support the failing heart and the overall circulation. Ideally, mechanical support is used as a bridge to either recovery or to other therapies such as a surgically implanted ventricular assist device (LVAD) or heart transplantation. It can be used in cardiogenic shock to prevent the development of multi-organ failure. In cardiac arrest patients, mechanical circulatory support enables treatment of the underlying cause while maintaining adequate perfusion.
A multitude of mechanical support devices have been developed over the past decades and this field is attracting increasing attention, especially after clinical trials did not show any clinical benefit for the intra-aortic balloon pump (IABP). Current European guidelines on cardiogenic shock no longer support routine IABP therapy, whereas short-term mechanical circulatory support holds a class IIb recommendation [5, 6].
Percutaneous cannulation techniques facilitate rapid insertion and initiation of ECLS therapy in emergency situations, such as cardiac arrest. Although ECLS usage has increased and several observational studies suggest that it has had a beneficial effect in both cardiac arrest and cardiogenic shock, no randomised controlled trials have been performed to date. Therefore, the actual evidence for its efficacy remains limited.
The main purpose of our study was to conduct a systematic review and meta-analysis of the available literature comparing ECLS with conventional therapy with regard to survival and neurological outcome in patients with cardiogenic shock after acute myocardial infarction (AMI) and patients with refractory cardiac arrest.
Methods
Selection criteria
Studies were considered for inclusion if they described outcome data from (A) patients with ECLS support and (B) a control group without ECLS support. Also, to qualify for inclusion, patients must have been diagnosed with either (1) refractory in-hospital or out-of-hospital cardiac arrest or (2) cardiogenic shock after AMI. Studies that did not report on survival to discharge, 30-day outcome or 6-month outcome were excluded. This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [7].
Search strategy
A medical librarian (J.L.) conducted a systematic search of OVID MEDLINE, OVID EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) and the publisher subset of PubMed from inception to 7 December 2015. The search strategy consisted of controlled vocabulary (i.e. MeSH) and free text words for two basic concepts: (1) ECLS and (2) cardiogenic shock, cardiac arrest or myocardial infarction (see Appendix 1 in the electronic supplementary material for the entire MEDLINE search). Non-human studies, paediatric studies, case reports and reviews were excluded by double negation (NOT animals/NOT humans/) and/or excluding words in the title. We cross-checked the reference lists and the cited articles of the identified relevant papers for additional references. The bibliographic records retrieved were downloaded, imported and de-duplicated in ENDNOTE.
Data extraction and quality assessment
The retrieved articles were screened for relevance on title and abstract, followed by full-text screening by two independent investigators (D.O. and J.S.). In the event of overlapping patient cohorts the study with the longest follow-up period was included.
The prespecified patient and outcome data were independently extracted by two investigators (D.O. and J.S.). Differences between reviewers regarding study selection or data extraction were resolved by consensus. The quality of the studies was assessed using a modified version of the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies [8].
Data analysis
The primary endpoint was 30-day survival. Secondary outcomes were long-term survival and 30-day and long-term favourable neurological outcome. Parameters describing the clinical course and complications were extracted, e.g. successful weaning from the cardiac assist device, bridging to destination therapy (long-term ventricular assist device or heart transplantation), timing of device placement, the occurrence of renal failure, stroke, peripheral vessel access complications and the need for blood transfusions (erythrocyte and fresh frozen plasma). If 30-day outcome data were not reported, in-hospital outcome data were used. For long-term data, the longest available follow-up was used. Neurological status was considered favourable when reported as either Pittsburgh Cerebral Performance Category (CPC) 1 or 2, or Modified Glasgow Outcome Score (MGOS) ≥4. Studies were grouped and presented by patient category: cardiac arrest or cardiogenic shock. A subcategory of propensity-matched studies is reported separately. Propensity score matching is a method used to balance observed covariates in the two treatment arms by matching the propensity score which represents the probability of receiving ECLS therapy.
Results are presented as absolute risk differences with a 95 % confidence interval (CI) and a number needed to treat (NNT) or number needed to harm (NNH) and were combined by a Mantel-Haenzel random effects model. Heterogeneity across studies was examined by the I 2 statistic. Potential publication bias was assessed by visual assessment of constructed funnel plots. Tests were two-tailed and a p value of less than 0.05 was considered statistically significant. An I 2 of greater than 40 % was considered to be an indication of substantial heterogeneity. Review Manager (version 5.3) was used for statistical analysis.
Results
Search results
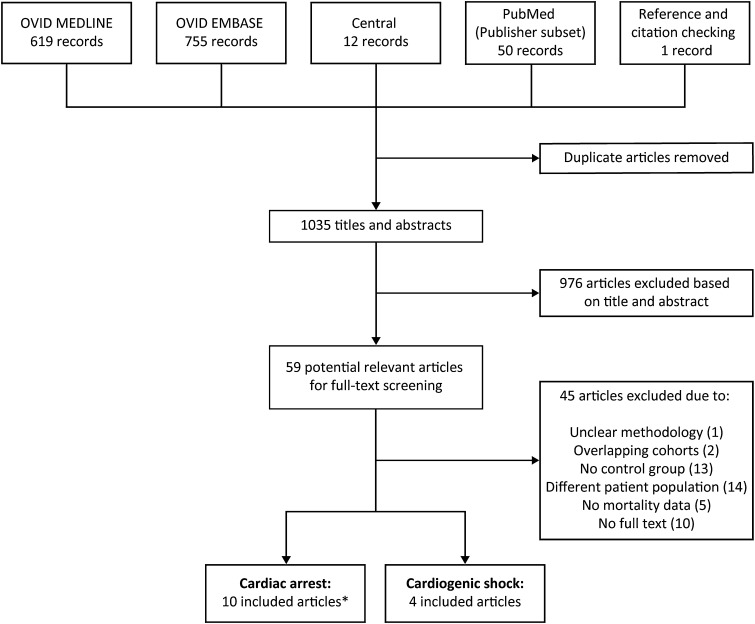
The de-duplicated results yielded a total of 1403 abstracts. A total of 59 relevant articles were identified and the full-text article was independently reviewed. Figure 1 shows a flowchart for selection of studies. One article was excluded as the intervention group contained both ECLS and IABP patients [9]. Fourteen articles were identified. Ten articles consisted of patients in refractory cardiac arrest [10–19]. However, two articles described the same cohort but with additional analysis [17 (Shin 2013 Int J Card) ] [19 (Shin 2011 Crit Car Med]. This resulted in a total of 9 included cardiac arrest cohorts with a total of 3098 patients (708 ECLS versus 2390 control patients) (Table 1). Five of the cardiac arrest studies reported a propensity-matched analysis, including a total of 438 patients (219 in both groups) [10, 11, 13, 15, 19]. Four studies consisted of patients with cardiogenic shock with a total of 235 patients (151 ECLS versus 84 control patients) [20–23] (Table 1).
Fig. 1.
Flowchart of the search strategy and selection of studies. Asterisk: 1 article reported on the same patient cohort as another included article, but provided additional data on propensity-matched analysis and was therefore included
Table 1.
Summary of included cohort studies on cardiogenic shock and cardiac arrest patients
| References | Country | Study period | Setting | Follow-up duration | Number of patients |
|---|---|---|---|---|---|
| Cardiac arrest | |||||
| Blumenstein et al. [10] | Germany | 2009–2013 | Retrospective, single centre | Long terma | 353 |
| Chen et al. [11] | Taiwan | 2004–2006 | Prospective, single centre | 1 year | 172 |
| Chou et al. [12] | Taiwan | 2006–2010 | Retrospective, single centre | 1 year | 66 |
| Kim et al. [13] | Korea | 2006–2013 | Prospective, single centre | 3 months | 499 |
| Lee et al. [14] | Korea | 2009–2014 | Retrospective, single centre | In-hospital | 955 |
| Maekawa et al. [15] | Japan | 2000–2004 | Prospective, single centre | 3 months | 162 |
| Sakamoto et al. [16] | Japan | 2008–2011 | Prospective, multi-centre | 6 months | 454 |
| Shin et al. [17] | Korea | 2003–2009 | Retrospective, single centre | 2 years | 406 |
| Siao et al. [18] | Taiwan | 2011–2013 | Retrospective, single centre | 1 year | 60 |
| Cardiogenic shock | |||||
| Chamogeorgakis et al. [20] | USA | 2006–2011 | Retrospective, single centre | In-hospital | 79 |
| Lamarche et al. [21] | Canada | 2000–2009 | Retrospective, single centre | 30 days | 61 |
| Sattler et al. [22] | Germany | 2011–2012, 2012–2013 | Retrospective, single centre | 30 days | 24 |
| Sheu et al. [23] | Taiwan | 1993–2002, 2002–2009 | Prospective, single centre | 30 days | 71 |
aNot defined, median long-term follow-up was 1136 (823–1415) days
Quality of studies
As all studies were cohort studies and no randomised controlled trials were available, the quality of the studies was low with a high risk of bias (Appendix 2). However, funnel plots did not show skewed distributions, suggesting that no publication bias was involved (Appendix 3).
Cardiac arrest
Patient characteristics
Table 2 shows the baseline characteristics of the studies on ECLS in the setting of cardiac arrest. A total of nine studies were included with 3098 patients in total, 708 in the ECLS group and 2390 in the control group. All studies included cardiac arrest patients, although with different inclusion criteria such as in-hospital cardiac arrest (IHCA), out-of-hospital cardiac arrest (OHCA), witnessed or non-witnessed cardiac arrest and differing durations of cardiopulmonary resuscitation (CPR). Overall, ECLS patients were more likely to be younger, male, suffer from acute myocardial infarction and to undergo primary PCI.
Table 2.
Baseline characteristics of the studies on ECLS-assisted cardiac arrest
| References | Patient population | Criteria for ECLS allocation/placement | Control arm | Number of patients (n) | Mean age (years) | Male (%) | Acute myocardial infarction (%) | Revascularisation (%) | CPR duration (min) | Interval between arrest and CPR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECLS | Control | ECLS | Control | ECLS | Control | ECLS | Control | ECLS | Control | ECLS | Control | ECLS | Control | ||||
| Blumenstein et al. [10] | Witnessed IHCA | ECLS was considered by the ECLS team if CPR >10 min and cardiac aetiology | Conventional CPR | 52 | 272 | 72 | 75 | 54 | 61 | 29 | 21 | – | – | 33 (19–47) | 20 (6–40) | –d | –d |
| Chen et al. [11] | Witnessed IHCA of cardiac origin, CPR >10 min | The decision was made by the attending doctors in charge. Exclusion for ECLS: failure to wean from bypass due to post-cardiotomy shock and patients who experienced shock requiring elective ECLS | Conventional CPR | 59 | 113 | 57 | 60 | 85 | 65 | 63 | 71 | 44 | 6a | 53 ± 37 | 43 ± 31 | –d | –d |
| Chou et al. [12] | IHCA due to AMI, CPR >10 min | The decision to carry out ECPR is determined by the cardiovascular surgeon | Conventional CPR | 43 | 23 | 61 | 70 | 93 | 74 | 100 | 100 | 100 | 43a | 60 ± 34 | 49 ± 35 | –e | –e |
| Kim et al. [13] | Cardiac arrest patients with CPR (no trauma) | ECPR was indicated when presumed correctable cause of CA, witnessed arrest or presumed short no-flow time when unwitnessed arrest and informed consent of the family and in-hospital CPR >10 min | Conventional CPR | 55 | 444 | 53 | 69 | 75 | 64 | – | – | – | – | 62 (47–89) | 35 (21–50) | 7 (0–13) | 8 (5–12) |
| Lee et al. [14] | IHCA and OHCA CPR | Judgment of ECLS team. Only ECLS if CPR >10 min or repetitive arrest events without ROSC >20 min. No ECLS if unwitnessed OHCA or no bystander CPR | Conventional CPR | 81 | 874 | 59 | 64 | 69 | 65 | 67 | 41 | – | – | 43 (21–60) | 30 (15–48) | – | – |
| Maekawa et al. [15] | Witnessed OHCA of presumed cardiac origin, CPR >20 min | Initiation of ECPR was dependent on the attending physicians | Conventional CPR | 53 | 109 | 54 | 71 | 83 | 73 | – | – | 40 | 6b | 49 (41–59) | 56 (47–66) | 2 (0–8) | 5 (0–9) |
| Sakamoto et al. [16] | OHCA based on VF/VT, no ROSC >15 min after hospital arrival, <45 min between emergency call and hospital arrival; cardiac origin | Assignment of facility to ECPR or CPR group | Conventional CPR | 260 | 194 | 56 | 58 | 90 | 89 | 64 | 59 | 38 | 11b | – | – | – | – |
| Shin et al. [17] | IHCA, witnessed, CPR >10 min | According to the discretion of the CPR team leader | Conventional CPR | 85 | 321 | 60 | 62 | 62 | 63 | 45 | 26 | 41 | 7a | 42 ± 26 | 41 ± 37 | –d | –d |
| Siao et al. [18] | Cardiac arrest with initial VF (start CPR <5 min), no ROSC after 10 min CPR | Judgment of the attending physician | Conventional CPR | 20 | 40 | 55 | 60 | 90 | 70 | 60 | 40 | 60 | 40c | 70 ± 50 | 34 ± 18 | –f | –f |
Values are presented as mean ± standard deviation or as median (IQR)
CPR cardiopulmonary resuscitation, PCI percutaneous coronary intervention, OHCA out-of-hospital cardiac arrest, IHCA in-hospital cardiac arrest, ROSC return of spontaneous circulation, AMI acute myocardial infarction, VF ventricular fibrillation, VT ventricular tachycardia, CA cardiac arrest, ECPR ECLS-assisted cardiopulmonary resuscitation
aReported as subsequent interventions (PCI or CABG)
bReported as primary PCI
cReported as subsequent interventions (PCI)
dConsidered to be minimal as the inclusion criterion is (witnessed) IHCA
eIHCA so minimal no-flow time. In this study CPR duration was defined as time from collapse till ROSC, death or running of ECMO machine
fNot mentioned, but inclusion criteria state no-flow less than 5 min
Survival
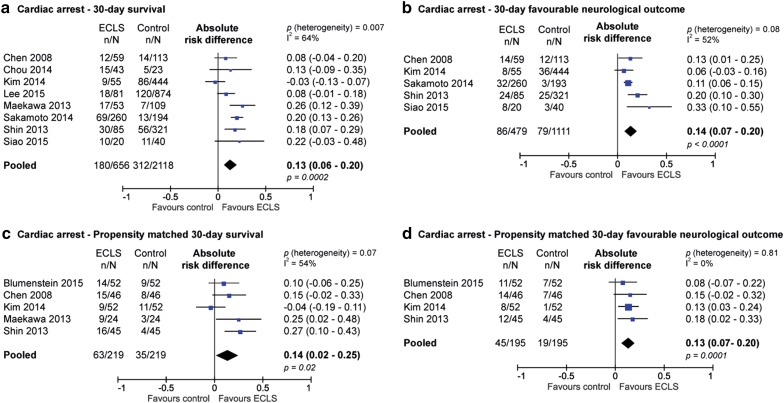
Figure 2a shows 30-day survival of patients with refractory cardiac arrest. The usage of ECLS in this setting was associated with increased survival at 30 days (absolute risk difference 13 %; 95 % CI 6–20 %; p < 0.001; NNT 7.7). The long-term difference in survival was 15 % in favour of the ECLS treated patients (see supplementary file) (absolute difference 15 %; 95 % CI 11–20 %; p < 0.0001; NNT 6.7). Short-term outcome data displayed substantial heterogeneity (I 2 = 64 %), but long-term survival did not (I 2 = 28 %).
Fig. 2.
Risk difference of 30-day survival (a) and favourable neurologic outcome (CPC 1 or 2) (b) and propensity-matched risk difference in 30-day survival (c) and favourable neurologic outcome (CPC 1 or 2) (d) in patients with cardiac arrest
Neurological outcomes
Favourable neurological outcomes, defined as CPC score 1 or 2, are shown in Fig. 2b. The use of ECLS was associated with a higher rate of favourable neurological outcome at both 30 days (risk difference 14 %; 95 % CI 7–20 %; p < 0.0001; NNT 7.1) and during long-term follow-up (risk difference 11 %; 95 % CI 6–16 %; p < 0.0001; NNT 9.1) (supplementary data). Short-term outcome data were moderately heterogeneous (I 2 = 52 %) but the long-term survival data did not show substantial heterogeneity (I 2 = 28 %).
Other outcomes
Peripheral vessel complications were only reported by two studies. Blumenstein reported 17.3 % of patients with leg ischaemia or malperfusion in the ECLS arm and 2.9 % in the control arm. Maekawa et al. reported 7.7 % cannulation site infection, 15.4 % leg ischaemia requiring reperfusion and 2.9 % compartment syndrome in the ECLS patient group (supplementary data) [15]. Complication rates were very poorly reported. Only one of the cardiac arrest studies reported on renal failure (1.9 % in the ECLS patients versus 7 % in the control patients) [10]. Stroke and blood transfusions were not reported.
Propensity score matching
Five studies performed a propensity-matched analysis to balance observed covariates in the two treatment groups. The propensity score reflects the probability of receiving ECLS therapy. The baseline characteristics, after matching based on propensity score, can be seen in the supplementary data. The included patient population differed between studies in terms of location of the arrest (IHCA versus OHCA), witnessed or unwitnessed arrest, presumed cardiac origin and duration of CPR. After propensity matching, the patients treated with ECLS and control patients were comparable in terms of age and gender. There were more patients in the ECLS arm than in the control arm receiving primary PCI, as only one of the five propensity-matched studies included primary PCI as a matching variable. The use of ECLS was associated with a higher survival rate at 30 days (difference 14 %; 95 % CI 2–25 %; p = 0.02; NNT 7.1) and in the long-term (difference 13 %; 95 % CI 6–20 %; p = 0.001; NNT 7.7) (Fig. 2c and supplementary data). Also, the use of ECLS was associated with a higher rate of favourable neurological outcome at both 30 days (risk difference 13 %; 95 % CI 7–20 %; p = 0.0001; NNT 7.7) and in the long-term (risk difference 14 %; 95 % CI 8–20 %; p < 0.0001; NNT 7.1) (Fig. 2d and supplementary data). In the propensity-matched analysis, short-term survival showed substantial heterogeneity (I 2 = 54 %), but long-term survival and the neurological outcomes showed no substantial heterogeneity (I 2 = 0 %).
Cardiogenic shock
Patient characteristics
Table 3 shows the baseline characteristics of the studies on ECLS in cardiogenic shock patients. A total of four studies were included with 235 patients in total, 151 in the ECLS group and 84 in the control group. All studies included cardiogenic shock patients after myocardial infarction, albeit with different inclusion criteria such as refractory CS, progressive CS or decompensated cardiomyopathy. In two studies, the control arm consisted of IABP support, and in two other studies, the control arm consisted of patients supported by Impella 5.0, Impella RD or TandemHeart. Patients in the ECLS arm were generally younger and were less likely to suffer from acute myocardial infarction (Table 3). In the two studies with IABP support in the control group, all patients were diagnosed with STEMI and treated with primary PCI.
Table 3.
Baseline characteristics of the studies on ECLS in cardiogenic shock patients
| References | Patient population | Criteria for ECLS allocation/placement | Control arm | Number of patients (n) | Mean age (years) | Male (%) | Acute myocardial infarction (%) | Primary PCI (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECLS | Control | ECLS | Control | ECLS | Control | ECLS | Control | ECLS | Control | ||||
| Chamogeorgakis et al. [20] | Post/infarction or decompensated cardiomyopathy (ischaemic or non-ischaemic) cardiogenic shock | Patients receiving heart compressions, ECLS is the only option. For more stable patients, TandemHeart or Impella. For isolated right ventricular failure, TandemHeart is favoured. In left ventricular failure, Impella 5.0 or TandemHeart can be used | Impella 5.0/TandemHearta | 61 | 18 | 53 | 58 | 80 | 72 | 53 | 78 | – | – |
| Lamarche et al. [21] | Acute, refractory, cardiogenic shock with potential for recovery and systemic perfusion did not improve with IABP and inotropes | Biventricular failure and oxygenation problems: ECLS. Unilateral failure: Impella | Impella 5.0/Impella RD | 32 | 29 | 50 | 54 | 63 | 83 | 41 | 38 | – | – |
| Sattler et al. [22] | Progressive cardiogenic shock due to acute myocardial ischaemia, and successful PCI | Enrolment during period with ECLS availability and ECLS is technically feasible | IABP | 12 | 12 | 55 | 68 | 83 | 83 | 100 | 100 | 100 | 100 |
| Sheu et al. [23] | STEMI with primary PCI and profound cardiogenic shockb | Enrolment date in period with ECLS availability | IABP | 46 | 25 | 65 | 67 | – | – | 100 | 100 | 100 | 100 |
CPR cardiopulmonary resuscitation, PCI percutaneous coronary intervention, AMI acute myocardial infarction, VF ventricular fibrillation, VT ventricular tachycardia, CA cardiac arrest, ECPR ECLS-assisted cardiopulmonary resuscitation
a7 Impella, 11 TandemHeart
bProfound shock: systolic blood pressure <75 mmHg despite inotropic agents and IABP
Survival outcomes
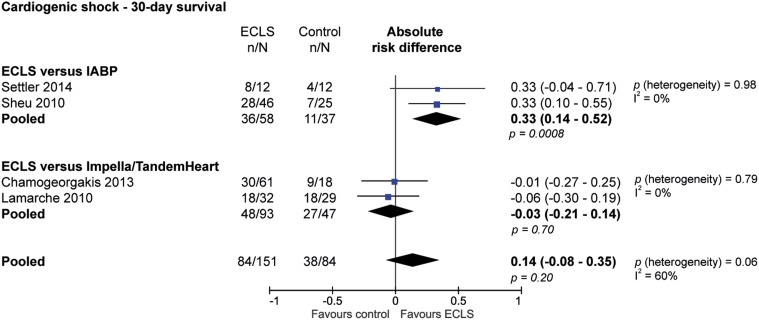
Figure 3 shows the absolute number of survivors among patients with and without ECLS treatment, with the absolute risk difference for each study, stratified by the different control arms. The studies with IABP in the control arm showed that ECLS support in the setting of cardiogenic shock was associated with improved 30-day survival (risk difference 33 %; 95 % CI 14–52 %; p = 0.0008; NNT 3). When ECLS was compared with Impella or TandemHeart, ECLS was not associated with a significant difference in 30-day survival (risk difference −3 %; 95 % CI −21 to 14 %; p = 0.70; NNH 33). When combining the control groups (IABP and Impella/TandemHeart), the use of ECLS was not associated with a change in 30-day survival in patients with cardiogenic shock (risk difference 14 %, 95 % CI - 8-35 %; p = 0.20; NNT 7.1). The analysis stratified according to control arm did not show any heterogeneity (I 2 = 0 %), but the overall effects were substantially heterogeneous (I 2 = 60 %). The long-term survival and neurological outcomes were not described in these studies.
Fig. 3.
Difference of 30-day survival of patients with cardiogenic shock, stratified according to different control therapies (IABP or Impella/TandemHeart)
Other outcomes
The percentage of patients who were successfully weaned from ECLS and the percentage of patients who were bridged to long-term ventricular assist device or heart transplant are shown in the supplementary data. Only Sattler et al. reported the time of device placement: in one patient, ECLS was placed before PCI, in nine patients immediately after PCI and in two patients ECLS therapy was initiated within 24–48 h after PCI with IABP support. Peripheral vessel complications and blood transfusions are shown in the supplementary data. Only one study reported the incidence of renal failure, with renal failure occurring in 58.3 % of patients treated with ECLS and in 25.0 % of the control patients [22]. Stroke was not reported by any study.
Discussion
We conducted two meta-analyses of cohort studies comparing ECLS therapy with varying control groups in the settings of cardiac arrest and cardiogenic shock. In the setting of cardiac arrest, the usage of ECLS showed an increase in survival of 13 % and an increase of favourable neurological outcome of 14 % at 30-days compared with no usage of ECLS. This effect was still prominent after baseline characteristics were adjusted by propensity matching. In patients with cardiogenic shock, ECLS was associated with higher 30-day survival compared with IABP, but there was no difference in survival when compared with Impella or TandemHeart.
In the absence of randomised controlled trials, we included non-randomised studies and therefore cannot rule out the influence of confounders. As a result, there was a difference in baseline characteristics between ECLS and control patients. ECLS-treated patients were more likely to be male, younger, suffer from acute myocardial infarction and were more likely to undergo primary PCI—all factors known to be associated with increased survival in this setting [24–26]. Another potentially important bias towards poor outcomes in the ‘control/no-ECLS’ group may be due to the fact that sicker patients may have been considered too ill to benefit from ECLS therapy and others may have died before they could receive ECLS therapy. As it is difficult to reliably distinguish between the effect of ECLS therapy and the effect of the bias and confounding inherent to cohort studies, the results of this analysis should be interpreted with caution. Nevertheless, the propensity-matched analysis in cardiac arrest, with matching baseline characteristics, showed results comparable with the outcome of the cohort studies.
In addition to the difference in baseline characteristics of the patients, differences in the treatment of patients might have influenced the results. Patients with cardiac arrest treated with ECLS were more likely to be revascularised. This finding suggests that the use of ECLS allows for more frequent revascularisation. Kagawa et al. investigated the effectiveness of intra-arrest PCI during ECLS, and they reported a higher survival rate in the intra-arrest PCI groups compared with delayed PCI (36 versus 12 %) [27]. The fact that ECLS-assisted CPR allowed for timely treatment of the underlying cause, such as intra-arrest PCI, might partly explain the increased survival in ECLS-assisted CPR.
In the cardiogenic shock patients, the difference in treatment effect may be explained by the amount of haemodynamic support that is generated by the mechanical support device. The used Impella devices (5.0 and RP) and TandemHeart actively support the circulation with around 4 L/min, which is comparable to ECLS, whereas the IABP only passively supports the overall circulation with ca. 0.5 L/min. However, a small meta-analysis of randomised trials comparing IABP (n = 47) with Impella/TandemHeart (n = 53) in CS complicating AMI did not show any difference in outcome [28]. This seems to contradict the previous hypothesis that ECLS, TandemHeart and Impella 5.0 might all be superior to IABP as they provide more haemodynamic support. This apparent contradiction may be explained by the different characteristics of the patients included, the differences in definition of (profound) CS and the low number of patients included in both meta-analyses. Although the support level of the used devices may be similar (around 4 L/min), they have different specifications and therefore different clinical indications [4, 5].
The variety of inclusion criteria in the included studies is likely to have contributed to the heterogeneity. Although we aimed to include patients with acute myocardial infarction, some cardiogenic shock studies included patients with a wide variety of aetiologies (100 % AMI in the IABP studies, but lower in the Impella/Tandemheart studies (no exact number reported)). In the cardiac arrest studies, there was variation in the location of the arrest, duration of no-flow and CPR. The inclusion criteria resulted in relatively low no-flow times as most studies included IHCA arrest, witnessed OHCA with bystander CPR, or mandatory low no-flow times. It is not known whether shorter no-flow and CPR duration before deploying ECLS results in a better outcome compared with conventional CPR. However, survival and outcome deteriorate as duration of no-flow and CPR increases [11].
Although vascular and bleeding complications are known to occur frequently during ECLS therapy, only a few of the included studies reported on these complications. Two previously published pooled analyses of complications of ECLS both reported high complication rates [29, 30]. They did not compare those rates with non-ECLS-treated patients. In these pooled analyses, lower limb ischaemia occurred in 16.9 and 10.7 %, which is comparable with our range of peripheral vessel complications, which is between 8.7 and 25 %. The occurrence of events may be directly related to ECLS therapy, or indirectly to the critical conditions of patients treated with ECLS. Either way we must keep in mind that survival with good neurological outcome might outweigh the risk for complications. In addition, complications during ECLS can only occur when patients are still alive for complications to occur. Therefore, the value of complications in these extremely high-risk patients is a relative one. The current meta-analysis found a survival rate of 45.2 % in cardiogenic shock patients and 27.4 % in the cardiac arrest patients treated with ECLS. These numbers are consistent with data from Xie et al., who performed a pooled analysis of observational cohort studies (without control arm) on patients treated with ECLS for refractory cardiogenic shock (n = 659) or for cardiac arrest (n = 277), and demonstrated a 30-day survival of 52.5 % in CS and 36.2 % in cardiac arrest [31].
Currently, ECLS has a class IIb recommendation (may be considered) in the European and American guidelines on myocardial revascularisation [6, 32]. The European Resuscitation Council (ERC) guidelines recommend that ECLS-assisted CPR should be considered to facilitate interventions [33]. Although the guidelines recommend consideration of ECLS, ECLS requires multidisciplinary expertise, which is often only available in a limited number of specialised centres. Experience is gained by providing ECLS support in remote locations and in the prehospital field to allow transfer to an experienced ECLS centre [27, 34–36]. In addition, the high cost of ECLS is a limiting factor, which mandates appropriate case selection.
Although the findings of this meta-analysis were limited by the heterogeneity of included studies, in the absence of large randomised trials, this pooled analysis represents the best available method for evaluating ECLS. These data should be taken into account when updating the clinical guidelines on cardiac arrest. Ultimately, to clarify the role of ECLS in cardiogenic shock and cardiac arrest, a randomised controlled trial should be undertaken; however, many randomised trials in this patient category have been aborted as a result to low inclusion rates [37]. Therefore, while aiming for a randomised trial, large multicentre registries could be the first step towards identifying patients that may benefit from ECLS or other circulatory support devices.
In conclusion, the current meta-analysis aggregated all available evidence on the effectiveness of ECLS in the continuous field of cardiac failure, ranging from cardiogenic shock to cardiac arrest. In the setting of refractory cardiac arrest, the meta-analysis showed increased survival and favourable neurological outcomes in the ECLS-treated patients. In the setting of cardiogenic shock there was an increased survival with ECLS compared with IABP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
J.P.S. Henriques reports research grants outside the submitted work. The other authors do not declare any conflicts of interest.
References
- 1.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 2.Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, Muller T, Windisch W. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975–2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35:156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 5.Ouweneel DM, Henriques JP. Percutaneous cardiac support devices for cardiogenic shock: current indications and recommendations. Heart. 2012;98:1246–1254. doi: 10.1136/heartjnl-2012-301963. [DOI] [PubMed] [Google Scholar]
- 6.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 13 Jan 2016
- 9.Tsao NW, Shih CM, Yeh JS, Kao YT, Hsieh MH, Ou KL, Chen JW, Shyu KG, Weng ZC, Chang NC, Lin FY, Huang CY. Extracorporeal membrane oxygenation-assisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial infarction complicated by profound cardiogenic shock. J Crit Care. 2012;27:530.e1–530.e11. doi: 10.1016/j.jcrc.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Blumenstein J, Leick J, Liebetrau C, Kempfert J, Gaede L, Gross S, Krug M, Berkowitsch A, Nef H, Rolf A, Arlt M, Walther T, Hamm CW, Mollmann H (2015) Extracorporeal life support in cardiovascular patients with observed refractory in-hospital cardiac arrest is associated with favourable short and long-term outcomes: a propensity-matched analysis. Eur Heart J Acute Cardiovasc Care. doi:10.1177/2048872615612454 [DOI] [PubMed]
- 11.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 12.Chou TH, Fang CC, Yen ZS, Lee CC, Chen YS, Ko WJ, Wang CH, Wang SS, Chen SC. An observational study of extracorporeal CPR for in-hospital cardiac arrest secondary to myocardial infarction. Emerg Med J. 2014;31:441–447. doi: 10.1136/emermed-2012-202173. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Jung JS, Park JH, Park JS, Hong YS, Lee SW. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out-of-hospital cardiac arrest: a propensity-matched study. Crit care. 2014;18:535. doi: 10.1186/s13054-014-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH, Jung JS, Lee KH, Kim HJ, Son HS, Sun K. Comparison of extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: is extracorporeal cardiopulmonary resuscitation beneficial? Korean J Thorac Cardiovasc Surg. 2015;48:318–327. doi: 10.5090/kjtcs.2015.48.5.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41:1186–1196. doi: 10.1097/CCM.0b013e31827ca4c8. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, Hase M, Tahara Y, Atsumi T. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Shin TG, Jo IJ, Sim MS, Song YB, Yang JH, Hahn JY, Choi SH, Gwon HC, Jeon ES, Sung K, Lee YT, Choi JH. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol. 2013;168:3424–3430. doi: 10.1016/j.ijcard.2013.04.183. [DOI] [PubMed] [Google Scholar]
- 18.Siao FY, Chiu CC, Chiu CW, Chen YC, Chen YL, Hsieh YK, Lee CH, Wu CT, Chou CC, Yen HH. Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation. 2015;92:70–76. doi: 10.1016/j.resuscitation.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, Song YB, Hahn JY, Choi SH, Gwon HC, Jeon ES, Sung K, Kim WS, Lee YT. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011;39:1–7. doi: 10.1097/CCM.0b013e3181feb339. [DOI] [PubMed] [Google Scholar]
- 20.Chamogeorgakis T, Rafael A, Shafii AE, Nagpal D, Pokersnik JA, Gonzalez-Stawinski GV. Which is better: a miniaturized percutaneous ventricular assist device or extracorporeal membrane oxygenation for patients with cardiogenic shock? ASAIO J. 2013;59:607–611. doi: 10.1097/MAT.0b013e3182a8baf7. [DOI] [PubMed] [Google Scholar]
- 21.Lamarche Y, Cheung A, Ignaszewski A, Higgins J, Kaan A, Griesdale DE, Moss R. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J Thorac Cardiovasc Surg. 2011;142:60–65. doi: 10.1016/j.jtcvs.2010.07.075. [DOI] [PubMed] [Google Scholar]
- 22.Sattler S, Khaladj N, Zaruba MM, Fischer M, Hausleiter J, Mehilli J, Kaab S, Hagl C, Massberg S, Theiss HD. Extracorporal life support (ECLS) in acute ischaemic cardiogenic shock. Int J Clin Pract. 2014;68:529–531. doi: 10.1111/ijcp.12380. [DOI] [PubMed] [Google Scholar]
- 23.Sheu JJ, Tsai TH, Lee FY, Fang HY, Sun CK, Leu S, Yang CH, Chen SM, Hang CL, Hsieh YK, Chen CJ, Wu CJ, Yip HK. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810–1817. doi: 10.1097/CCM.0b013e3181e8acf7. [DOI] [PubMed] [Google Scholar]
- 24.Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Schmidt M, Mastroianni C, Chastre J, Leprince P, Anselmi A, Combes A. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42:370–378. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36:2246–2256. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Bougouin W, Geri G, Dumas F, Champigneulle B, Legriel S, Charpentier J, Mira JP, Sandroni C, Cariou A. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42:1128–1136. doi: 10.1007/s00134-016-4349-9. [DOI] [PubMed] [Google Scholar]
- 27.Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura K, Sera A, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–1613. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- 28.Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, Serruys PW. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30:2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 29.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, Patroniti N, Antonelli M, Pesenti A, Pappalardo F. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 30.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1866 adult patients. Ann Thorac Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Xie A, Phan K, Tsai YC, Yan TD, Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a meta-analysis. J Cardiothorac Vasc Anesth. 2015;29:637–645. doi: 10.1053/j.jvca.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Cave DM, Gazmuri RJ, Otto CW, Nadkarni VM, Cheng A, Brooks SC, Daya M, Sutton RM, Branson R, Hazinski MF. Part 7: CPR techniques and devices: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S720–S728. doi: 10.1161/CIRCULATIONAHA.110.970970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soar J, Nolan JP, Bottiger BW, Perkins GD, Lott C, Carli P, Pellis T, Sandroni C, Skrifvars MB, Smith GB, Sunde K, Deakin CD, Adult advanced life support section collaborators European Resuscitation Council guidelines for resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, Pavie A, Combes A, Leprince P. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program) Eur Heart J. 2013;34:112–120. doi: 10.1093/eurheartj/ehs081. [DOI] [PubMed] [Google Scholar]
- 35.Lamhaut L, Jouffroy R, Soldan M, Phillipe P, Deluze T, Jaffry M, Dagron C, Vivien B, Spaulding C, An K, Carli P. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation. 2013;84:1525–1529. doi: 10.1016/j.resuscitation.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41:2039–2056. doi: 10.1007/s00134-015-4051-3. [DOI] [PubMed] [Google Scholar]
- 37.Ouweneel DM, Engstrom AE, Sjauw KD, Hirsch A, Hill JM, Gockel B, Tuseth V, van der Schaaf RJ, Henriques JP. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol. 2016;202:894–896. doi: 10.1016/j.ijcard.2015.10.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.