Abstract
Background:
Radiotherapy is one of the most important factors which results in negative effects on wound healing and increases anastomosis leakage. Diverting loop ileostomy has been usually performed after colorectal anastomosis in cases of colorectal cancer with a history of neoadjuvant radiotherapy to decrease the chance of leakage. Considering the side effects of diverting loop ileostomy, the objective of the present study is to investigate the effect of human amniotic membrane (HAM) on colorectal anastomosis leakage after neo-adjuvant radiotherapy.
Methods:
In this experimental animal study, 20 crossbreed rabbits were randomly divided into two groups (case group: 13 rabbits, control group: 7 rabbits) after receiving an equal dose of external beam radiation. Four weeks after irradiation, resection of 4 cm of colorectal segment and end-to-end single layer anastomosis were conducted. In the case group, a 2×2 cm wrap of HAM applied around the site of anastomosis. Eight weeks later, all the survived rabbits were sacrificed. A segment of anastomotic sites was resected in all expired and survived rabbits and sent for pathological evaluation. Mann-Whitney U Test (SPSS for Windows, Ver. 16, Chicago, IL) was applied to analyze healing scores between the two groups.
Results:
Due to anastomosis dehiscence, 5 rabbits expired in the control group, but all the 13 rabbits (case group) survived after 8 weeks and showed no leakage. In addition, pathological evaluation revealed significant epithelialization and neovascularization in the case group. Statistically, healing score was higher in the case group rather than the control group (P<0.001).
Conclusion:
To prevent post irradiation colorectal anastomosis leakage, the use of HAM might play a significant role and a feasible technical approach.
Keywords: Human amniotic membrane, Colonic anastomosis, Radiotherapy, Anastomotic leak, Rectal neoplasms
What’s Known
Radiotherapy exerts negative effects on wound healing and increases anastomosis leakage.
Diverting loop ileostomy has been usually performed after colorectal anastomosis in cases of colorectal cancer with a history of neoadjuvant radiotherapy to decrease the chance of leakage.
What’s New
Considering the side effects of diverting loop ileostomy, we investigated the effect of human amniotic membrane on colorectal anastomosis leakage after neoadjuvant radiotherapy: this approach is a feasible technique to prevent colorectal anastomosis leakage and its subsequent complications.
Introduction
Anastomosis leakage is still one of the most serious complications in colorectal surgery, causing significant mortality and morbidity despite improved preoperative and postoperative managements in recent years. Various factors including the patient, tumor, and therapy-related parameters are involved in anastomotic leakage. Age, gender, nicotine abuse, alcohol abuse, obesity, hypertension diabetes mellitus, coronary heart disease (CHD), and chronic obstructive pulmonary disease (COPD) are some of the patient-related variables. Tumor-related variables include tumor stage and tumor height from the dentate line. Neoadjuvant therapy, the extent of mesorectal excision, type of reconstruction, anastomotic technique, and protective ileostomy are therapy-related variables. The rate of anastomotic leakage was reported from 2.4% to 69%.1-3 The mortality and morbidity rates caused by anastomotic leakage were respectively estimated 7% to12%, and 20% to 30%.4
Neoadjuvant radiotherapy (with or without chemotherapy) has been applied in advanced rectal cancer to achieve down staging, en-bloc resection, tumor shrinkage, and decreasing the rate of local recurrence. Additionally, this modality allows the surgeons to perform sphincter saving procedure instead of abdominoperineal resection and permanent ileostomy construction. Preoperative radiotherapy may lead to delayed wound healing and subsequent wound complication.5 Overexpression of factors such as tissue growth factor beta (TGFβ), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and pro-inflammatory cytokines like interleukin-1 and interleukin-8, after irradiation, leads to uncontrolled matrix accumulation and fibrosis.6
Changes in fibroblast function, vasculature, and regulatory growth factors after radiotherapy lead to abnormal wound healing. HAM includes three sections, namely a single epithelial layer, a thick basement membrane, and a vascular stroma, which make the inner layer of placenta. HAM has important properties, including anti-inflammatory, antimicrobial, antifibrosis, antiscarring, low immunogenicity, and high potency of differentiation. Considering these features, HAM has already been used as a biologic dressing to promote wound healing process and treatment for tissue reconstruction, abdominal adhesiolysis, neurolysis, tenolysis and injuries of the vagina and dura-matter since more than 100 years ago.1,7 The precise mechanism of HAM in wound healing remains unknown. Various mechanisms of anti-inflammatory effect of HAM such as inducing apoptosis, decreasing lipid peroxidation and inhibiting the chemotactic activity of polymorphonuclear neutrophil (PMN) have been proposed.8 HAM has already been applied in multiple sites of gastrointestinal tract, such as duodenum, colon, and in rectovaginal fistula showing significant effects on epithelialization, neovascularization, proper wound healing, and better outcome.9
Classic surgical approach to rectal cancer is neoadjuvant radiotherapy, segmental resection of the involved rectum with free margins, colorectal anastomosis, and diverting loop ileostomy except in stage I.10 The side effects, complications, and limitations of ileostomy are dehydration, delay in the start of adjuvant therapy, and needing the second admission for the closure of colostomy. Some complications are ileostomy-related such as skin irritation, paraostomal hernia, ostoma necrosis, prolapse, bleeding, and electrolytes imbalance.11
Owing to adverse effects of pelvic irradiation on wound healing and colorectal anastomosis leakage as well as significant complications of fecal diversion by temporary loop ileostomy, we aimed to investigate the effect of HAM on the prevention of colorectal anastomosis leakage after neoadjuvant radiotherapy.
Materials and Methods
Animals and Ethic
In collaboration with the Animal Laboratory of Shiraz University of Medical Sciences, 20 crossbreed rabbits were selected and initially evaluated by veterinarian surgeon for any underlying problems. The age of rabbits was between 6-8 months and their weights were between 2-2.5 kg. The Animal Care Committee of Iran Veterinary Organization and the Headquarters of Animal Laboratory of Shiraz University of Medical Sciences supervised the process (e.g. animal selection, procedures, preoperative, and postoperative cares) according to their guidelines. All procedures were performed under aseptic conditions in the Department of Laboratory Animal Medicine of Shiraz University of Medical Sciences in accordance with the standards of Laboratory Biosafety Guidelines. The HAM was provided by Shiraz Burn Research Center from Shiraz Zeinab Hospital and primarily evaluated for viral markers (HIV, HCV, HBV). It was then preserved in gluteraldehyde and frozen in 20°C. This study was approved by the Research and Ethics Committee of Shiraz University of Medical Sciences.
Experimental Design and Operative Procedure
Step 1- Neoadjuvant Radiation: The rabbits were randomly divided into two groups (case group: 13 rabbits and control group: 7 rabbits). External beam irradiation was performedby a 6 MV linear accelerator unit and under general anesthesia in the Department of Radiotherapy, Namazi Teaching Hospital, Shiraz University of Medical Sciences, Shiraz, Iran. All rabbits received a single fraction dose of 10 Gy through two parallel-opposed portals encompassing rabbit colons. The abdominal region containing approximately a 10 cm length of colorectum was exposed.
Step 2- Procedure: Four weeks post-irradiation, after intravenous sedation by Ketamine-Xylazine (50 mg/kg administered 10-20 minutes in advance) followed by preparation and draping, laparotomy was performed layer by layer. Resection of 4 cm of colorectal segment 3 cm above dentate line and end-to-end single layer anastomosis with Vicryl 3-0 were conducted by interrupted stitches. A 2×2 wrap of HAM sutured around anastomosis line and fixed by Vicryl 3-0 with simple interrupted sutures. Abdominal irrigation was performed by warm saline. Abdominal fascia and skin sutured one by one with nylon 3-0 with continuous lock sutures. Surgical diet was started after 24 hours in both groups. Protocol of anesthesia, all procedures, preoperative, and postoperative cares (standard cages, temperature 20-24°C, humidity 55±5%, ventilation 12 times per hour, 12:12 hours light-dark cycle, and ad libitum feeding) were similar for all the rabbits.
Step 3- Follow-Up Analysis of Anastomosis: The survived rabbits were sacrificed after 8 weeks and specimens were fixed in formaldehyde 10% solution (formalin). Additionally, exploration was performed in expired rabbits and the site of anastomosis was resected. Samples labeled blindly and sent to the Department of Histopathology at Shiraz Medical School, Faghihi Teaching Hospital. After gross examination, histological sampling, tissue processing, slide preparation with hematoxylin and eosin staining were performed. The slides were blindly studied by a single pathologist. Histopathological findings were discussed for each sample on the basis of our modified scoring system. The scoring system was based on the previous version as suggested by Abramov and colleagues.12 The main modified items used in this system were histological evidence of epithelialization, collagenization, and neovascularization.
The statistically significant difference in healing score between HAM and standard groups was determined using Mann-Whitney U test (SPSS for Windows, Ver. 16, Chicago, IL).
Results
Macroscopic Evaluation
After 8 weeks, no anastomosis leakage was found in 13 rabbits (case group). After laparotomy, no evidence of abscess formation, pussy discharge or anastomosis site leakage was detected. Due to anastomosis leakage, 5 rabbits in the control group were expired, laparotomies were performed and subsequent peritonitis detected. Anastomotic sites resected and labeled for pathologic evaluation.
Pathological Evaluation
Histopathological assessment was performed by a single pathologist in the Department of Histopathology (figures 1 and 2). A modified scoring system for surgical wound healing12 was used to determine the grade of healing in each sample. Scoring was performed according to multiple factors such as epithelialization, inflammation, neovascularization, necrosis, and granulation tissue formation (table 1). Pathological evaluation revealed significant epithelialization and neovascularization in the case group (table 2).
Figure 1.
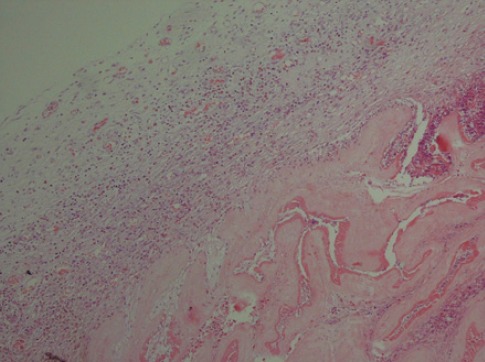
Hematoxylin and eosin staining (HPF×100) revealed human amniotic membrane and adjunct tissue reaction.
Figure 2.
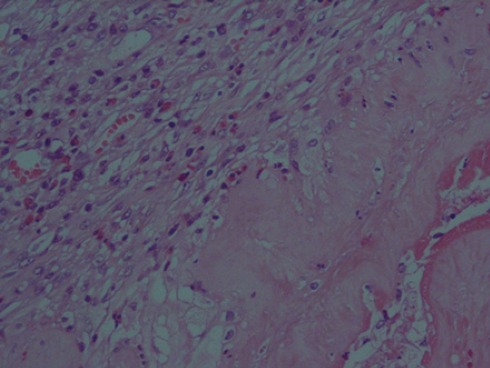
Hematoxylin and eosin staining (HPF×400) revealed human amniotic membrane and repair process beneath it with angiogenesis, edema, and fibroblast prolifration.
Table 1.
Modified scoring system for surgical wound healing
| Score | Epithelialization | Collagenization | Inflammation | Neovascularization | Necrosis | Granulation tissue |
|---|---|---|---|---|---|---|
| 1 | None | None | Severe | None | Extensive | None |
| 2 | None | None | Moderate | None | Focal | Immature |
| 3 | Partial | Partial | Mild | <5/HPF | None | Mild mature |
| 4 | Complete, immature | Complete, irregular | None | 6-10/HPF | None | Mod mature |
| 5 | Complete, mature | Complete, regular | None | >10/HPF | None | Fully mature |
Table 2.
Healing scores in single anastomosis and human amniotic membrane (HAM) wrapped groups
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 | #12 | #13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group healing score | 2 | 1 | 2 | 1 | 1 | 1 | 1 | ||||||
| HAM group healing score | 4 | 4 | 3 | 4 | 2 | 4 | 4 | 4 | 3 | 3 | 4 | 3 | 4 |
The percentage of healing score is presented in figure 3. The mean healing score in the case and control groups was 3.539±0.183 and 1.28±0.184, respectively. The healing score was significantly higher in the HAM group (P<0.001).
Figure 3.
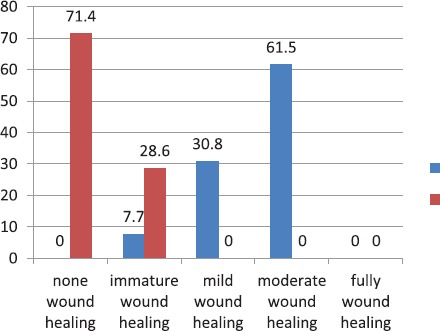
The percentage of healing scores in the case (13 rabbits) and control (7 rabbits) groups to assess the effect of human amniotic membrane on the prevention of colorectal anastomosis leakage in cases with neoadjuvant radiotherapy.
Discussion
This experimental study was performed as the first investigation to assay the effects of HAM on wound healing and bowel anastomosis leakage in an animal model that underwent neoadjuvant radiotherapy. Our findings showed a better histological surgical outcome and the prevention of colorectal anastomosis leakage in the HAM wrapped group. In addition, in cases without applying HAM and no ileostomy construction, we had a significant poor outcome.
The incidence of wound dehiscence in our study was approximately 72% (5 cases of 7) in the control group. In our investigation, we applied HAM on colonic anastomosis in cases with neoadjuvant radiation. In the HAM group, any evidence of anastomosis leakage was not detected. Anastomotic leakage is one of the most serious complications affecting 2-10% of patients undergoing gastrointestinal surgery.4 Anastomotic dehiscence could be considered as the main cause of substantial mortality and morbidity and poor long-term outcome.13,14
In a study by Uludag et al.,1 they revealed amniotic membrane (AM) had a significant effect on the prevention of colonic anastomosis leakage in cases with secondary peritonitis. They also mentioned AM had a protective effect on colorectal anastomosis leakage in early adjuvant intraperitoneal chemotherapy in other investigations.8 The higher incidence of anastomotic leakage in the control group may be caused by adverse effects of neoadjuvant radiotherapy on our colorectal anastomosis.
Problematic wound healing caused by radiotherapy is frequently overlooked during the treatment of cancer. Post-irradiation poor wound healing has multiple clinical sequels such as ulceration, pain, psychological distress, and wound infection.15 To prevent anastomosis leakage and wound complications, classically, temporary loop ileostomy and fecal diversion are mandatory in patients with colorectal anastomosis after preoperative pelvic irradiation. Diverting loop ileostomy has added serious adverse effects such as electrolytes imbalance, dehydration, surgical site complication, and psychosocial problems. Furthermore, complications of diverting ileostomy would affect postoperative management; such as delay in the start of adjuvant chemoradiation or failure to complete the courses.11 In the control group, anastomosis without ileal diversion had more leakage and subsequently secondary peritonitis and mortality. However, in the other groups, we had no evidence of such complications and no mortality despite any construction of diverting ileostomy.
In our study, pathological evaluation revealed more granulation tissue formation, neovascularization, epithelialization, and less necrosis in the case group compared with the control group. Various studies assessed the effect of HAM on wound healing of multiple sites of the GI tract. Barlas et al.14 mentioned the effectiveness of HAM as an intestinal patch for neomucosal growth in rabbits. In a study on dogs, Najibpour et al.16 noted that applying HAM led to better histological outcomes and wound healing in colonic anastomosis compared to the simple anastomosis. Roshanravan et al.17 applied HAM as a bioprosthesis to repair rectovaginal fistula in dogs, better surgical and histological outcomes were obtained. A study on 50 Wistar rats proved that HAM with contributing re-establishment of duodenal wall structure led to accelerated wound healing.18 Uludag et al.1 reported lower dehiscence rate, anastomotic leakage, and better wound healing after applying HAM in colonic anastomosis of rats. In addition, the research of Kuriu et al.19 noted that the intraperitoneal adhesion was reduced by HAM graft in rats. Moreover, HAM has also been used in the reconstruction of conjunctival defects, reconstruction of the oral cavity and prevention of postoperative adhesions.20 Although the precise mechanism of HAM to reduce inflammation and increase wound healing process has not been completely clear yet, it seems that HAM works by suppressing inflammatory cytokines, expressing anti-inflammatory proteins, tissue growth factor, multipotent cells, increasing angiogenesis and fibroblast activity.21 The findings of these studies are in line with the present study, but none of them assessed the effects of HAM in cases with external beam irradiation.
This trial was conducted on a small size animal. Because of differences between humans and rabbits (regarding GI tract), it is appropriate to apply this method on large animals and human beings. It is noteworthy that anastomosis leakage in humans has multiple risk factors such as underlying disease, obesity, diabetes mellitus, anemia, smoking, and is variable in different procedures. These factors were not evaluated in the present study.
Conclusion
The present study is the first assessment of applying HAM after external beam irradiation in colorectal surgery. Considering the obtained result, applying HAM on colorectal anastomosis in cases with neoadjuvant external beam radiotherapy might be useful in preventing anastomosis leakage and its subsequent complications. Unknown probable side effects of HAM, especially in long-term follow-up should be evaluated in further investigations. Moreover, other prospective studies are warranted in applying HAM on colonic anastomosis instead of diverting loop ileostomy construction and more definitive conclusion.
Acknowledgment
The authors would like to thank the Vice Chancellor of Shiraz University of Medical Sciences for supporting this research (grant number: 5733) and Dr. Atefeh Yousefi Pourdargah, Dr. Alireza Safarpour, and Dr. Hajar Khazraei. This article is based on a thesis by Sam Moslemi and Sajjad Ahmadi Joraghi.
Conflict of Interest: None declared.
References
- 1.Uludag M, Citgez B, Ozkaya O, Yetkin G, Ozcan O, Polat N, et al. Effects of amniotic membrane on the healing of primary colonic anastomoses in the cecal ligation and puncture model of secondary peritonitis in rats. Int J Colorectal Dis. 2009;24:559–67. doi: 10.1007/s00384-009-0645-y. [DOI] [PubMed] [Google Scholar]
- 2.Corman ML, I. Allison S, P. Kuehne J. Colon and Rectal Surgery. New York: Lippincott Williams & wilkins; 2005. Colon and Rectal Surgery; pp. 177–277. [Google Scholar]
- 3.Kruschewski M, Rieger H, Pohlen U, Hotz HG, Buhr HJ. Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Colorectal Dis. 2007;22:919–27. doi: 10.1007/s00384-006-0260-0. [DOI] [PubMed] [Google Scholar]
- 4.Telem DA, Chin EH, Nguyen SQ, Divino CM. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg. 2010;145:371–6. doi: 10.1001/archsurg.2010.40. [DOI] [PubMed] [Google Scholar]
- 5.Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438–43. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 6.Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997;42:99–106. doi: 10.1016/S0167-8140(96)01880-4. [DOI] [PubMed] [Google Scholar]
- 7.Ghahramani L, Jahromi AB, Dehghani MR, Ashraf MJ, Rahimikazerooni S, Rezaianzadeh A, et al. Evaluation of repair in duodenal perforation with human amniotic membrane: An animal model (dog) Adv Biomed Res. 2014;3:113. doi: 10.4103/2277-9175.131029. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uludag M, Ozdilli K, Citgez B, Yetkin G, Ipcioglu OM, Ozcan O, et al. Covering the colon anastomoses with amniotic membrane prevents the negative effects of early intraperitoneal 5-FU administration on anastomotic healing. Int J Colorectal Dis. 2010;25:223–32. doi: 10.1007/s00384-009-0833-9. [DOI] [PubMed] [Google Scholar]
- 9.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 10.Bell SW, Walker KG, Rickard MJ, Sinclair G, Dent OF, Chapuis PH, et al. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg. 2003;90:1261–6. doi: 10.1002/bjs.4219. [DOI] [PubMed] [Google Scholar]
- 11.Phatak UR, Kao LS, You YN, Rodriguez-Bigas MA, Skibber JM, Feig BW, et al. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol. 2014;21:507–12. doi: 10.1245/s10434-013-3287-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramov Y, Golden B, Sullivan M, Botros SM, Miller JJ, Alshahrour A, et al. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007;15:80–6. doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 13.Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29:907–16. doi: 10.1097/BCR.0b013e31818b9e40. [DOI] [PubMed] [Google Scholar]
- 14.Barlas M, Gokcora H, Erekul S, Dindar H, Yucesan S. Human amniotic membrane as an intestinal patch for neomucosal growth in the rabbit model. J Pediatr Surg. 1992;27:597–601. doi: 10.1016/0022-3468(92)90456-H. [DOI] [PubMed] [Google Scholar]
- 15.Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J. 2005;2:112–27. doi: 10.1111/j.1742-4801.2005.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najibpour N, Jahantab MB, Hosseinzadeh M, Roshanravan R, Moslemi S, Rahimikazerooni S, et al. The effects of human amniotic membrane on healing of colonic anastomosis in dogs. Annals of Colorectal Research. 2013;1:97–100. doi: 10.5812/acr.16139. [DOI] [Google Scholar]
- 17.Roshanravan R, Ghahramani L, Hosseinzadeh M, Mohammadipour M, Moslemi S, Rezaianzadeh A, et al. A new method to repair recto-vaginal fistula: Use of human amniotic membrane in an animal model. Adv Biomed Res. 2014;3:114. doi: 10.4103/2277-9175.131033. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schimidt LR, Cardoso EJ, Schimidt RR, Back LA, Schiazawa MB, d’Acampora AJ, et al. The use of amniotic membrane in the repair of duodenal wounds in Wistar rats. Acta Cir Bras. 2010;25:18–23. doi: 10.1590/S0102-86502010000100006. [DOI] [PubMed] [Google Scholar]
- 19.Kuriu Y, Yamagishi H, Otsuji E, Nakashima S, Miyagawa K, Yoshikawa T, et al. Regeneration of peritoneum using amniotic membrane to prevent postoperative adhesions. Hepatogastroenterology. 2009;56:1064–8. [PubMed] [Google Scholar]
- 20.von Versen-Hoynck F, Hesselbarth U, Moller DE. Application of sterilised human amnion for reconstruction of the ocular surface. Cell Tissue Bank. 2004;5:57–65. doi: 10.1023/B:CATB.0000022222.41304.de. [DOI] [PubMed] [Google Scholar]
- 21.Uludag M, Citgez B, Ozkaya O, Yetkin G, Ozcan O, Polat N, et al. Effects of amniotic membrane on the healing of normal and high-risk colonic anastomoses in rats. Int J Colorectal Dis. 2009;24:809–17. doi: 10.1007/s00384-009-0691-5. [DOI] [PubMed] [Google Scholar]


