Abstract
Background:
Drug nano-carriers are one of the most important tools for targeted cancer therapy so that undesired side effects of chemotherapy drugs are minimized. In this area, the use of ultrasound can be helpful in controlling drug release from nanoparticles to achieve higher treatment efficiency.
Objective:
Here, we studies the effects of ultrasound irradiation on the release profile of 5-fluorouracil (5-Fu) loaded magnetic poly lactic co-glycolic acid (PLGA) nanocapsules.
Methods:
5-Fu loaded magnetic PLGA nanocapsules were synthesized by multiple emulsification method. Particle size was measured by dynamic light scattering (DLS) and transmission electron microscope (TEM). The pattern of drug release was assessed with and without 3 MHz ultrasound waves at intensities of 0.3, 0.5 and 1 w/cm2 for exposure time of 5 and 10 min in phosphate-buffered saline (PBS).
Results:
The size of nanoparticles was about 70 nm. Electron microscope images revealed the spherical shape of nanoparticles. The results demonstrated that the intensity and exposure time of ultrasound irradiation have significant effects on the profile of drug release from nanoparticles.
Conclusion:
It may be concluded that the application of ultrasound to control the release profile of drug loaded nanocapsules would be a promising method to develop a controlled drug delivery strategy in cancer therapy.
Keywords: Nanoparticle , Ultrasound , Cancer , PLGA , 5-Fu
Introduction
Cancer is one of the major causes of mortality in the world. Surgery, radiotherapy and chemotherapy are the most common approaches for cancer treatment. Since radiotherapy and surgery are limited to local treatment of cancer which has not spread to nearby tissue, systemic chemotherapy is the main treatment for many types of cancer[1]. Distribution of drug in the body is non-selective and undesired side effects are usually seen due to normal tissue damages. Considering the poor response of cancer to the chemotherapy agents, there exists an essential need to develop some certain ways to increase drug delivery efficiency and to reduce the side effects[2-6].
Nanoparticles are promising platforms to increase the chance of selective delivery of chemotherapeutic agents to cancer cells. To develop good drug delivery carriers, it is desirable to have systems with good capability of drug loading, controlled release profile, high biological half-life and low toxicity[7,8-11]. As a biocompatible drug carrier, iron oxide nanoparticles have recently received food and drug administration (FDA) approval and extensively utilized in the field of targeted chemotherapy[8,12]. On the other hand, there are many studies reporting that polymer-coated magnetic nanoparticles may have higher capabilities in the area of targeted drug delivery[13]-[15]. Polymers which are used to design and synthesize polymer-coated magnetic nanoparticles must have the unique properties of biocompatibility and biodegradation; poly lactic co-glycolic acid (PLGA) is one of the most important polymers in this area[7]. We have recently reported the synthesis and characterization of a novel PLGA-based magnetic nanocapsule containing 5-floururacil (5-Fu) as an appropriate drug carrier[16]. Using an external magnetic field, we targeted this new nanocapsule towards colon tumors in vivo and reported significantly better results in comparison to 5-Fu alone. We also suggested the combination of magnetic drug targeting and ultrasound wave has significant effects on increasing the therapeutic ratio of magnetic nanocapsules[17,18].
Ultrasound is considered a mediator for targeted drug delivery through mechanical interaction with both drug carrier and cell membrane[19]. The mechanical vibrations caused by interaction of ultrasound with environment can increase the movement of cell membrane in smaller scales which is called macro-molecular massage. This process can modify the properties of biological tissues such as the permeability of cell membrane. Moreover, macro-molecular massage can change the profile of drug release from nano-carriers[20]. The investigation done by Ninomiya et al. shows the rate of drug release for doxorubicin loaded temperature-sensitive liposome speeds up about 40 percent if liposome is exposed by 1 MHz ultrasonic waves with nominal intensity of 0.5 W/cm2 for 2 min[21]. Burke et al. demonstrated that the use of drug loaded PLGA and application of 1 MHz ultrasound radiation increased in-vivo tumor retardation rate rather than the free drug prescription[22]. In another study, Rappaport et al. incubated cancer cells with pluronic micelles containing Doxorubicin. The incubated cells were exposed to 69 KHz ultrasound at intensity of 2.3 W/cm2 for 10 min. The growth of cells receiving both micelles and ultrasound was much slower than the cells receiving micelles alone[23]. In addition to these studies, we have recently studied the synergistic effects of 3 MHz ultrasound waves at intensity of 0.3W/cm2 and 5-Fu loaded PLGA nanocapsules on colon tumor in vivo. It was demonstrated that combination of nanocapsules with ultrasound irradiation significantly increased the therapeutic ratio rather than the prescription of the free drug alone[17]. We had an important question related to the effect of ultrasound parameters on the release rate of 5-Fu from PLGA nanocapsules and finding a good response for this question motivated us to conduct the present study. Here, for the first time, we investigated how ultrasound parameters affect the release rate of a drug from nanocapsules designed for magnetic drug targeting. The original data obtained from this study may be helpful to optimize ultrasound parameters and to suggest an appropriate in-vivo scientific paradigm.
Material and Methods
Chemicals
5-Fu was purchased from Sigma Chemical Company (St Louis, MO, USA). PLGA polymer (Resomer RG502H) was purchased from Boehringer-Ingelheim (Ingelheim, Germany). The mole ratio of glycolic acid to lactic acid in PLGA was 50:50 and its molecular weight was 12000 g/mol. Ferrous chloride (FeCl2.4H2O), ferric chloride (FeCl3.6H2O), oleic acid, ammonia solution (25 vol%), dichloromethane (DCM), glycerin, span60 and tween60 were purchased from Merck Chemical Company (Darmstadt, Germany). All chemicals were used without further purification.
Nanocapsule Preparation
5-Fu-loaded magnetic PLGA nanocapsules were synthesized according to the method which has been recently reported by our research group[16,24]. Briefly, magnetic nanocapsules were prepared using a modified O1/W1/O2/W2 multiple emulsion solvent evaporation procedure. Glycerin was used as a surfactant to stabilize the dispersed phase. First, 20mg of magnetite nanoparticles (12 nm in diameter and modified by oleic acid) was dispersed in 0.5 ml of DCM to prepare a primary organic phase (O1) using an ultrasonic bath. Then, the inner aqueous solution was prepared by dissolving 10 mg 5-Fu as a hydrophilic drug and 15 mg tween60 as a surfactant in 1.5 ml of double-distilled water (W1). The magnetic dispersion (O1) was emulsified in the inner aqueous solution (W1) by ultrasonication using the sonicator probe at an output of 50W for 30 s in an ice-bath to obtain an O1/W1 emulsion (dispersion of magnetite). This primary emulsion was emulsified in an organic solution (O2) of the polymer (50 mg PLGA and 250 mg span 60 in 6 ml DCM) by ultrasonication for 30 s (50 W) in an ice-bath to obtain O1/W1/O2 double emulsion. Next, this double emulsion was immediately poured into 15 ml of W2 aqueous solution, which was made of 150 mg tween60 dissolved in 7.5 ml of distilled water and 7.5 ml of glycerin, and the mixture was ultrasonicated again for 30 s. The resulting multiple emulsion (O1/W1/O2/W2) was diluted in 24 ml of aqueous solution composed of 12 ml distilled water and 12 ml glycerin under mechanical stirring, and the DCM was removed by solvent evaporation. The stirring was continued for a period of 3 h at room temperature to allow evaporation of the organic solvent. The resulting magnetic nanocapsules were cleaned by repeating the procedure of centrifuging and re-suspending in distilled water three times and were then collected with a magnet. Most of the non-encapsulated drug remained in the outer aqueous solution, and the remaining loosely adsorbed drug on the surface of the nanocapsules was removed through washing with double-distilled water. Finally, the obtained nanocapsules were dried by freeze-drying and stored at 4˚C.
Determination of Particle Size and Morphology
The morphological investigation of 5-Fu-loaded nanocapsules was performed using a Zeiss LEO 906 transmission electron microscope (TEM). The nanocapsules were suspended in distilled water and sonicated for 10 min. A drop of the nanoparticle suspension was placed on a carbon coated grid. The grid was perfectly dried and observed under TEM at 100 kV. The size distribution and the effective diameter of nanocapsules were measured using a Brookhaven 90 plus Nanoparticle Size Analyzer (Brookhaven Instruments, Holtsville, NY, USA).
Determination of Drug Content and Encapsulation Efficiency
To evaluate drug concentration in a nanocapsule, a UV absorption measurement was performed. 5-Fu concentration was calculated by application of Beer’s law in which the absorption is proportional to concentration. The drug content in the magnetic nanocapsules is defined as the ratio of the encapsulated drug to the total weight of solid magnetic nanocapsule. The encapsulation efficiency is defined as the ratio of the encapsulated drug content to the total amount of drug used for nanocapsule preparation. First, the solid (freeze-dried) nanocapsules were weighed (5 mg) and re-dissolved in 5 ml acetone (spectroscopy grade). Next, the insoluble magnetite particles were removed from the solution by magnetic separation. With respect to Equation (1) which is a previously established calibration curve, the 5-Fu concentration in the acetone solution was determined by UV absorption at a wavelength of 210 nm (characteristic absorption band of 5-Fu in acetone):
Y= 2.9875x - 0.014, R2= 0.998 (1)
Then, the amount of encapsulated drug in the nanocapsules was calculated according to Equations (2) and (3):
Drug Content = (weight of encapsulated drug in nanocapsules /weight of nanocapsules) × 100 (2)
Encapsulation Efficiency = (weight of encapsulated drug in nanocapsules/weight of initial loading of drug) × 100 (3)
Experimental Setup
Study of Drug Release Profile in the Absence of US Irradiation
At first, the following calibration equation for 5-Fu in phosphate buffered saline (PBS) was obtained using dilution technique:
Y= 3.173x - 0.061, R2= 0.997 (4)
where Y is 5-Fu concentration and X is absorbance at the wavelength of 269 nm. Then, in- vitro release of 5-Fu from magnetic nanocapsules was studied in 0.01 M PBS (pH 7.4) using the dialysis method. The dialysis bag (molecular weight cut-off 12,400 Da) was soaked in preheated double-distilled water before use. A weighed amount of freeze-dried magnetic nanocapsules
(1 mg) was re-suspended in 1 ml PBS and transferred into a dialysis bag. Both ends of the tube were affixed with clamps. The bag was placed into 50 ml of PBS that acted as a release medium. The release study was performed in an incubator shaker at 37 ˚C. At selected time intervals, the solution outside the dialysis bag was removed for UV-visible analysis and replaced with fresh buffer solution Figure 1. Based on the absorbance of 5-Fu at 269 nm, drug concentration was calculated using Equation (5):
Figure1.
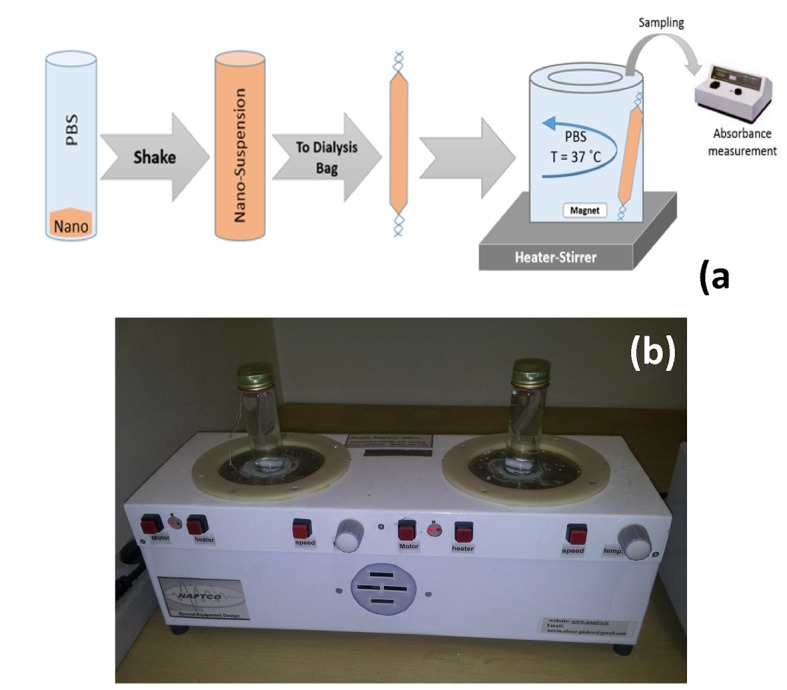
(a) A schematic illustration and (b) an image of experimental setup when ultrasound was absent.
Y= 3.173x - 0.061, R2= 0.997 (5)
In the study of drug release behavior, the cumulative amount of released drug was calculated, and the percentage of released drug from the magnetic nanocapsules was plotted as a function of time.
Study of Drug Release Profile in the Presences of Ultrasound Irradiation
To investigate the effects of ultrasound exposure on drug release profile, above procedure (the protocol mentioned in section 2.5.1) was similarly performed, but an additional pre-treatment step was considered. The additional step was included (i) the insertion of nanocapsules loaded dialysis bag in a customized container made of Plexiglas Figure 2 and (ii) ultrasound irradiation to nano-suspension with different parameters using a plane circular transducer with an area of 1 cm2 operating at 3 MHz in the continuous wave mode (Phyaction 190i, Germany). Ultrasound power output was measured using the acoustic radiation force method16,24. A linear relationship between nominal intensity (I1) and measured intensity (I2) was obtained as demonstrated below:
Figure2.
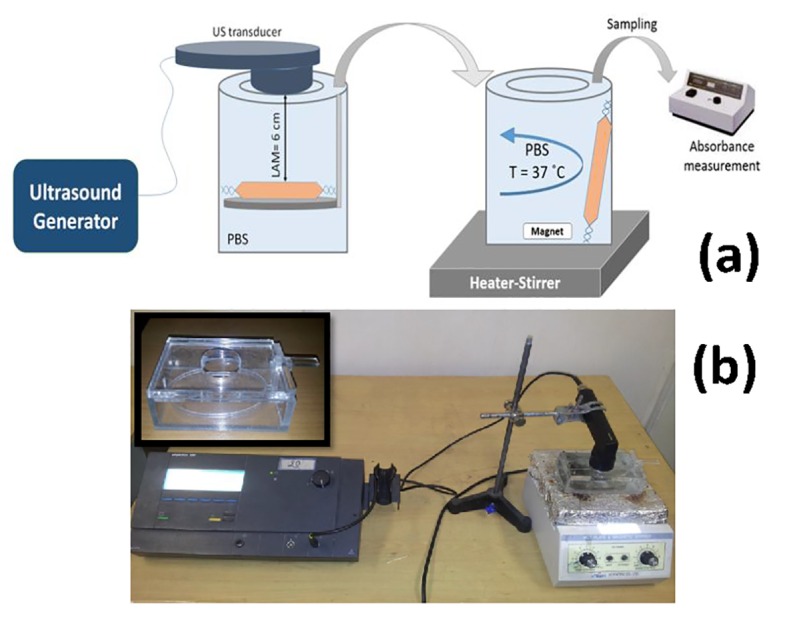
(a) A schematic illustration and (b) an image of experimental setup when ultrasound was present (inset: designed container).
I1= 0.942 I2+ 0.185, R² = 0.974 (6)
The ratio of peak intensity to average intensity (Ip/Ia) was calculated using the dye paper method[16,24]and Kossoff approximation method which was found to be approximately 5.
After ultrasound exposure, dialysis bag was removed from container and placed into 50 ml of PBS at 37˚C as a release medium, and samples were taken at pre-selected time intervals. Based on the status of ultrasound exposure to nanocapsules, 7 different groups were considered in this study (Table 1). Control group did not receive any ultrasound radiation. In other 6 groups, nanocapsules were ultrasonicated for 5 min and 10 min at various intensities from 0.3 to 1 w/cm2. Samples were collected for 24 hours and the absorption of each sample was measured through UV spectrophotometer Ray leight UV-1601 and then the cumulative release curves were plotted.
Table 1.
Considered groups in this study
| Group | Ultrasound parameter | |
|---|---|---|
| (Exposure time and Intensity) | ||
| 1 | Control (No Exposure) | |
| 2 | 5 min | 0.3 w/cm2 |
| 3 | 0.5 w/cm2 | |
| 4 | 1 w/cm2 | |
| 5 | 10 min | 0.3 w/cm2 |
| 6 | 0.5 w/cm2 | |
| 7 | 1 w/cm2 | |
Statistical Methods
All experiments were conducted in triplicate. Statistical analysis was performed by one-way ANOVA test and used the Tukey test at 95% confidence level as a post-test. Statistical analyses were performed using SPSS software (version 11). A value of P < 0.05 was considered statistically significant.
Results
Nanoparticle Characteristics
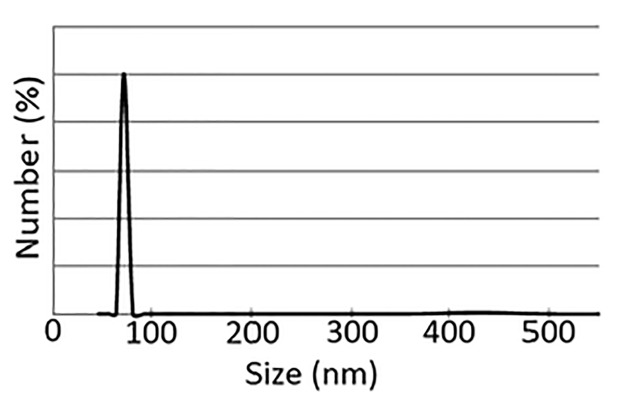
DLS results revealed that nanocapsules had good monodispersity and their effective diameter was about 70 nm (Figure 3). A typical TEM micrograph of magnetic nanocapsules is presented in Figure 4, the dark regions may correspond to the magnetic nanoparticles incorporated into PLGA nanocapsules.
Figure3.
Size distribution of magnetic PLGA nanocapsules containing 5-Fu (Effective diameter was approximately of 70 nm).
Figure4.
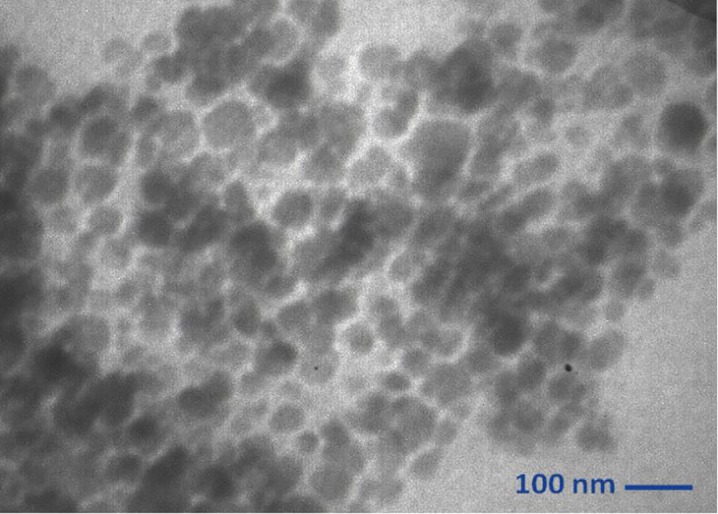
TEM image of magnetic nanocapsules
According to spectrophotometry studies and considering equations 2 and 3, drug content and encapsulation efficiency of synthesized nanocapsules were calculated which were 5.8% and 46.4%, respectively.
Effects of Ultrasound Irradiation on Drug Release
Effect of Intensity
Figure 5a and 5b show the effect of ultrasound at different intensities on the rate of drug release from nanocapsules. Figure 5a illustrates the effect of 5-minute ultrasound irradiation of dialysis bag at different intensities on the rate of drug release from nanoparticles. The drug released 24 hours after sonication for 1W/cm2 intensity (16.4± 0.93)% was almost 3 fold higher than the control (5.8± 0.32)%. This signifies that sonication period and intensity are the two parameters affecting the rate of drug release from nanoparticles. Figure 5b shows the effect of 10 min ultrasound irradiation on drug release profile. After 24 hours, the amount of drug release from nanocapsules when exposed to intensity of 1W/cm2 was (22.6± 1.16) %. The 24-hour drug release at intensities of 0.3 and 0.5W/cm2 were (13.6± 0.79) % and (17.7 ± 0.92) %, respectively.
Figure 5a.
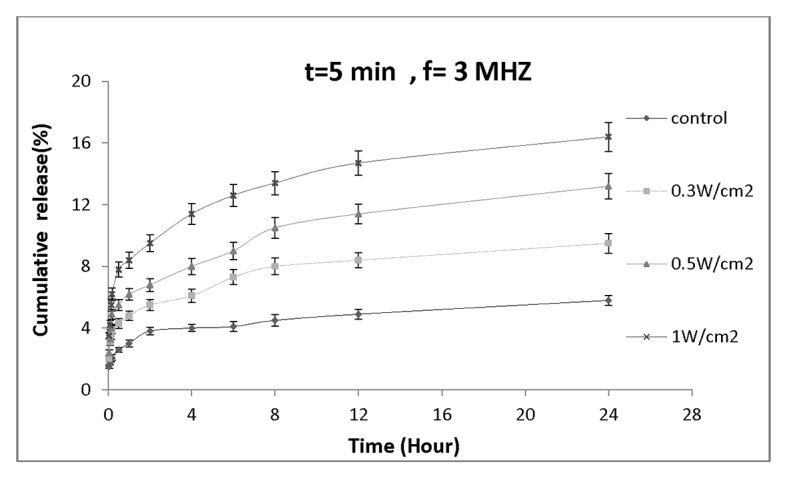
Drug release profile of nanocapsules exposed by various ultrasound intensities for 5min.
Figure 5b.
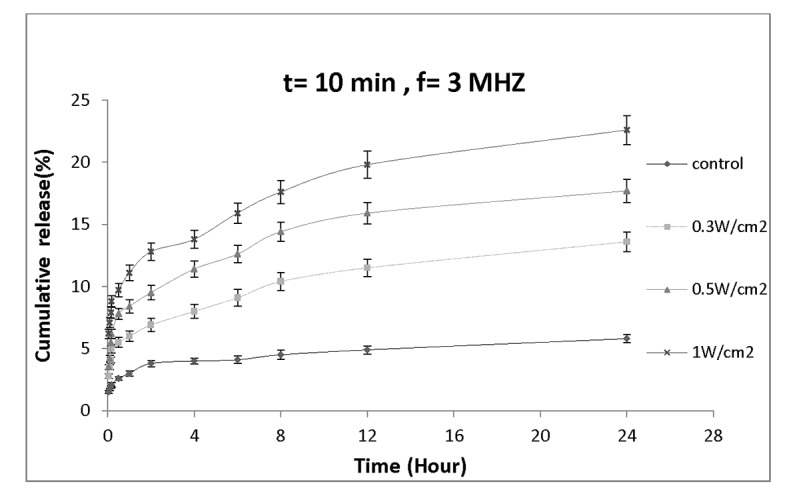
Drug release profile of nanocapsules exposed by various ultrasound intensities for 10 min.
Effect of Exposure Time
To evaluate the effect of ultrasound exposure time on the rate of drug release, we re-sorted our findings and re-plotted drug release profile from point of view of exposure time at certain intensity. Figure 6demonstrates the amount of drug release versus time for the nanocapsules treated with the same frequency but different sonication period. Figure 6a shows the sonication at intensity of 0.3 W/cm2; it is evident that an increase in ultrasound exposure time from 5 min to 10 min, significantly enhances the amount of released drug from (9.5± 0.63) % to (13.6± 0.79) %. Figure 6b shows the elevation of drug release at 0.5 W/cm2 for 5 and 10-minute sonication period. As it can be seen, the amount of drug release 24 hours after ultrasound exposure significantly increased from (13.2± 0.81) % to (17.7± 0.92) %. Finally, Figure 6c presents the results obtained for ultrasound intensity at 1W/cm2 at two different exposure times. It illustrates that the percentage of drug released from nanocapsules has significantly increased (16.4± 0.93)% and (22.6± 1.16)% for 5 min and 10 min exposure periods, respectively.
Figure 6a.
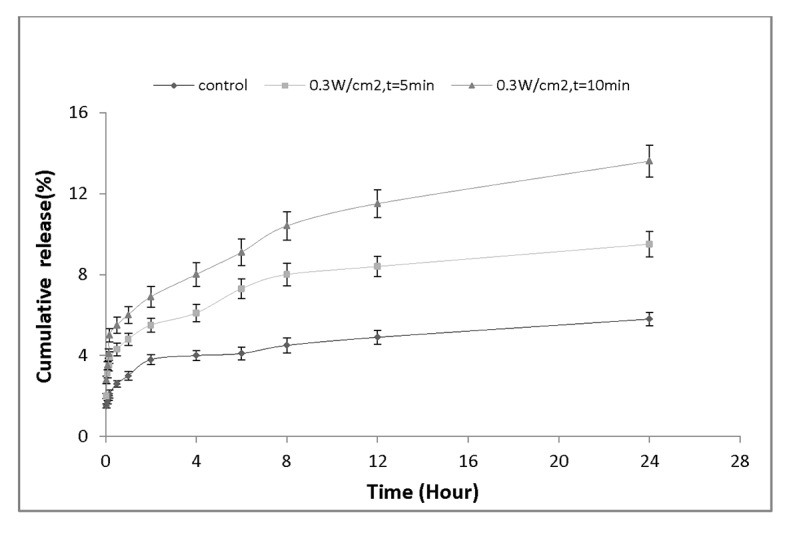
Drug release profile of nanocapsules exposed by ultrasound intensity of 0.3 W/cm2 for 5 min and 10 min.
Figure 6b.
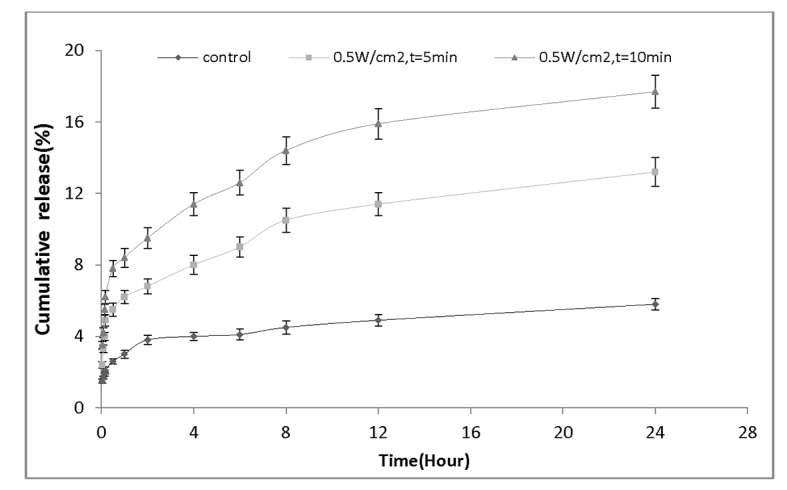
Drug release profile of nanocapsules exposed by ultrasound intensity of 0.3 W/cm2 for 5 min and 10 min.
Figure 6c.
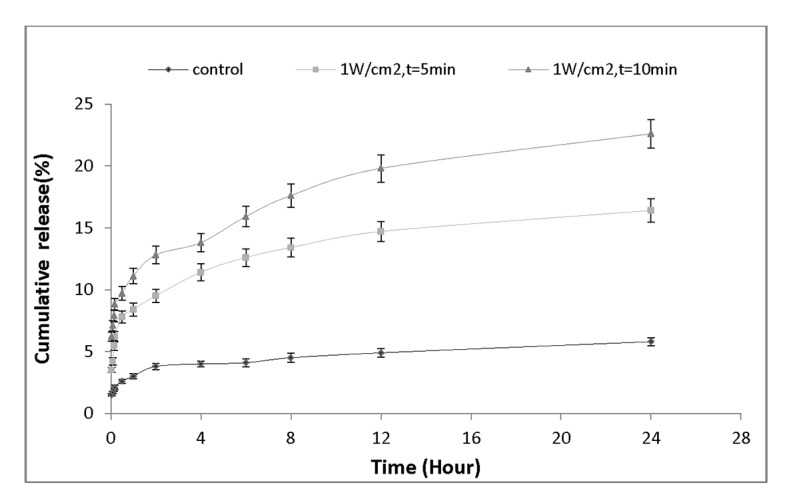
Drug release profile of nanocapsules exposed by ultrasound intensity of 1 W/cm2 for 5 min and 10 min.
Discussion
Characteristics of Nanocapsules
The size of nanoparticles is of great importance in drug delivery, and influences their biodistribution profile[25]. This dependence is nonlinear and different from one organ to another. Table 2 presents a comparison between the synthesized nanocapsules in this study and nanocapsules reported by other researchers. As it is seen in Table 2, the structure of nanocapsules affects the size of nanocapsules and encapsulation efficiency.
Table 2.
A comparison between the characteristics of nanocapsules synthesized in current study and some other nanocapsules containing 5-Fu.
| Reference | Structure of nanocapsules | Size(nm) | Drug content(%) | Encapsulation efficiency(%) |
|---|---|---|---|---|
| Current study | PLGA | 70 | 46.4 | 5.8 |
| Ashjari et al[28] | PLGA | 91-235 | 62-84 | 4.3-7.5 |
| Wang et al[29] | PLGA-Chitosan | 197-340 | 28.6-24.1 | 22-28 |
| Lei et al[30] | PEG-PBLG* | 230 | 61.5 | 27 |
| Bruke et al[22] | PLGA-BSA** | 130 | 8 | 8 |
PBLG: Poly-Benzyl L-Glutamate
BSA: Bovine serum albumin
Effect of Ultrasound Irradiation on Drug Release Profile
The results presented in this study show that ultrasound waves affect the profile of drug release from nanocapsules. To make an in-depth discussion, we present Table 3 to summarize what may be found according to Figures 5 and 6. The ratios reported in Table 3 are calculated according to Equation 7:
Table 3.
Summary of enhancement ratios for cumulative drug release due to ultrasound irradiation
| Ultrasound parameter((W/cm2)/min) | 0.3/5 | 0.3/10 | 0.5/5 | 0.5/10 | 1/5 | 1/10 |
|---|---|---|---|---|---|---|
| Enhancement ration | 1.64 | 2.34 | 2.28 | 3.05 | 2.83 | 3.90 |
Enhancement ratio=CRi/CRj (7)
Where CRi is cumulative release in the presence of ultrasound and CRj is cumulative release in the absence of ultrasound. Based on Table 3, it may be inferred that the amount of energy density delivered to the nanocapsules directly changes the amount of drug release. This finding is in agreement with results reported in other studies.
Rappaport et al. studied the effect of ultrasound intensity on drug release from doxorubicin- loaded polymeric micelles. They concluded that the intensity had a linear relationship with drug release so that the higher the intensity, the faster the rate of drug release. For example, the drug release is about 3% for 1MHz frequency and 2W/cm2 intensity while it reaches about 8% for 6W/cm2 in the same frequency. It means that drug release increased 2.6 folds[23].
In another study performed by Afadzi et al, the rate of drug release was investigated at different ultrasound intensities. They also showed that the drug release increased with increasing of intensity. For example, the drug release reaches about 60% for 2-minute exposure and 57.24 W/cm2 whereas, the drug release was not as much for the same exposure time and 1.64 W/cm2 intensity[23].
Another parameter which affects the rate of drug release is ultrasound exposure time. It is obvious that the amount of delivered energy to nanocapsules in longer periods is higher. As a result, higher amounts of energy imparted to the medium cause turbulence into it influencing the nanoparticle membrane. This may accelerate the rate of drug release from nanocapsules.
A similar result was reported by Cohen-levi et al. They demonstrated that the longer the ultrasound exposure time, the greater the drug release from liposome. They reported drug release reached 38% and 60% for 10 min and 30 min ultrasound exposure, respectively[26].
In a study performed by Affadzi et al., the effect of ultrasound exposure time on the rate of drug release from Liposomes was investigated. Keeping intensity constant, they demonstrated that drug release was 30% for 20 sec ultrasound exposure while this amount reached 60% for 120 sec exposure[27].
As stated in Introduction section, we have recently reported the effects of 3 MHz ultrasound irradiation on therapeutic ration of PLGA-based magnetic nanocapsules containing 5-Fu[17,18]. Using an external magnetic field, we targeted the nanocapsules towards the induced colon tumors in BALB/c mice and reported significantly better results in comparison with 5-Fu alone. We also demonstrated that the combination of magnetic drug targeting and ultrasound wave had significant effect on increasing the therapeutic ratio of nanocapsules[17,18]. Considering the data obtained and discussed in the present study (Table 3), it may be concluded that ultrasound enhances cumulative drug release and consequently increases the therapeutic ratio of nanocapsules in vivo.
Conclusion
In this study, we reported the effects of 3 MHz ultrasound on the rate of drug release from PLGA nanocapsules. The results demonstrated that drug release profile could be modified by controlling the physical parameters of ultrasound waves. It was concluded the amount of drug released from nanocapsules significantly rose by increasing the exposure time and the intensity of ultrasound. This study may be a good indication to prove the high capabilities of ultrasound waves in the field of controlled release of drugs using novel nano-carriers.
Conflict of Interest:Nothing to be declared.
References
- 1.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 2.Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C, et al. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J. 2006;35:446–50. doi: 10.1007/s00249-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 3.Alexiou C, Jurgons R, Schmid R, Hilpert A, Bergemann C, Parak F, et al. In vitro and in vivo investigations of targeted chemotherapy with magnetic nanoparticles. Journal of Magnetism and Magnetic Materials. 2005;293:389–93. doi: 10.1016/j.jmmm.2005.02.036. [DOI] [Google Scholar]
- 4.Voltairas PA, Fotiadis DI, Michalis LK. Hydrodynamics of magnetic drug targeting. J Biomech. 2002;35:813–21. doi: 10.1016/S0021-9290(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 5.Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95:200–6. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- 6.Arias JL. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13:2340–69. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayal S, Ramanujan R. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Materials Science and Engineering: C. 2010;30:484–90. doi: 10.1016/j.msec.2010.01.006. [DOI] [Google Scholar]
- 8.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005;172:1487–90. doi: 10.1164/rccm.200504-613PP. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehdizadeh A, Pandesh S, Shakeri-Zadeh A, Kamrava SK, Habib-Agahi M, Farhadi M, et al. The effects of folate-conjugated gold nanorods in combination with plasmonic photothermal therapy on mouth epidermal carcinoma cells. Lasers Med Sci. 2014;29:939–48. doi: 10.1007/s10103-013-1414-2. [DOI] [PubMed] [Google Scholar]
- 10.Shakeri-Zadeh A, Kamrava SK, Farhadi M, Hajikarimi Z, Maleki S, Ahmadi A. A scientific paradigm for targeted nanophotothermolysis; the potential for nanosurgery of cancer. Lasers Med Sci. 2014;29:847–53. doi: 10.1007/s10103-013-1399-x. [DOI] [PubMed] [Google Scholar]
- 11.Khoei S, Mahdavi SR, Fakhimikabir H, Shakeri-Zadeh A, Hashemian A. The role of iron oxide nanoparticles in the radiosensitization of human prostate carcinoma cell line DU145 at megavoltage radiation energies. Int J Radiat Biol. 2014;90:351–6. doi: 10.3109/09553002.2014.888104. [DOI] [PubMed] [Google Scholar]
- 12.Chao X, Zhang Z, Guo L, Zhu J, Peng M, Vermorken AJ, et al. A novel magnetic nanoparticle drug carrier for enhanced cancer chemotherapy. PLoS One 2012;7:e40388. doi: 10.1371/journal.pone.0040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3:169–80. doi: 10.2147/ijn.s1608. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scialabba C, Licciardi M, Mauro N, Rocco F, Ceruti M, Giammona G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. Eur J Pharm Biopharm. 2014;88:695–705. doi: 10.1016/j.ejpb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Ramasamy T, Tran TH, Choi JY, Cho HJ, Kim JH, Yong CS, et al. Layer-by-layer coated lipid-polymer hybrid nanoparticles designed for use in anticancer drug delivery. Carbohydr Polym. 2014;102:653–61. doi: 10.1016/j.carbpol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Shakeri-Zadeh A, Shiran MB, Khoee S, Sharifi AM, Ghaznavi H, Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29:548–56. doi: 10.1177/0885328214536940. [DOI] [PubMed] [Google Scholar]
- 17.Shakeri-Zadeh A, Khoee S, Shiran M-B, Sharifi AM, Khoei S. Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumor irradiation by ultrasound on CT26 tumors in BALB/c mice. Journal of Materials Chemistry B. 2015;3:1879–87. doi: 10.1039/C4TB01708K. [DOI] [PubMed] [Google Scholar]
- 18.Shakeri-Zadeh A, Khoei S, Khoee S, Sharifi AM, Shiran M-B. Targeted, monitored, and controlled chemotherapy: a multimodal nanotechnology-based approach against cancer. ISRN Nanotechnology. 2013;2013 [Google Scholar]
- 19.Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 20.Jafarian Dehkordi F, Shakeri-Zadeh A, Khoei S, Ghadiri H, Shiran M-B. Thermal distribution of ultrasound waves in prostate tumor: comparison of computational modeling with in vivo experiments. ISRN Biomathematics. 2013;2013:1–4. doi: 10.1155/2013/428659. [DOI] [Google Scholar]
- 21.Ninomiya K, Kawabata S, Tashita H, Shimizu N. Ultrasound-mediated drug delivery using liposomes modified with a thermosensitive polymer. Ultrason Sonochem. 2014;21:310–6. doi: 10.1016/j.ultsonch.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Burke CW, Alexander Et, Timbie K, Kilbanov AL, Price RJ. Ultrasound-activated agents comprised of 5FU-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Mol Ther. 2014;22:321–8. doi: 10.1038/mt.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapoport N, Pitt WG, Sun H, Nelson JL. Drug delivery in polymeric micelles: from in vitro to in vivo. J Control Release. 2003;91:85–95. doi: 10.1016/S0168-3659(03)00218-9. [DOI] [PubMed] [Google Scholar]
- 24.Shakeri-Zadeh A, Khoei S, Khoee S, Sharifi AM, Shiran MB. Combination of ultrasound and newly synthesized magnetic nanocapsules affects the temperature profile of CT26 tumors in BALB/c mice. J Med Ultrason (2001) 2015;42:9–16. doi: 10.1007/s10396-014-0558-4. [DOI] [PubMed] [Google Scholar]
- 25.Avgoustakis K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr Drug Deliv. 2004;1:321–33. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- 26.Cohen-Levi D, Kost J, Barenholz Y. Ultrasound for targeted delivery of cytotoxic drugs from liposomes . Beer Sheva, Israel: Ben Gurion University; 2000. [Google Scholar]
- 27.Afadzi M, Davies Cde L, Hansen YH, Johansen T, Standal OK, Hansen R, et al. Effect of ultrasound parameters on the release of liposomal calcein. Ultrasound Med Biol. 2012;38:476–86. doi: 10.1016/j.ultrasmedbio.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Ashjari M, Khoee S, Mahdavian AR. A multiple emulsion method for loading 5-fluorouracil into a magnetite-loaded nanocapsule: a physicochemical investigation. Polymer International. 2012;61:850–9. doi: 10.1002/pi.4154. [DOI] [Google Scholar]
- 29.ang Y, Li P, Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14:585–92. doi: 10.1208/s12249-013-9943-3. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Wang A, Jiang W, Guan Z. Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer. 2008;8:103. doi: 10.1186/1471-2407-8-103. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]