Abstract
Background:
Oxidative stress plays an important role in the pathogenesis and progression of γ-irradiation-induced cellular damage, Lung is a radiosensitive organ and its damage is a dose-limiting factor in radiotherapy. The administration of dietary antioxidants has been suggested to protect against the succeeding tissue damage. The present study aimed to evaluate the radioprotective efficacy of Hesperidin (HES) against γ-irradiation-induced tissue damage in the lung of male rats.
Materials and Methods:
Thirty two rats were divided into four groups. Rats in Group 1 received PBS and underwent sham irradiation. Rats in Group 2 received HES and underwent sham irradiation. Rats in Group 3 received PBS and underwent γ-irradiation. Rats in Group 4 received HES and underwent γ-irradiation. These rats were exposed to γ-radiation 18 Gy using a single fraction cobalt-60 unit, and were administered HES (100 mg/kg/d, b.w, orally) for 7 days prior to irradiation. Rats in each group were sacrificed 24 hours after radiotherapy (RT) for the determination of superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA) and histopathological evaluations.
Results:
Compared to group 1, the level of SOD and GSH significantly decreased and MDA level significantly increased in group 3 at 24 h following irradiation, (p=0.001, p<0.001, p=0.001), respectively. A statistically significant difference in all parameters was observed for rats in group 4 as compared to group 3 (p<0.05). Histopathological results 24 hours after RT showed that radiation has increased inflammation, lymphocyte, macrophage and neutrophil compared to group 1 ( p<0.0125). Oral administration of HES before RT significantly decreased macrophage and neutrophil when compared to group 3 (p<0.0125), but partly there was inflammation and lymphocyte that indicated there was no significant difference when compared to group 3 (p>0.0125).
Conclusion:
Oral administration of HES was found to offer protection against γ-irradiation- induced pulmonary damage and oxidative stress in rats, probably by exerting a protective effect against inflammatory disorders via its free radical scavenging and membrane stabilizing ability.
Keywords: Hesperidin , Radioprotector , Oxidative Stress , Inflammation
Introduction
Radiotherapy (RT) is one of the most common and important techniques for cancer treatment which is performed with the intent to cure or to palliate[1,2]. Lung is a radiosensitive organ. In patients with thorax and chest wall malignancies including breasts, lung, esophagus, lymphomas or any other mediastinal neoplasms, irradiation of the lungs is inevitable[3]. Lung injuries are divided into two distinct phases. The first or early phase is called radiation pneumonitis (acute syndrome) as evidenced by alveolar edema, alveolar neutrophils, alveolar erythrocytes and foamy macrophages according to histopathological evaluation. According to studies of histopathological changes in this phase, extensive alveolar damage is considered as the first symptom of a lung injury[4]. The second or latent phase (chronic syndrome) is the occurrence of pulmonary fibrosis months to years after RT.
However, ionizing radiation can break chemical bonds and damage living cells due to free radical generation during water radiolysis in the cells. The ruinous effect of ionizing radiation is generally due to reactive oxygen species (ROS) including superoxide radical (O2º¯), hydroxyl radical (OHº) and hydrogen peroxide (H2O2) generated by the decomposition of water[6]. One of the indices of oxidative damage is the malondialdehyde (MDA) formation as an end product of lipid peroxidation. Because of the serious damaging potentials of ROS, cells depend on the elaboration of antioxidant defense system (AODS), both enzymatic and non-enzymatic oxidant defense mechanisms[7]. These antioxidant systems consist of low molecular weight antioxidants like glutathione (GSH) and various antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). SOD enzyme catalyzes the dismutation of O2º¯into H2O2. H2O2 can be transformed into H2O and O2 by CAT and GSH-Px enzymes. GSH acts as a substrate or cofactor for the antioxidant enzymes, GSH peroxidase, GSH transferase and reductase which are involved in the termination of hydroperoxides[5,6].
In spite of the improvements in the development of clinical RT treatment planning and treatment delivery technologies, there remains a significant toxicity of RT to normal tissues and organs[7]. In order to obtain better tumor control with a higher dose, normal tissues should be protected against radiation injury. Thus, the role of radioprotective compounds is very important in clinical RT[1]. Though, a large variety of compounds has shown promise as radioprotectors in laboratory studies, most of them failed even before reaching the preclinical stage due to their toxicity and side effects. The search for less toxic radiation protectors has spurred interest in the development of natural products.
Flavonoids are a family of polyphenolic compounds discovered in fruits and vegetables. Flavonoids have wide biological properties including antibacterial, antiviral, anticancer, immunostimulant and antioxidant effects[8]. Hesperidin (Hesperetin-7-rhamnoglucoside) is a flavonone glycoside belonging to flavonoid family. Hesperidin (HES) is the main flavonoid in sweet orange and lemon. In young unripe oranges, it accounts for up to 14% of the fresh weight of fruit[9]. Several investigations have demonstrated that HES has radioprotective properties to protect normal tissue against RT[10,12]. The present investigation was performed with the objective of determining the protective effect of HES against γ-irradiation induced acute lung injury and oxidative stress in the lung tissue of male Sprague-Dawley rats.
Materials and Methods
Chemicals and Animals
All chemicals including Hesperidin [CAS registry number: 520-26-3], phosphate buffered saline tablet (PBS), thiobarbituric acid, trichloroacetic acid, 1,1,3,3 tetraethoxypropane, ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), HEPES buffer, mannitol and sucrose were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Healthy adult male Sprague-Dawley rats were purchased from Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Rats weighing 220±5 gr were housed in accordance with the principles outlined in “The Guide for The Care and Use of Laboratory Animals” prepared by Shiraz University of Medical Sciences in the university animal house, and given standard pellet diet and water ad libitum. All animals were kept under controlled conditions of temperature (23±2°C), humidity (55±5%) and light (12 h of light and dark cycle). Four animals were housed together in polypropylene cages containing sterile husk bedding throughout the experiment.
Irradiation of Animals
Prior to radiation exposure, rats received anesthesia using ketamine at a dose of 80 mg/kg and xylazine at a dose of 5 mg/kg via an intraperitoneal injection. The rats were immobilized in the supine position by taping the extremities on a well-ventilated plexiglas container. Irradiation of animals was carried out with a cobalt-60 γ-radiation source (Theratron Phoenix, Canada) in the department of radiotherapy, Namazi Teaching Hospital, Shiraz, Iran. Anesthetized animals were irradiated locally on thorax in groups of five rats, simultaneously. The source-to-skin distance was 55 cm with a dose rate of 30 cGy/min at room temperature. The Rats were irradiated with a single dose of 18 Gy γ-rays.
Administration of HES
HES was dissolved in PBS (pH 7.6) and administered orally using a ball tipped needle for 7 consecutive days prior to exposure to γ-irradiation. The drug was freshly prepared on each day. The dose 100 mg/kg of HES was selected for this study based on the reports of Hosseinimehr, et al.13 where they had shown the protective effect of various doses of HES against γ-irradiation induced damage to mouse bone marrow cells.
Experimental Design
Male rats were randomly divided into 4 groups. Group 1 (Control): Eight rats served as controls only received PBS for 7 days. Group 2 (HES): Eight rats were treated with HES for 7 days. Group 3 (Radiation): Eight rats received PBS for 7 days and one hour after the last dose of PBS exposed to γ-irradiation. Group 4 (HES+R): Eight rats were treated with HES for 7 days and one hour after the last dose of HES exposed to γ-irradiation. After the last administration of HES or PBS on the seventh day, the animals in Control and HES groups were anesthetized like rats in Radiation and HES+R groups. Rats were sacrificed 24 hours after RT for biochemical assay and acute histopathological evaluation.
Supernatant Preparation
Twenty-four hours after the last dose of specific treatment, animals were anesthetized with ketamine and xylazine, and a laparotomy was performed then, the chest was opened. The lung was perfused in situ through the right ventricle of the heart with sodium chloride 0.9 % and diced with scissors. The perfused tissue was removed to any red blood cells and clot with PBS, pH 7.4, and for determination of SOD activity 0.3 gram of the lung tissue was homogenized in 1.5 ml of cold 20 mM HEPES buffer, pH 7.2, (containing 1 mM EGTA, 210 mM mannitol and 70 mM sucrose). The crude homogenate was centrifuged 1500x g for five minutes at 4ºC. For determination of total GSH and MDA concentrations, 0.2 gram of the lung tissue was homogenized in 1ml of cold buffer (50 mM PBS, pH 7 containing 1 mM EDTA) and the crude homogenate was allocated both MDA and GSH assay. GSH and MDA homogenates were centrifuged 10000x g for 15 minutes and 1500x g for 10 minutes at 4ºC, respectively. Homogenate was performed using an IKA T 10 basic ULTRA-TURRAX (Germany) homogenizer. And then, the clear supernatant was used for biochemical analysis.
Biochemical Assay
Total SOD activity and total GSH content were assayed using commercial assay kits (Cayman, USA) as per manufacturer’s instruction. The SOD activity was measured using a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine and produced a yellow color that was measured by absorbent reading at 440nm. The GSH concentration was quantified using glutathione reductase. The sulfhydryl group of GSH reacts with DTNB (5,5’-dithio-bis-2-nitrobenzoic acid, Ellman’s reagent) and produces a yellow colored 5-thio-2-nitrobenzoic acid (TNB). The mixed disulfide, GSTNB (between GSH and TNB) that is concomitantly produced, is reduced by glutathione reductase to recycle the GSH and produce more TNB. Measurement of the absorbance of TNB at 405nm provides an accurate estimation of GSH in the sample. MDA levels were assayed for products of lipid peroxidation according to TBARS method. MDA content in samples and standards reacted with TBA (Thiobarbituric Acid) at 95°C for 20 minutes and 5 minutes incubated at 25ºC then centrifuged 5000rpm for 15 minutes at 4ºC. MDA concentration was read spectrophotometrically at 532nm and determined by comparison of predetermined MDA standard curve. SOD activity was expressed as U/ml and the concentrations of GSH and MDA are expressed as µM.
Histopathological Evaluation
After the removal of lung from chest, lung pieces were fixed, inflated with 10% neutral buffered formalin introduced through the airways, and then embedded in paraffin. Whole mount sections of the lung were cut (5µm), processed and stained with hematoxylin & eosin (H&E). All histological work was performed at the unit of pathology, Faghihee Teaching Hospital, Shiraz, Iran. Acute histopathological changes were evaluated under the light microscope (Olympus, Japan) by personnel blinded to the samples. Semi-quantitative scoring of each variable was performed by a histopathologist using the following scale: 1 (no change), 2 (mild), 3 (moderate) and 4 (severe) injuries. In the acute phase, the descriptive items for radiation-induced lung injuries were the presence of neutrophils, macrophages, lymphocytes, incidence of inflammation, erythrocytes (RBC), hyaline arteriosclerosis, vascular thickness, alveolar thickness and collapse.
Statistical Analysis
Data were analyzed using a commercially available statistics software package (SPSS® for Windows v. 19, Chicago, USA). Biochemical assay was analyzed by ANOVA test and post hoc Tukey HSD. Results are presented as mean ± standard error of mean (SEM); P< 0.05 was regarded as statistically significant. Histopathological evaluations were analyzed by Pearson Chi-square test (P< 0.05 was regarded as statistically significant) and a pair-wise comparison with the Pearson Chi-square test (P< 0.015 was regarded as statistically significant).
Results
Effect of HES on Endogenous Antioxidant Status and Lipid Peroxidation
The effect of gamma irradiation with or without HES on SOD activity and concentration of GSH and MDA in lung tissue, 24 h after γ-irradiation, is shown in Table 1. The activity of SOD and concentration of GSH in the lung tissue significantly decreased at 24 h following irradiation as compared to Control group (p=0.001, p<0.001). Treatment with Hesperidin for 7 consecutive days prior to exposure of γ-irradiation significantly increased level of HES+R group to near normal compared to Radiation group (p=0.015, p=0.005) and Control group (p<0.001, p=0.007) (Figure 1, A and B). MDA levels in radiation group of lung tissue showed a significant increase (p=0.001) compared to control. Hesperidin-treated rats showed significantly decreased (p=0.002) levels of MDA in HES+R group compared to Radiation group. In addition, we observed Hesperidin alone treatment significant difference (p<0.001, p<0.001, p<0.001) when compared to control (Figure 1, C).
Table 1.
SOD Activities, GSH and MDA Levels in Control, HES, Radiation and HES+R Groups (mean ± SEM).
| Treatment | SOD (U/ml) | GSH (µM) | MDA (µM) |
|---|---|---|---|
| Control | 0.733±0.080 | 0.349±0.002 | 0.0607± 0.0016 |
| HES | 0.767±0.083 | 0.350±0.004 | 0.0605±0.0015 |
| Radiation | 0.462±0.066 | 0.257±0.004 | 0.0817±0.0044 |
| HES+R | 0.699±0.059 | 0.326±0.006 | 0.0628±0.0030 |
Figure1.
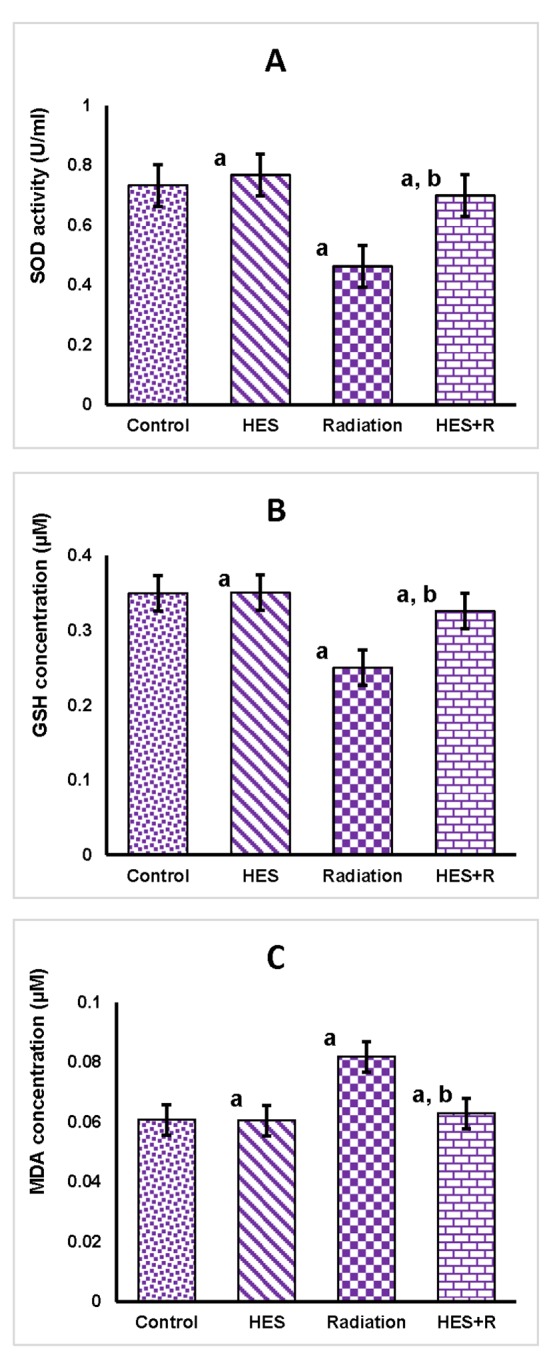
Changes in SOD activities (A), GSH concentrations (B) and MDA Level (C) in the lung tissue of male rats in Control, HES, Radiation and HES+R groups, 24 hrs after the last treatment. Values were expressed as mean ± SEM (n = 8). a. Indicated significant difference when each group is compared to control group (p< 0.05). b. Indicated significant difference when HES+R group is compared to Radiation group (p< 0.05).
Radiation Damage and Effects of HES in Acute Phase
The histopathological observation of lung sections of the Control, HES, Radiation and HES+R groups at 24 hrs post irradiation were evaluated (Figure 2). The descriptive factors were examined including the presence of neutrophils, macrophages, lymphocytes, incidence of inflammation, erythrocytes (RBC), hyaline arteriosclerosis, vascular thickness, alveolar thickness and collapse. P-values less than 0.05 refer to significant differences between the groups by Pearson Chi-Square test. According to the results, only a significant difference was observed in the inflammation, lymphocyte, macrophage and neutrophil (p=0.003, p=0.01, p=0.001, p<0.001), respectively. Among groups, analysis was performed by a pair-wise comparison with Pearson Chi-square test and p-value <0.0125, which indicates a significant difference (Table 2). The results showed significant increases in inflammation, lymphocyte, macrophage and neutrophil frequency in R group compared with control group (p=0.004, p=0.004, p=0.005 and p=0.004). In HES+R group, treatment with HES resulted in a decrease in these factors to near normal compared to Radiation group. Macrophage and neutrophil significantly decreased (p<0.0125) but partly, there were remains of inflammation and lymphocyte indicating there was no significant difference (p=0.036, p=0.053). In addition, in HES and HES+R groups, a significant difference was not observed when compared to control (p>0.0125).
Figure2.

Histopathological investigation of radioprotective effects of HES and radiation damage in the acute phase (24 hours). Inflammation and an increase in lymphocyte, macrophages and neutrophils are significant at 18 Gy of γ-irradiation. Flashes indicate an accumulation of lymphocyte, macrophages and neutrophils in lung tissue. (Magnification ×100, H& E staining). A: Control, B: HES, C: Radiation, D: HES+R.
Table 2.
Effect of HES Treatment at 24 hrs Post Irradiation on Histopathological Factors in the Lung Tissue of Rats
| Control | HES | Radiation | HES+R | |
|---|---|---|---|---|
| Inflammation | 1.13±0.354 | 1.75±0.886 | 3.13±0.835 a | 1.63±0.518 |
| Lymphocyte | 1.13±0.354 | 1.75±0.886 | 3.13±0.835 a | 1.88±0.835 |
| Macrophage | 1.38±0.744 | 2.13±0.835 | 3.63±0.518 a | 1.75±0.707 b |
| Neutrophil | 1.25±0.463 | 1.38±0.518 | 3.25±0.707 a | 1.63±0.518 b |
kjvgk
Conclusion
The effects of ionizing radiation are mediated through the generation of ROS in cells as a result of water radiolysis and also the depletion of endogenous antioxidants[14]. ROS can increase the expression of chemokines, cytokines and endothelial leukocyte adhesion molecules amplifying the cascade eliciting inflammatory response[15]. Since ROS plays a major role in radiation toxicity, supplementation of antioxidants will be of great significance to patients undergoing RT to prevent damage to normal tissues.
Free radicals generated by radiation attack the fatty acid component of membrane lipids leading to lipid peroxidation and finally resulting in cell death[16]. In accordance with previous reports[10,17,18], our results indicated that lipid peroxidation increased in the lung tissues after localized irradiation. On the other hand, the observed lowered levels of MDA level upon oral administration of HES indicated its ability to scavenge ROS which in-turn prevents further peroxidation of membrane lipids. It was also reported that hesperidin offers protection by terminating the lipid peroxidative side chain rather than scavenging extra cellular non-lipid radicals that initiate lipid peroxidation[19].
SOD constitutes the first enzymatic antioxidant defense and GSH constitutes one of the major non-enzymatic antioxidant defenses converting active oxygen molecules into non-toxic compounds. Exposure to 18 Gy radiation decreased the activities of these antioxidant enzymes in the lung indicative of oxidative stress. The decline in the levels of these enzymes in the present study could be elucidated by the fact that excessive superoxide radicals may inactivate H2O2 scavengers, thus resulting in the inactivation of SOD[20]. Depletion of GSH in vitro and in vivo is known to cause an inhibition of the glutathione peroxidase activity and has been shown to increase lipid peroxidation[21,22]. Oral administration of HES restored the activities of these enzymes in the lung tissue exposed to radiation. Thus, HES contributes significantly to the intracellular antioxidant defense system by acting as a powerful consumer of superoxide anion and hydroxyl radicals which induced oxidative stress in the lung. Hesperidin has also been reported to act as a powerful consumer of superoxide, singlet oxygen and hydroxyl radicals[10,22], thereby contributing significantly to the intracellular antioxidant defense system. Thus, the increase in antioxidant status during HES pre-administration might have further decreased the attack of free radicals on bio-molecules including DNA and membrane lipids thereby decreasing the deleterious effects of radiation on lung tissue.
Radiation-induced inflammatory response is associated with several molecular and cellular pathways. Molecular pathways bearing secretion of some cytokines include IL-1, IL-6, IL-8, TNFα, INFγ and TGFβ. These cytokines increase the expression of MAPKs, NFkB, NADPH Oxidase, iNOS and COX-2. NADPH Oxidase is produced by neutrophils, and iNOS is produced by macrophages[23]. Inflammation response in lung tissue is characterized by vascular thickness change, leukocytes infiltration and increased number of macrophages, neutrophils and lymphocytes.
In the current study, radiation increased the number of macrophages, neutrophils, lymphocytes which resulted in the incidence of inflammation at 24h after lung exposure (Table 2). Supplementation with HES prior to exposure to radiation reduces macrophages, neutrophils (Figure 2). This shows HES can reduce acute inflammation pathways induced by high exposure radiation. These results demonstrate that HES administration alleviates inflammation signs including reduction of macrophages accumulated especially. Macrophages have an important role in acute inflammation and oxidative damages in lung tissues. So, administration of supplements that ameliorate macrophage activity can be very useful. On the other hand, HES has been reported to exert a protective effect that includes modulate expression of pro-inflammatory cytokines and chemokines, reduction of polymorphonuclear neutrophils secretion in the airway and improved pulmonary edema and lung morphology. Hesperidin effectively reduces pulmonary vascular permeability leading to amelioration of pulmonary edema[24]. So, HES may protect the integrity of alveolar membrane resulting in the prevention of inflammatory cells and significantly improved lung morphology. Some studies showed HES supplement reduces oxidative and pathologic damages induced by ionizing radiation in liver[10,11]. Thus, HES has anti-inflammatory and specific protective effects against inflammatory disorders done through a mechanism involving the antioxidant activity of free radicals.
According to the reports, HES contains pharmacological effects that include antioxidant, anti-inflammatory and anticarcinogenic actions. The results of the current study show that HES significantly diminishes γ-radiation-induced damage in the lung tissue of rats. Hesperidin acts by scavenging free radicals and by maintaining intracellular SOD and GSH levels, thereby preventing lipid peroxidation and subsequent tissue damage. It seems that HES may be used as a natural radiaoprotector with anti-inflammatory and antioxidant role to prevent oxidative stress damage caused by ionizing radiation in a short time after exposure of lung tissue. Based on the results of our study, we conclude that HES can be a candidate for treating and preventing radiation-induced damage to the tissues particularly for patients undergoing RT. The results of the present study could be a basis for further studies that attempt to reduce lung injuries in patients who have undergone thorax irradiation. However, further experiments are required to investigate the molecular mechanisms related to the radioprotective functions of HES in lung.
Acknowledgement
This study was funded by grant number 93-7082 from the Vice-Chancellor of Research at Shiraz University of Medical Sciences. This paper presented the results of the MSc Thesis of Abolhasan Rezaeyan. Authors gratefully acknowledge Radiobiology Laboratory and Diagnostic Laboratory Science and Technology Research Center (DLSTRC) in School of Paramedical Sciences, Shiraz University of Medical Sciences for providing facilities throughout this study. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific study.
Conflict of Interest:None
References
- 1.Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 2.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 3.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/S1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 4.Gross NJ. Experimental radiation pneumonitis. IV. Leakage of circulatory proteins onto the alveolar surface J Lab Clin Med 1980;95:19–31. [PubMed] [Google Scholar]
- 5.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/S0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 6.Sies H, Wahllander A, Waydhas C, Soboll S, Hoberle D. Functions of intracellular glutathione in hepatic hydroperoxide and drug metabolism and the role of extracellular glutathione. Adv Enzyme Regul. 1980;18:303–20. doi: 10.1016/0065-2571(80)90022-9. [DOI] [PubMed] [Google Scholar]
- 7.Greenberger JS. Radioprotection. In Vivo. 2009;23:323–36. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari AK. Imbalance in antioxidant defence and human diseases: Multiple approach of natural antioxidants therapy. Current Science-Bangalore. 2001;81:1179–87. [Google Scholar]
- 9.Barthe GA, Jourdan PS, McIntosh CA, Mansell RL. Radioimmunoassay for the quantitative determination of hesperidin and analysis of its distribution in Citrus sinensis. Phytochemistry. 1988;27:249–54. doi: 10.1016/0031-9422(88)80625-3. [DOI] [Google Scholar]
- 10.Pradeep K, Park SH, Ko KC. Hesperidin a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in Sprague-Dawley rats. Eur J Pharmacol. 2008;587:273–80. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Kalpana KB, Devipriya N, Srinivasan M, Vishwanathan P, Thayalan K, Menon VP. Evaluating the radioprotective effect of hesperidin in the liver of Swiss albino mice. Eur J Pharmacol. 2011;658:206–12. doi: 10.1016/j.ejphar.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Pradeep K, Ko KC, Choi MH, Kang JA, Chung YJ, Park SH. Protective effect of hesperidin, a citrus flavanoglycone, against gamma-radiation-induced tissue damage in Sprague-Dawley rats. J Med Food. 2012;15:419–27. doi: 10.1089/jmf.2011.1737. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinimehr SJ, Nemati A. Radioprotective effects of hesperidin against gamma irradiation in mouse bone marrow cells. Br J Radiol. 2006;79:415–8. doi: 10.1259/bjr/40692384. [DOI] [PubMed] [Google Scholar]
- 14.Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Robbins SL, Kumar V. Robbins and Cotran pathologic basis of disease . Philadelphia: Saunders/Elsevier; 2010. p. 60. [Google Scholar]
- 16.Lee JH, Lee YM, Park JW. Regulation of ionizing radiation-induced apoptosis by a manganese porphyrin complex. Biochem Biophys Res Commun. 2005;334:298–305. doi: 10.1016/j.bbrc.2005.06.102. [DOI] [PubMed] [Google Scholar]
- 17.Tahamtan R, Shabestani*Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, et al. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015;17:111–20. doi: 10.22074/cellj.2015.517. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koc M, Taysi S, Emin*Buyukokuroglu M, Bakan N. The effect of melatonin against oxidative damage during total-body irradiation in rats. Radiat Res. 2003;160:251–5. doi: 10.1667/3034. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi S, Sakurada S, Nagumo M. Role of intracellular SOD in protecting human leukemic and cancer cells against superoxide and radiation. Free Radic Biol Med. 1994;17:389–95. doi: 10.1016/0891-5849(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 20.Blech DM, Borders CL*Jr. Hydroperoxide anion, HO-2, is an affinity reagent for the inactivation of yeast Cu, Zn superoxide dismutase: modification of one histidine per subunit. Arch Biochem Biophys. 1983;224:579–86. doi: 10.1016/0003-9861(83)90245-X. [DOI] [PubMed] [Google Scholar]
- 21.Jagetia GC, Reddy TK. Modulation of radiation-induced alteration in the antioxidant status of mice by naringin. Life Sci. 2005;77:780–94. doi: 10.1016/j.lfs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG. Flavonoids as antioxidants. Journal of the American Chemical Society. 1994;116:4846–51. doi: 10.1021/ja00090a032. [DOI] [Google Scholar]
- 23.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–56. doi: 10.2174/13894501113149990198. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh CC, Kao SJ, Lin CC, Wang SD, Liu CJ, Kao ST. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007;80:1821–31. doi: 10.1016/j.lfs.2007.01.052. [DOI] [PubMed] [Google Scholar]


