Summary
Recent studies have implicated keratin 5 (KRT5)+ cells in repopulation of damaged lung tissue following severe H1N1 influenza virus infection. However, the origins of the cells repopulating the injured alveolar region remain controversial. We sought to determine the cellular dynamics of lung repair following influenza infection and define whether nascent KRT5+ cells repopulating alveolar epithelium were derived from pre-existing alveolar or airway progenitor cells. We found that the wound-healing response begins with proliferation of SOX2+ SCGB1A1− KRT5− progenitor cells in airways. These cells generate nascent KRT5+ cells as an early response to airway injury and yield progeny that colonize damaged alveolar parenchyma. Moreover, we show that local alveolar progenitors do not contribute to nascent KRT5+ cells after injury. Repopulation of injured airway and alveolar regions leads to proximalization of distal airways by pseudostratified epithelium and of alveoli by airway-derived epithelial cells that lack the normal characteristics of mature airway or alveolar epithelium.
Keywords: lung repair, keratin 5, wound repair, lung injury, SCGB1A1 lineage, SOX2 lineage, progenitor cells, influenza virus
Graphical Abstract
Highlights
-
•
Influenza induces KRT5+ cell appearance in remodeled distal lung epithelium
-
•
Alveolar progenitor cells do not contribute to nascent KRT5+ cells
-
•
Multiple airway progenitor cells give rise to nascent KRT5+ cells in airways
-
•
SOX2 lineage-labeled cells are the major cellular source of nascent KRT5+ cells
Stripp and colleagues report that H1N1 influenza virus infection in mice induces distal lung epithelial remodeling marked by the appearance of nascent KRT5+ cells in injured airways and alveoli. Rather than pre-existing basal, club, and alveolar progenitor cells, they traced the cellular origin of these nascent KRT5+ cells to a population of airway-resident SOX2+ Lin− progenitor cells.
Introduction
The mammalian respiratory tract is normally maintained by abundant progenitors that differ in molecular phenotype, differentiation potential, and regulation between airway regions along the proximodistal axis (Hogan et al., 2014, Rackley and Stripp, 2012). Basal cells maintain the pseudostratified epithelium of proximal airways of mice by self-renewal and differentiation into club cells and ciliated cells (Rock and Hogan, 2010, Rock et al., 2009). In contrast, a self-renewing subset of club progenitors maintains the basal cell-deficient simple epithelium of distal bronchial and bronchiolar airways (Evans et al., 1978, Rawlins et al., 2009). Alveolar type II (AT2) cells function as local progenitors to maintain the alveolar epithelium and differentiate into alveolar type I (AT1) cells (Barkauskas et al., 2013, Evans et al., 1978). Although each of these progenitors contribute to epithelial homeostasis and repair following mild injury, their contribution to repair following severe injury remains unclear.
Influenza virus infection causes widespread lung epithelial cell death. However, patients and animals typically recover from influenza virus infection, implicating a role for stem-cell-mediated repair mechanisms. After influenza virus infection in mice focal clusters of cytokeratin 5 immunostained (KRT5+), transcription factor TRP63 immunostained (TRP63+) cells appear in the alveoli (referred to as nascent KRT5+ pods) (Kumar et al., 2011). However, the cellular origin of nascent KRT5+ pods and their contribution to repair remain controversial (Kumar et al., 2011, Mahoney and Kim, 2015, McQualter and Laurent, 2015, Rawlins, 2015, Vaughan et al., 2015, Zuo et al., 2015). Clarifying the identity and distribution of progenitor cells from which nascent KRT5+ cells are derived is a prerequisite toward understanding the wound-healing response to influenza infection. In this study we define the cellular dynamics of the wound-healing response to influenza infection and reveal that nascent KRT5+ cells in both the airways and alveolar parenchyma descend from airway-resident progenitor cells.
Results
Ectopic Appearance of Nascent KRT5+ Cells in Distal Airways Precedes KRT5+ Pod Appearance following PR8 Infection
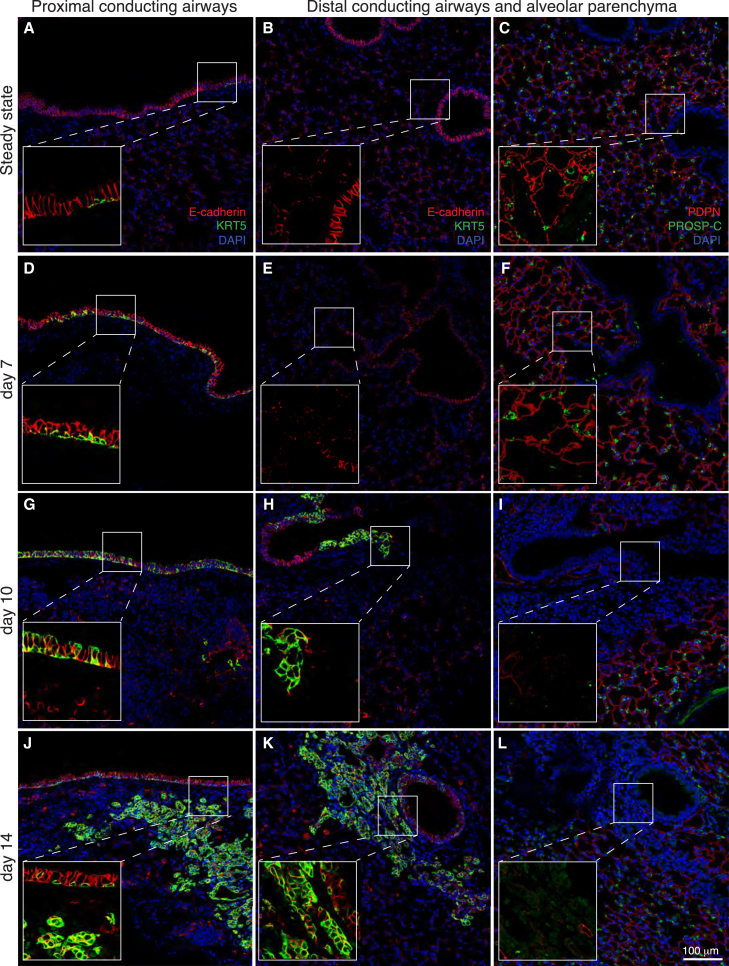
To systematically map the emergence of nascent KRT5+ cells, we performed immunofluorescence staining of mouse lung tissue at time points associated with active epithelial repair and remodeling following PR8 infection (Kumar et al., 2011, Vaughan et al., 2015, Zuo et al., 2015). Figure 1 shows that KRT5+ cells were very sparse in uninjured lungs, with distribution largely restricted to the proximal regions of large conducting airway epithelium (Figure 1A) and absent in distal airways and alveolar regions (Figure 1B). Immunostaining for the AT1 and AT2 cell markers, podoplanin (PDPN) and prosurfactant protein C (PROSP-C), respectively, showed intact alveolar structures at this time point (Figure 1C). Conversely, 7 days after PR8 infection proximal airways were lined by a pseudostratified epithelium that included numerous KRT5+ cells extending along the basement membrane with KRT5− cells at the epithelial luminal surface (Figure 1D), whereas distal conducting airways and alveolar regions remained devoid of KRT5+ cells (Figure 1E) and showed no evidence of epithelial damage (Figure 1F). Ten days after PR8 infection we observed a further increase in the abundance of KRT5+ cells in the proximal airways, which appeared hyperplastic with KRT5+ cells at both the basal surface and airway lumen (Figure 1G). Notably, at this time we also observed a hyperplastic epithelium containing KRT5+ basal and luminal cells in distal conducting airways (Figure 1H) adjacent to focal areas of alveolar damage characterized by dense infiltrates of DAPI+ cells and loss of immunostaining for PDPN and PROSP-C (Figure 1I). By day 14 after PR8 infection, KRT5+ cells were abundant along the entirety of the proximal (Figure 1J) and distal (Figure 1K) conducting airways. Moreover, regions of alveolar damage included clusters of nascent KRT5+ cells (Figures 1K and 1L) similar to those described previously by Kumar et al. (2011). Our data show that PR8-induced remodeling of the respiratory epithelium initiates in the proximal conducting airways and proceeds in a proximal to distal wave leading to the appearance of ectopic KRT5+ cells in small airways and alveoli.
Figure 1.
Nascent KRT5+ Cell Appearance Occurs in a Proximal to Distal Pattern following PR8 Influenza Virus Infection in Mice and Results in Remodeling of the Distal Lung
Photomicrographs of lung tissue sections from control mice (A–C) and mice recovered for 7 days (D–F), 10 days (G–I), or 14 days (J–L) following infection with PR8 influenza virus. For each time point the distal conducting airways and alveolar parenchyma panels are serial sections. Sections are immunostained for the basal cell marker KRT5 (green) and the pan-epithelial cell marker, E-cadherin (red), or the AT1 cell marker, PDPN (red), and the AT2 cell marker PROSP-C (green). For each panel the zoomed insert shows magnified boxed region without DAPI staining to highlight co-stained regions. At least three animals were analyzed per time point. Scale bar, 100 μm.
Resident AT1 and AT2 Cells Do Not Contribute to the Formation of KRT5+ Alveolar Pods following PR8 Infection
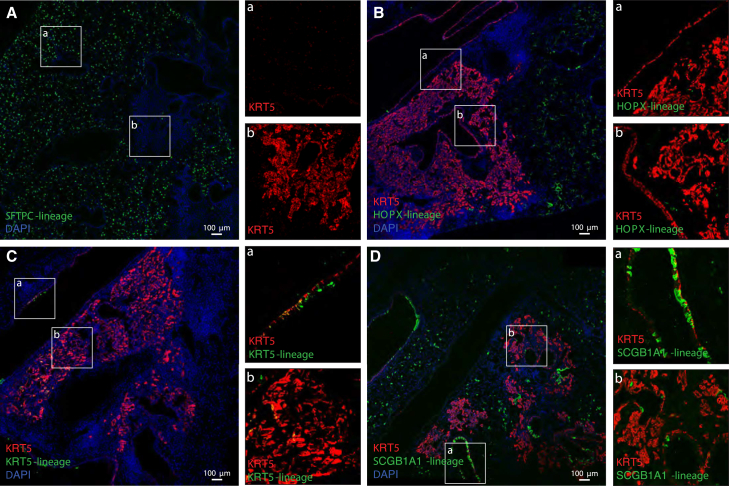
We reasoned that the appearance of ectopic KRT5+ epithelial cells within injured alveolar regions after PR8 infection might result from changes in the molecular phenotype of resident alveolar epithelial cell types or by recruitment of progenitors from the airways. In the alveoli, AT2 cells serve as a major progenitor cell for normal repair of the alveolar epithelium (Barkauskas et al., 2013). To determine whether pre-existing resident AT2 cells contributed to the appearance of KRT5+ pods following PR8 infection we used Sftpc-CreER;Rosa-26-TdTomato mice to indelibly tag AT2 cells and all of their progeny by tamoxifen injection. Immunostaining for the reporter shows extensive lineage labeling of PROSP-C+ AT2 cells in steady-state adult mice (Figure S1A). Fourteen days after PR8 infection, damaged alveolar regions with cellular infiltration (dense DAPI+ nuclei) and focal clusters of KRT5+ alveolar pods were notably devoid of SFTPC lineage-labeled cells (Figures 2A and 3F). In contrast, uninjured areas showed robust SFTPC lineage labeling (Figure 2A). Note that due to the incompatibility of primary antibodies, co-staining for TDTOMATO and KRT5 was not possible, therefore TDTOMATO and KRT5 staining was performed on serial sections. Nevertheless, the complete absence of overlapping staining between the SFTPC lineage label and KRT5 suggests that SFTPC lineage cells are not the cells of origin for nascent KRT5+ alveolar pod cells.
Figure 2.
Contribution of Pre-existing SFTPC, HOPX, KRT5, and SCGB1A1 Lineage-Labeled Cells to KRT5+ Cells after PR8 Infection
Sftpc-CreER, Rosa-tdtomato (A), Hopx-CreER, Rosa-mTmG (B), Krt5-CreER, Rosa-mTmG (C), and Scgb1a1-CreER, Rosa-mTmG (D) mice treated with tamoxifen to lineage label AT2, AT1, basal, or club cells, respectively, were infected with PR8 influenza virus and killed at day 14 post infection. Photomicrographs showing representative lung tissue sections immunostained for KRT5 (red), lineage markers (green), and DAPI (blue). In (A) RFP (green) and KRT5 (red) staining was performed on serial sections due to the incompatibility of primary antibodies. For each panel, the boxed region is magnified to highlight representative KRT5+ airway cells and alveolar pods. At least three animals were analyzed per time point. Scale bars, 100 μm. See also Figure S1.
Figure 3.
Pre-existing SOX2+ Cells Are the Major Cellular Source of KRT5+ Cells after PR8 Infection
Sox2-CreER, Rosa-mTmG mice treated with tamoxifen to lineage label airway epithelial cells were infected with PR8 influenza virus and killed 14 and 21 post infection. Photomicrographs (A–D) showing representative lung tissue sections immunostained for KRT5 (red), lineage markers (green), and DAPI (blue) (A–D). Scale bars, 100 μm. Scatterplots and pie charts show the quantitation and relative contribution, respectively, of HOPX, SFTPC, SCGB1A1, KRT5, and SOX2 cell lineages toward airway (E) and alveolar (F) KRT5+ cells after PR8 infection. Individual dots represent cells per animal (E) or cells per pod (F). Data include means ± SEM. Photomicrographs (G and H) show representative lung tissue sections from uninjured animals immunostained for KRT5, SCGB1A1, and FOXJ1 (red), lineage marker (green), and DAPI (blue). Arrows identify SOX2+ Lin− cells. At least three animals were analyzed per time point. See also Figures S1–S3 and Table S1.
Although AT2 cells are considered the dominant progenitor cell population in the alveoli recent data have also suggested that AT1 cells, demarked by expression of the transcription factor HOPX, can also function as progenitors for alveolar epithelium (Jain et al., 2015). To test if a surviving pool of AT1 cells could contribute to the KRT5+ alveolar pods, we used Hopx-CreER knockin mice that have previously been shown to label adult AT1 cells under steady state (Jain et al., 2015). As previously reported (Jain et al., 2015), we observed efficient lineage labeling of AT1 epithelial cells following tamoxifen treatment, many of which showed co-localization of the membrane-localized GFP lineage reporter with PDPN (Figure S1B). Fourteen days after PR8 infection of HOPX lineage-labeled mice we observed the characteristic pattern of injury with cellular infiltration and focal clusters of KRT5+ alveolar pods (Figure 2B). However, even though rare HOPX lineage-labeled cells were observed within injured alveolar regions we did not observe co-localization of KRT5 and lineage label within KRT5+ airways (Figures 2Ba and 3E) or alveolar pods (Figures 2Bb and 3F). These data demonstrate that HOPX lineage-labeled cells do not directly contribute to the formation of KRT5+ alveolar pods within the 14 day period after PR8 infection.
Resident Basal and Club Cells Make Minor Contributions to Nascent KRT5+ Cells following PR8 Infection
In the trachea, basal cells serve as local self-renewing progenitor cells (Rock et al., 2009). Thus it is reasonable to speculate that pre-existing KRT5+ basal cells may undergo atypical expansion and migration to generate nascent KRT5+ basal-like cells. However, previous studies assessing the contribution of resident airway basal cells to the generation of KRT5+ alveolar pods have provided varied results, which, at least in part, could be attributed to differences in the Krt5-CreER driver line and reporter systems used. Here we have used the Krt5-CreER knockin line to efficiently lineage label resident basal cells in the proximal airways prior to injury (Figure S1C). Fourteen days after PR8 infection, we observed only a fraction of the nascent KRT5+ cells that were KRT5 lineage labeled in the airways (15.5% ± 4.2%; Figures 2Ca and 3E) and alveolar pods (2.0% ± 0.43%; Figures 2Cb and 3F).
Alternatively, recent evidence suggests that KRT5+ basal cells can also be generated by de-differentiation of SCGB1A1+ club cells (Tata et al., 2013). Thus it is reasonable to speculate that pre-existing club cells might also contribute to nascent KRT5+ cells in airway and alveolar compartments after PR8 infection. Indeed, a study by Zheng et al. (2014) reported that SCGB1A1+ club cells are a major source of nascent KRT5+ cells after influenza infection. This was later refuted by Vaughan et al. (2015) who suggest that SCGB1A1 lineage cells might be mislabeled due to tamoxifen persistence, whereby the progeny of upstream progenitors are labeled rather than pre-existing club cells. To assess the fate of club cells we used a Scgb1a1-CreER knockin line which provided robust lineage labeling of airway columnar epithelial cells and some alveolar epithelial cells (Figure S1D). Fourteen days after PR8 infection we observed significant depletion of club cells in injured areas of the lung and only a very minor contribution of SCGB1A1 lineage-labeled cells to the generation of nascent KRT5+ cells in the airways (0.32% ± 0.27%; Figures 2Da and 3E) and alveolar pods (0.7% ± 0.6%; Figures 2Db and 3F). Overall, our results support a predominant role of an SCGB1A1 lineage-negative pool of progenitors in the generation of nascent KRT5+ cells following PR8 infection.
Resident SOX2+ Airway Epithelial Progenitor Cells Repopulate Injured Airway and Alveolar Epithelium after PR8 Infection
Since our lineage-tracing experiments showed that known lung progenitors (i.e., basal, club, AT2, and AT1 cells) make modest/no contribution to nascent KRT5+ cells following PR8 infection, we sought to determine whether an undefined pool of airway progenitors might be a source of nascent KRT5+ cells. To test this, lineage tracing was performed using mice harboring a Sox2-CreER knockin allele and a Rosa-26-mTmG fluorescent reporter. SOX2 labels basal, secretory, and ciliated epithelial cells in the airways of adult lung, but not alveolar cells (Figures S1E and S2 and Table S1).
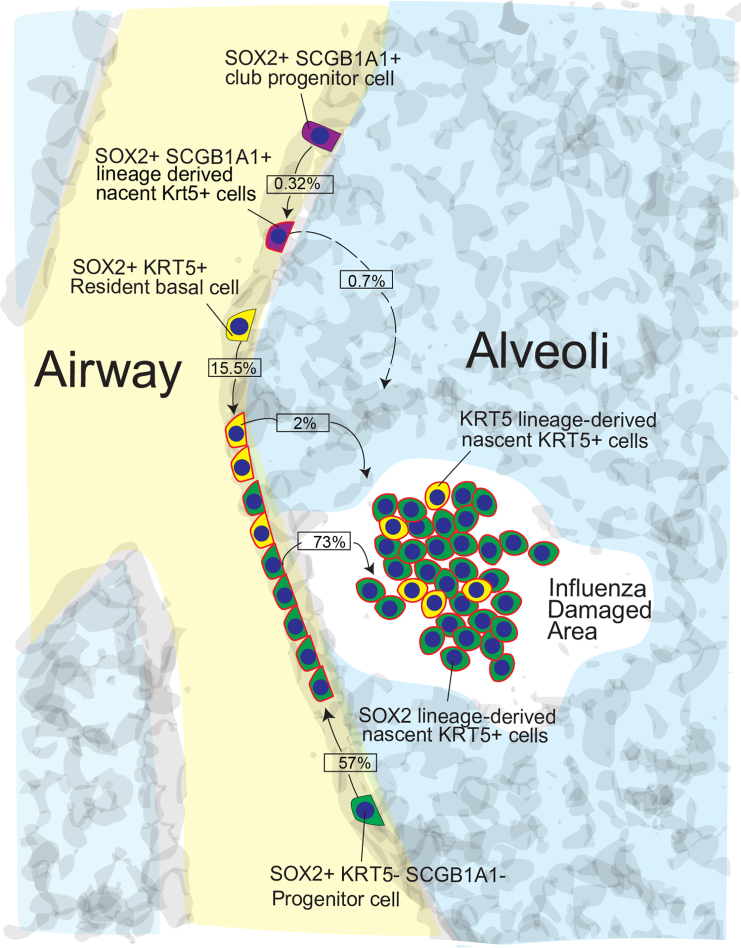
Interestingly, unlike other reporter mice that responded to PR8 infection similar to wild-type mice, comparison of Sox2-CreER/wt and Sox2-wt/wt control mice revealed an allele-specific defect in response to PR8 infection. Mice harboring the Sox2-CreER allele consistently displayed a significantly reduced incidence of KRT5+ alveolar pods after PR8 infection (Figure S3). Nevertheless, lineage analyses of nascent KRT5+ airway and alveolar pod cells revealed that the majority of nascent KRT5+ cells appearing in alveolar pods (73% ± 9.4%; Figures 3A–3F) and hyperplastic airways (57% ± 7.6%; Figures 3D–3F) were indeed SOX2 lineage labeled. However, it is important to note that while many KRT5+ alveolar pods were composed entirely of SOX2 lineage-labeled cells (Figure 3Aa), we also observed KRT5+ alveolar pods that were of mixed lineage (i.e., containing SOX2 lineage-negative and SOX2 lineage-positive cells; Figure 3Ba) as well as some cells that were entirely SOX2 lineage negative (Figure 3Ca). This could reflect either incomplete lineage labeling of SOX2-expressing cells at the time of infection or be indicative of an alternative SOX2− progenitor cell population that also contributes to the repair and remodeling processes following PR8 infection. Comparison of the relative contributions of the various cell lineages toward nascent Krt5+ cells in the airways and alveolar pods after PR8 infection shows that while multiple pools of airway progenitors (i.e., SOX2+ SCGB1A1+ and SOX2+ KRT5+ cells) contribute to the generation of nascent KRT5+ cells in the airways (Figure 3E) and alveoli (Figure 3F), the predominant cell of origin is a population of SOX2+ SCGB1A1− KRT5− cells (SOX2+ Lin−) cells. Immunostaining and lineage labeling of uninjured lungs showed that SOX2+ FOXJ1− SCGB1A1− KRT5− cells were scattered sporadically throughout the entire airway epithelium (Figures 3G and 3H).
Discussion
Herein we show that the appearance of KRT5+ pods in the alveolar parenchyma of influenza-virus-infected mice (Kumar et al., 2011, Vaughan et al., 2015, Zuo et al., 2015) is preceded by an increase in KRT5+ cells and hyperplastic remodeling in airways. Epithelial remodeling occurs focally in airways and alveoli in response to severe epithelial cell depletion that includes loss of normally abundant local progenitor cells. The emergence of nascent KRT5+ cells in a proximal to distal wave suggests that the wound-healing response initiates in the airways. Given the severity of epithelial cell destruction following influenza virus infection this response of airway progenitor cells is not unlike basal cell hyperplasia that has been reported in tracheobronchial airways following exposure to naphthalene that specifically targets abundant secretory progenitor cells (Hong et al., 2004). However, naphthalene-induced depletion of airway cells was not associated with the appearance of basal cells or the formation of a pseudostratified epithelium in distal bronchiolar airways (Hong et al., 2001, Stripp et al., 1995). We find that remodeled pseudostratified or hyperplastic airway epithelium was adjacent to damaged alveolar regions, marked by loss of AT1 and AT2 cells, and included distal bronchiolar airways. Thus it is possible that signals emanating from the injured alveolar regions contribute to cell-fate decisions that were uniquely impacted by influenza virus infection.
To date, the question of whether cells migrate from the airways to populate the alveolar parenchyma after infection or if a pre-existing population of alveolar cells proliferate locally to replace lost alveolar epithelial cells has remained unclear. Previous reports have suggested that both KRT5 lineage-labeled cells (Vaughan et al., 2015, Zuo et al., 2015) and SCGB1A1 lineage-labeled cells (Zheng et al., 2012, Zheng et al., 2014) contribute, albeit to variable extents, to KRT5+ alveolar pod cells following influenza virus infection. Here we confirm previous studies by Vaughan et al. (2015) that SCGB1A1 lineage (club) cells and SFTPC lineage (AT2) cells have very minimal to no contribution to the generation of nascent KRT5+ cells in either the airways or alveolar space. In addition, we show here that HOPX lineage (AT1) cells also do not give rise to nascent KRT5+ cells. Rather, we show that resident alveolar epithelial cell types are ablated at focal sites of severe virus-induced parenchymal injury. Our data support the concept that rather than a local proliferative response by alveolar epithelial cells, nascent KRT5+ cells arise elsewhere in the lung and colonize regions of alveolar injury. As for pre-existing basal cells, we again confirm previous studies by Vaughan et al. (2015) that surviving KRT5 lineage cells do have a minor contribution to the generation of nascent KRT5+ cells. However, the collective contribution of all the pre-existing cell lineages assessed (i.e., KRT5, SCGB1A1, HOPX, and SFTPC lineages) accounts for only a nominal portion of the nascent KRT5+ cells, suggesting the existence of an alternative progenitor cell of distinct lineage. Our use of the PR8 strain of influenza virus to induce severe lung injury does not exclude the possibility that, under conditions of less severe injury or altered cellular tropism, more club and basal cells may survive and be able to generate nascent KRT5+ cells. These data suggest that the loss of more abundant epithelial progenitor cells from the airways and alveoli may be the driving force for recruitment of previously unidentified epithelial progenitor cells that typically make minimal contributions to normal tissue maintenance.
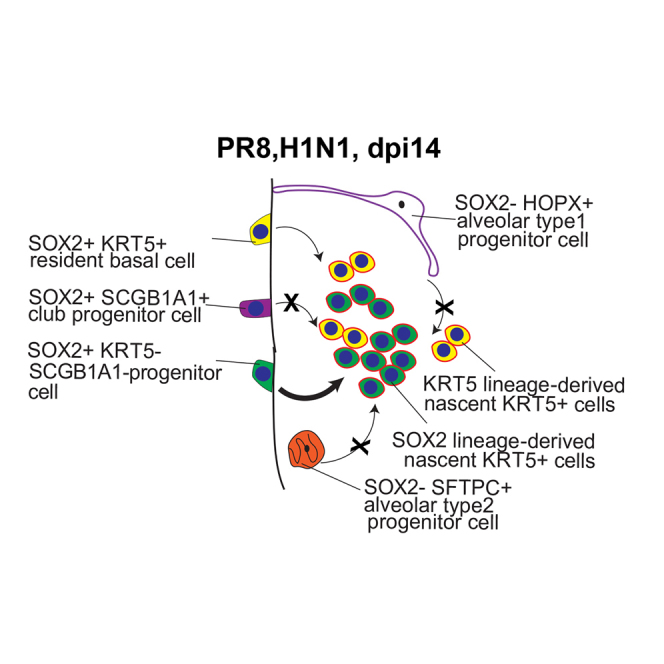
Here we provide the first definitive evidence that an alternative airway progenitor cell, marked by expression of SOX2, is the predominant cell of origin for nascent KRT5+ cells after influenza infection. This was true for nascent KRT5+ cells that emerge in the airways and those that appear later in the alveolar parenchyma, suggesting a common SOX2 lineage ancestry (Figure 4). Thus, given that SOX2 is exclusively expressed in the airway epithelium in the adult lung, these studies suggest that the wound-healing response initiates with SOX2+ SCGB1A1− KRT5− HOPX− SFTPC− (SOX2+ Lin−) cells giving rise to nascent KRT5+ cells in the airways, which then populate the damaged alveolar parenchyma.
Figure 4.
Activation of Airway-Resident SOX2+ Lin− Progenitor Cells in Response to Influenza-Induced Severe Lung Injury
The schematic shows that following PR8 influenza virus infection-induced lung injury SOX2+ Lin− progenitor cells are the major source of nascent KRT5+ cells in the airway and alveolar zones of cell loss. The percent contribution of SOX2+ Lin+ and SOX2+ Lin− progenitor cells has been included.
A limitation of this study is the broad expression of SOX2 in all airway epithelial cells, which prevents us from establishing the precise molecular identity of these progenitor cells. Thus, while we can rule out club cells, bronchioalveolar stem cells, and basal airway cells because of their SCGB1A1 or KRT5 lineages, it is unclear how the SOX2+ Lin− cells relate to previously identified α6+β4+ multipotent epithelial stem cells (Chapman et al., 2011, McQualter et al., 2010) and β4+ CD200+ CD14+ progenitor cells (Vaughan et al., 2015).
In summary, these studies suggest that the widespread destruction of airway and alveolar epithelial cells following severe influenza infection triggers a wound-healing response that initiates in the airways with the proliferation of rare airway-resident SOX2+ Lin− progenitor cells. These cells then give rise to nascent KRT5+ cells in remodeled airways and populate the damaged alveolar parenchyma. Overall, this study provides important insights into the selective use of different progenitor cell pools and their role in repair after severe tissue damage such as that induced by H1N1 influenza virus infection.
Experimental Procedures
Mouse Strains
Adult mice (2–4 months old) were used according to Institutional Animal Care and Use Committee-approved protocols. Hopx-CreER (Jain et al., 2015), Sftpc-CreER (Rock et al., 2011), Scgb1a1-CreER (Rawlins et al., 2009), Krt5-CreER (Rock et al., 2009), and Sox2-CreER mice (Arnold et al., 2011) were obtained from Jackson Laboratory. These mice were bred to Rosa-26-mTmG (Jackson Laboratory; Muzumdar et al., 2007) or Rosa-26- tdtomato (supplied by D. Jiang and P.W. Noble) for lineage-tracing experiments.
Tamoxifen Administration and Virus Infection
Tamoxifen, prepared by sonication in corn oil, was administered in three intraperitoneal injections (200 mg/kg per injection) every other day. A chase period of 3 weeks was allowed to eliminate residual levels of tamoxifen before virus infection. A dose of 200 pfu of a murine-adapted H1N1 influenza A (A/PR/8/34) virus was administered by intratracheal inhalation. Virus stock was obtained from ATCC (VR-95). Weights and behavioral activity were recorded daily.
Immunofluorescence Staining
Immunostaining was performed on 7 μm sections of formalin-fixed, paraffin-embedded tissue as described previously by Chen et al. (2012). The following primary antibodies were used: rabbit anti-Keratin 5 (1:1,000, Santa Cruz, sc-66856), mouse anti-E-Cadherin (1:1,000, BD Biosciences, 610182), chicken anti-GFP (1:1,000, Abcam, ab13970), Syrian hamster anti-Podoplanin (1:2,000, LifeSpan Biosciences, clone 8.1.1, LS-C143022), rabbit anti-Prosurfactant Protein C (1:1,000, Millipore, AB3786), rabbit anti-SOX2 (1:1,000, Seven Hills Bioreagents, WRAB-1236), rabbit anti-RFP (1:400, Rockland, 600-401-379), rabbit anti-SCGB1A1 (1:10,000, generated in-house by B. Stripp), and mouse IgG1 anti-FOXJ1 (1:300, eBioscience, 14-9965-82). Sections were incubated overnight at 4°C with respective primary antibodies. Sections were then washed with PBS to remove unbound antibody and incubated with Alexa Fluor-conjugated secondary antibodies and nuclear stain DAPI (1:1,000) at room temperature for 45 min. Immunostained slides were then washed with PBS to remove unbound secondary antibody and mounted with Fluormount-G (Electron Microscopy Sciences, catalog no. 17984-25).
Microscopy
Images were taken using a Zeiss LSM 780 confocal or a Zeiss Observer.Z1 microscope. Quantitation was done on at least three independent animals per condition.
Quantitation of Lineage-Tracing Analysis of KRT5+ Cells after PR8 Infection
Serial sections of entire caudal and cranial lobes were analyzed from surviving flu-infected animals at the indicated time points. KRT5+ alveolar pods were defined as a group of at least four contiguous KRT5-immunostained cells within the alveolar zone, which were not associated with bronchiolar epithelial cells. For each KRT5+ pod, the percentage of lineage-positive (Lin+) cells was assayed by counting the proportion of KRT5-immunostained cells that were co-immunostained for GFP (lineage marker). Similarly, the lineage contribution toward KRT5+ basal cells was scored by counting the percentage of KRT5+ basal cells that were co-immunostained with GFP (lineage marker).
Author Contributions
Conceptualization, S.R. and B.R.S.; Methodology, S.R., N.C., X.G., C.Y., and A.M.M.; Investigation, S.R., N.C., J.L.M., and A.M.M.; Writing, S.R., J.L.M., and B.R.S.; Funding Acquisition, B.R.S,; Resources, L.Q. and B.B.
Acknowledgments
We are grateful to T. Mizuno and G. Carraro for insightful discussions, support, and critical input during the study and manuscript preparation, and M. Kostelny for help with maintaining the animal colonies. This study was funded by CIRM LA1-06915 and LRRC-UO1HL111018.
Published: October 20, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.09.010.
Supplemental Information
References
- Arnold K., Sarkar A., Yram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.A., Li X., Alexander J.P., Brumwell A., Lorizio W., Tan K., Sonnenberg A., Wei Y., Vu T.H. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Matsumoto K., Brockway B.L., Rackley C.R., Liang J., Lee J.H., Jiang D., Noble P.W., Randell S.H., Kim C.F. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Cabral-Anderson L.J., Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab. Invest. 1978;38:648–653. [PubMed] [Google Scholar]
- Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C., Niklason L., Calle E., Le A., Randell S.H. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Giangreco A., Hurley C.M., Stripp B.R. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Barkauskas C.E., Takeda N., Bowie E.J., Aghajanian H., Wang Q., Padmanabhan A., Manderfield L.J., Gupta M., Li D. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D., Sun Y., Joo L.S., Dagher R., Zielonka E.M. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney J.E., Kim C.F. Tracing the potential of lung progenitors. Nat. Biotechnol. 2015;33:152–154. doi: 10.1038/nbt.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter J.L., Laurent G.J. Delineating the hierarchy of lung progenitor cells and their response to influenza. Eur. Respir. J. 2015;46:315–317. doi: 10.1183/09031936.00011915. [DOI] [PubMed] [Google Scholar]
- McQualter J.L., Yuen K., Williams B., Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Rackley C.R., Stripp B.R. Building and maintaining the epithelium of the lung. J. Clin. Invest. 2012;122:2724–2730. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E.L. Stem cells: emergency back-up for lung repair. Nature. 2015;517:556–557. doi: 10.1038/517556a. [DOI] [PubMed] [Google Scholar]
- Rawlins E.L., Okubo T., Xue Y., Brass D.M., Auten R.L., Hasegawa H., Wang F., Hogan B.L. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Hogan B.L. Developmental biology. Branching takes nerve. Science. 2010;329:1610–1611. doi: 10.1126/science.1196016. [DOI] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp B.R., Maxson K., Mera R., Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am. J. Physiol. 1995;269:L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- Tata P.R., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B.M., Vinarsky V., Cho J.L., Breton S., Sahay A. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.E., Brumwell A.N., Xi Y., Gotts J.E., Brownfield D.G., Treutlein B., Tan K., Tan V., Liu F.C., Looney M.R. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Limmon G.V., Yin L., Leung N.H., Yu H., Chow V.T., Chen J. Regeneration of alveolar type I and II cells from Scgb1a1-expressing cells following severe pulmonary damage induced by bleomycin and influenza. PLoS One. 2012;7:e48451. doi: 10.1371/journal.pone.0048451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Yin L., Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am. J. Respir. Cell Mol. Biol. 2014;50:595–604. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- Zuo W., Zhang T., Wu D.Z., Guan S.P., Liew A.A., Yamamoto Y., Wang X., Lim S.J., Vincent M., Lessard M. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.