Abstract
Background:
The purpose of this study was to create biomaterial scaffolds like platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) containing stromal cell-derived factor-1 (SDF1) as a chemokine to induce hyaline cartilage regeneration of rabbit knee in a full thickness defect.
Methods:
We created a full thickness defect in the trochlear groove of thirty-six bilateral knees of eighteen mature male rabbits. The knees were randomly divided into six groups (group I: untreated control, group II: PRP, group III: PRF, group IV: Gelatin+SDF1, group V: PRP+SDF1, and group VI: PRF+SDF1). After four weeks, the tissue specimens were evaluated by macroscopic examination and histological grading, immunofluorescent staining for collagen type II, and analyzed for cartilage marker genes by real-time PCR. The data were compared using statistical methods (SPSS 20, Kruskal-Wallis test, Bonferroni post hoc test and P<0.05).
Results:
Macroscopic evaluations revealed that international cartilage repair society (ICRS) scores of the PRF+SDF1 group were higher than other groups. Microscopic analysis showed that the ICRS score of the PRP group was significantly lower than other groups. Immunofluorescent staining for collagen II demonstrated a remarkable distribution of type II collagen in the Gel+SDF1, PRP+SDF1 and PRF+SDF1 groups compared with other groups. Real-time PCR analysis revealed that mRNA expression of SOX9 and aggrecan were significantly greater in the PRF+SDF1, PRP+SDF1, Gel+SDF1 and PRF groups than the control group (P<0.05).
Conclusion:
Our results indicate that implantation of PRF scaffold containing SDF1 led to the greatest evaluation scores of full-thickness lesions in rabbits.
Keywords: Platelet-rich plasma, Platelet rich fibrin, Chemokine CXCL12, Cartilage, Knee
What’s Known
Implantation of a combination of collagen І scaffold and ultrapurified alginate gel containing stromal cell-derived factor-1 (SDF1) improved the repairing process by conferring better support for the migration of cartilage mesenchymal stem cells (C-MSCs) and synovial membrane mesenchymal stem cells (SM-MSCs).
Adding platelet-rich fibrin (PRF) to bone allograft in sinus elevation surgery can reduce the healing time.
Some studies have shown that if platelet-rich plasma (PRP) is applied in chondral lesions, signs of healing will be observed.
What’s New
The effects of PRF+SDF1 and PRP+SDF1 were not studied so far.
We used PRP and PRF scaffolds and SDF1 to magnify their effects and reconstruct injured sites. These scaffolds are rich in slow-releasing growth factors and are easily harvested from autologous blood.
There is similarity between PRF fibrin network and the natural one.
SDF1, which is expressed by BMSCs, regulates the trafficking, homing, and migration of stem cells into the injury site.
Introduction
Adult articular cartilage has insufficient ability to repair after either erosion or damage and it is unlikely to be reformed to normal condition once it has been impaired.1 Functional repair of articular cartilage defects encountered a major challenge to cartilage tissue engineering.
Articular cartilage repair has been based on finding and resolving the primary reason for the injury, but it has been recognized that only treating the damage site is not adequate to gain good long-term functionality.2 The matrix environment of hyaline cartilage is different from that of subchondral bone in biological, physical and chemical characteristics.3 This led to the proposal that in order to gain positive results in tissue repair, the ‘microenvironment’ in which the repair process takes place should be retouched, such as those elements that play a pro-angiogenic, conductive, cell differentiation and chemotactic role.2 This tendency has been the reason behind a growth in using genetic and tissue engineering in the examination of articular cartilage repair.4
A number of natural and artificial biomaterials have been used as scaffolds for cartilage reformation, but their biological effectiveness as well as safety remains unresolved. Therefore, scaffold biomaterials extracted from the patients’ own body have long been a tempting choice.5 Platelet-rich plasma (PRP), which can be easily harvested from autologous blood, has been used in clinical experiments for decades.6 PRP is rich in growth factors, containing those that help the proliferation of chondrogenic cells and discharging of cartilaginous matrix, such as platelet-derived growth factor (PDGF), transforming growth factor (TGF-b), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and insulin-like growth factor (IGF).6,7
A new member in platelet concentrated products is platelet-rich fibrin (PRF), which can be prepared easily without biochemical blood handling. PRF is similar to PRP except in biochemical architectures because of slow polymerization during PRF preparation.8 The PRF fibrin network is similar to the natural one. Such a network leads to a more competent cell mobilization and repopulation and consequently healing.8 Stromal cell-derived factor-1 (SDF1), a chemokine factor expressed by BMSCs, regulates the trafficking, homing and migration of stem cell by mediating chemokinesis and chemotaxis into the injury site.9 Bone marrow, which is adjoining the chondro-osseous junction, discharges SDF1.10
We believe that autologous PRP and PRF could be used alone as a 3-D scaffold capable of cartilage tissue regeneration because of releasing endogenous growth factors. SDF1 stimulates cartilage mesenchymal stem cells (C-MSCs), synovial membrane mesenchymal stem cells (SM-MSCs), and bone marrow stromal cells (BMSCs) to migrate to the subchondral bone in full-thickness defects to make cartilage. The aim of the study was to evaluate tissue repair along with PRP and PRF or without treatment. The secondary objective was to evaluate the efficacy of PRP and PRF used with SDF1.
Materials and Methods
PRP Gel Preparation
Blood samples were taken from the hearts of six rabbits. The 9 mL of whole blood collected in a tube containing 1 mL an anticoagulant (3.8% sodium citrate solution) was centrifuged for 5 minutes at 2,000 rpm and the supernatant was placed in a new tube. The supernatant was centrifuged for 10 minutes at 3,000 rpm. The plasma at the top was discarded and the remaining 1mL of liquid PRP precipitate was collected. After mixing PRP (200 μL/defect) and 40 mmol/L calcium chloride (CaCl2), a gel was formed. The PRP gel bonded to the adjacent cartilage about 10 minutes after implantation.11
PRF Preparation
The 10 mL blood samples collected from the hearts of six rabbits without anticoagulant were immediately centrifuged at 3,000 rpm for 10 minutes. In the middle of the tube, a fibrin clot was formed between supernatant acellular plasma and the lower red corpuscles.8
SDF1 Incubation
Before transplantation, 600 μL of rhSDF1 alpha (120 ng/mL Peprotech) was incubated with PRP gel, PRF and gelatin separately for 2 hours at 37ºC.12
Study Design and Animal Experimentation
A total of 18 adult male white Japanese rabbits (weighing 2.5-3.0 kg) were used in this study. The animals were housed in metal wire cages, in a temperature-controlled room under a 12:12 h light-dark cycle at 22–24ºC and 50–60% relative humidity. They were fed ad libitum with standard laboratory chow and tap water. Animal interventional experiments were carried out at Animal Experiments of Tehran University of Medical Sciences under the rules and regulation of the Animal Care and Use Committee of Shiraz University School of Medicine.
The 36 bilateral knees of the 18 rabbits were randomly divided into 6 experimental groups of 6 knees per group. Each animal was sedated by intramuscular injection of ketamine hydrochloride 60 mg/kg and xylazine 6 mg/kg. In sterile conditions, a medial para-patellar arthrotomy was made in both knees. A full-thickness cylindrical cartilage defect of 4 mm in diameter and 3 mm in depth was created in the patellar groove using a standard size stainless biopsy punch. The joints were thoroughly rinsed with sterile saline solution before transplantation. In group I, the defects were left unfilled (control group). In group II, the defects were filled with platelet-rich plasma only (PRP group). In group III, the defects were filled with platelet-rich fibrin only (PRF group). In group IV, the defects were filled with gelatin containing stromal cell-derived factor-1 (Gel+SDF1 group). In group V, the defects were filled with platelet-rich plasma containing stromal cell-derived factor-1 (PRP+SDF1 group). In group VI, the defects were filled with platelet-rich fibrin containing stromal cell-derived factor-1 (PRF+SDF1 group) in a randomized manner. The rabbits were accommodated in separate cages and allowed unrestricted activity after surgery.9,11,12
Gross Morphology
Four weeks after implantation, the rabbits were sacrificed with an overdose of anesthesia. The distal parts of the femurs were excised, photographed and graded for cartilage repair, according to the international cartilage repair society score (ICRS) macroscopic assessment scores11 (table 1).
Table 1.
ICRS macroscopic evaluation of cartilage repair
| Categories | Score |
|---|---|
| Degree of defect repair | |
| In level with surrounding cartilage | 4 |
| 75% repair of defect depth | 3 |
| 50% repair of defect depth | 2 |
| 25% repair of defect depth | 1 |
| 0% repair of defect depth | 0 |
| Integration to border zone | |
| Complete integration with surrounding cartilage | 4 |
| Demarcating border <1 mm | 3 |
| 3/4 of graft integrated, 1/4 with a notable border >1 mm width | 2 |
| 1/2 of graft integrated with surrounding cartilage, 1/2 with a notable border >1 mm | 1 |
| From no contact to 1/4 of graft integrated with surrounding cartilage | 0 |
| Macroscopic appearance | |
| Intact smooth surface | 4 |
| Fibrillated surface | 3 |
| Small, scattered fissures or cracks | 2 |
| Several, small or few but large fissures | 1 |
| Total degeneration of grafted area | 0 |
| Overall repair assessment | |
| Grade I: normal | 12 |
| Grade II: nearly normal | 11-8 |
| Grade III: abnormal | 7-4 |
| Grade IV: severely abnormal | 3-1 |
Histological and Immunohistochemical Analysis
After gross evaluation, samples were fixed in 4% paraformaldehyde for 7 days, decalcified in 10% EDTA for 3 weeks and then embedded in paraffin and cut perpendicularly into 5 μm sections. Then, the sections were stained for general histology with hematoxylin and eosin (H&E) and with Toluidine blue to estimate the cartilaginous matrix distribution. The re-created tissue was scored blindly according to the ICRS scale9,13 (table 2).
Table 2.
ICRS visual histological assessment scale
| Feature | Score |
|---|---|
| I. Surface | |
| Smooth/continuous | 3 |
| Discontinuities/irregularities | 0 |
| II. Matrix | |
| Hyaline | 3 |
| Mixture: Hyaline/fibrocartilage | 2 |
| Fibrocartilage | 1 |
| Fibrous tissue | 0 |
| III. Cell distribution | |
| Columnar | 3 |
| Mixed/columnar-clusters | 2 |
| Clusters | 1 |
| Individual cells/disorganized | 0 |
| IV. Cell population viability | |
| Predominantly viable | 3 |
| Partially viable | 1 |
| <10% viable | 0 |
| V. Subchondral Bone | |
| Norma | 3 |
| Increased remodeling | 2 |
| Bone necrosis/granulation tissue | 1 |
| Detached/fracture/callus at base | 0 |
| VI. Cartilage mineralization (calcified cartilage) | |
| Normal | 3 |
| Abnormal/inappropriate location | 0 |
Immunofluorescent analysis was performed using a monoclonal antibody against Collagen II (Cat number: MA1-37493). The sections were deparaffinized by xylene and rehydrated by decreased concentrations of ethanol solutions. Heat-induced epitope retrieval was done in citrate buffer (sodium citrate, 10 mM; pH 6.0) in a pressure cooker for 4 minutes at full pressure. Subsequent to cooling, endogenous peroxidase was blocked using a 3% hydrogen peroxide solution for 20 minutes. The slides were then washed with phosphate buffer solution (10 mM; pH 7.4) and incubated with primary antibodies overnight at 4°C. The slides were washed again three times with PBS and subsequently incubated with an FITC-conjugated secondary antibody (Cat number: 11555100) according to the respective manufacturer’s instructions. The slides were washed three times with PBS and then covered with cover slips. Finally, samples were analyzed by florescent microscope.9
Real-time PCR Analysis
Total RNA was extracted from the regenerated tissues in the defect area. Total RNA extraction was performed using RNX-plus kit (CinnaGen) according to the protocol. The RNA samples were resuspended in 30 μL of nuclease-free water. The concentration and quantification of total RNA were measured with spectrophotometer, with the OD260/OD280 ratio of all RNA samples 1.9–2.0 and OD260/OD230 ratio up to 2. The first strand cDNA was synthesized with the first-strand cDNA synthesis kit (Bioneer kit, K-2101). For each reaction, 1 μg (1 μL) RNA was used for reverse transcription, in a mixture of 20 pmoles (1 μL) random primer, and 18 μL DEPC-D.W with a final volume of 20 μL. The mixture was incubated at 15ºC for 1 min, 50ºC for 60 minutes and heated at 95ºC for 5 minutes to terminate the reaction. The cDNA was subsequently stored at -20ºC. qPCR was performed in a volume 1 μL of primer and 1 μL from template also add 3 μL DEPC.D.W with 5 μL Master Mix (AccuPower® 2X GreenStarTMqPCR Master Mix, Bioneer kit). Real-time PCR was done by ABI (Applied Biosystems). All PCR reactions were in the following condition: initial 95ºC for 15 minutes, followed by 35 cycles at 95ºC for 15 s and 60ºC for 30 s. After completing the qPCR, their products run on agarose gels for quality check and real-time data analyzed by Livak rules.14 Beta actin was used for the internal control. The PCR primers for each gene are displayed below. Each sample was tested in duplicate. The sequences of primers used in real-time PCR analyses were as follows: Aggrecan forward GAGACCAAGTCCTCAAGCCC; reverse CTCTGTCTCCTGGCAGGTTC. Sox9 forward GCTCCGACACCGAGAATACA; reverse TTGACGTGGGGCTTGTTCTT.13
Data Analysis
Data are expressed as mean±SD (standard deviation). Significance was considered at the 0.05 level. Outcome variables were macroscopic evaluation scores, histological scores, and genes expression. A comparison between groups for each outcome was carried out with the Kruskal-Wallis test. Bonferroni post hoc tests were used for the comparison analysis of each two groups. Data were analyzed using SPSS (version 20) software.
Results
Macroscopic Observation
Four weeks after surgery, the regenerated regions of all groups were studied. The macroscopic evaluation of the treated areas in the PRF+SDF1 group revealed complete repair and integrated well with the surrounding cartilage as compared with other groups (figure 1). A post-hoc analysis showed that ICRS scores of all experimental groups were significantly higher than those in the control group and the scores of the PRF+SDF1 group were greater than those of the other groups (P<0.001) (figure 2).
Figure 1.
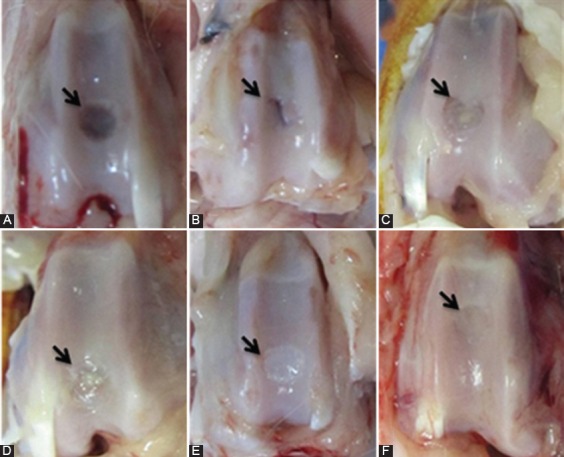
Gross appearance of defects in the trochlear groove at four weeks after surgery, respectively. A) Control group: Transplantation was not performed. The defect is partially vacant and clearly noticeable from the surrounding cartilage. B) PRP group: Healing tissue is thinner than normal surrounding cartilage and shows a large fissure. C) PRF group: Repaired tissue covers defect with a minor concavity. D) Gel+SDF1 group: Repaired tissue covers the defect almost completely, but is thin and distinguishable from the surrounding cartilage. E) PRP+SDF1 group: Healing tissue covers defect with smooth white tissue and distinguishable from the surrounding cartilage. F) PRF+SDF1 group: Repaired tissue covers the defect completely and no obvious margin was notable.
Figure 2.
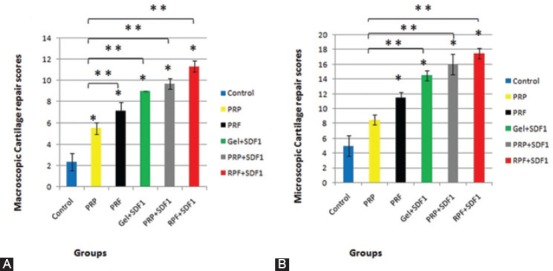
A) ICRS macroscopic and B) ICRS microscopic scores in the regenerated cartilage of defects in the trochlear groove at four weeks postoperatively. Data are presented as mean±SD. *Significant difference with non-treated control (P<0.05); **Significant difference with RPP group (P<0.05).
Microscopic Observation
Four weeks after surgery, the regenerated regions were evaluated by H&E and Toluidine blue staining. The regenerated regions of the PRF+SDF1 group were entirely filled by the repaired tissue and showed better results than the other groups (figures 3 and 4). A post-hoc analysis showed that ICRS scores of all groups, except for the PRP group, were significantly higher than the control group (P<0.001). The scores of the PRF+SDF1 group were greater than the other groups, but were not significant when compared with the Gel+SDF1 and PRP+SDF1 groups. The scores of the PRP group were significantly lower than the other groups except for the PRF group, which was not significant (figure 2). The qualitative study of immunofluorescent staining of collagen II demonstrated that neo-cartilages were minimally positive in the control, PRP, and PRF groups. In contrast, the Gel+SDF1, PRP+SDF1 and PRF+SDF1 groups exhibited a remarkable distribution of type II collagen (figure 5).
Figure 3.
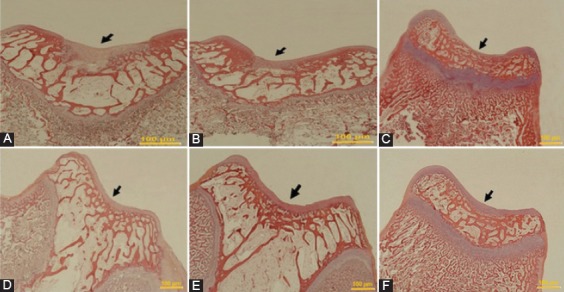
The microscopic appearance of H&E staining of defects in the trochlear groove at four weeks after surgery, respectively (Original magnification=200×). A) Control group: The defect is concave and a noticeable thin layer of non-cartilaginous tissue. B) PRP group: The defect is filled by fibrous tissue with a crack. C) PRF group: The defect is covered by fibrous tissue. D) Gel+SDF1 group and (E) PRP+SDF1 group: The defects in both groups are filled with a repaired tissue, which are thinner than the normal surrounding cartilage. F) PRF+SDF1 group: The defect is entirely filled by the repaired tissue, resembling the healthy cartilage surrounding the tissue.
Figure 4.
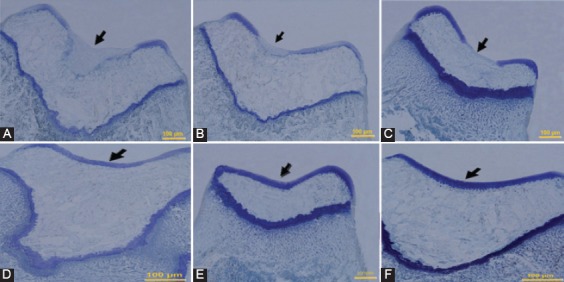
Histological appearance of Toluidine blue staining of defects in the trochlear groove at four weeks after surgery, respectively (Original magnification=200×). A) Control group: The defect is filled by no cartilage matrix. B) PRP group, and C) PRF group: The defects in both groups have poor staining fibrocartilagous tissues and poorly organized ECM. D) Gel+SDF1 group, and E) PRP+SDF1 group: The repaired tissues show a good staining in both groups. F) PRF+SDF1 group: The defect has an intense staining.
Figure 5.
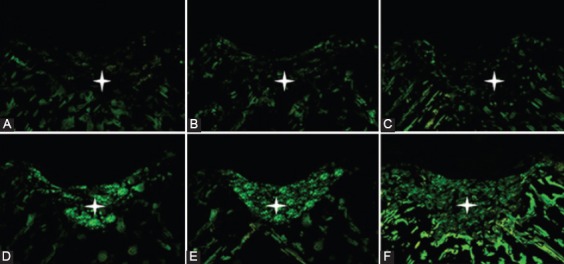
Immunofluorescent staining appearance of defects in trochlear groove at four weeks after surgery. A) Control group, B) PRP group, C) PRF group, D) Gel+SDF1 group, E) PRP+SDF1 group, and F) PRF+SDF1 group. The neo-cartilages are minimally positive in A, B and C. The neo-cartilages are positive in D and E. A remarkable distribution of type II collagen is seen in F.
Real-Time PCR Analysis
Four weeks after surgery, a real-time PCR analysis revealed that the mRNA expression of SOX9 was significantly greater in the regenerated tissue of all groups than the untreated control group (P<0.001). The level of gene expression of aggrecan in all groups was significantly higher than the control group, except for the PRP group that was not significant (P<0.001) (figures 6 and 7).
Figure 6.
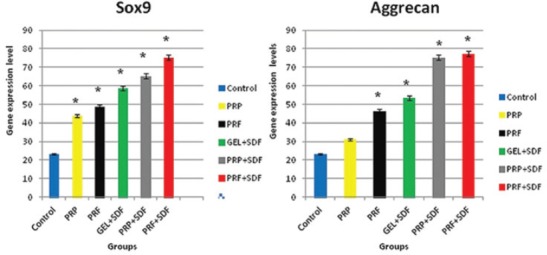
The amount of gene expression of SOX9 and aggrecan in the regenerated cartilage of defects in the trochlear groove at four weeks postoperatively. Data are presented as mean±SD. *Significant difference with non-treated control (P<0.05).
Figure 7.
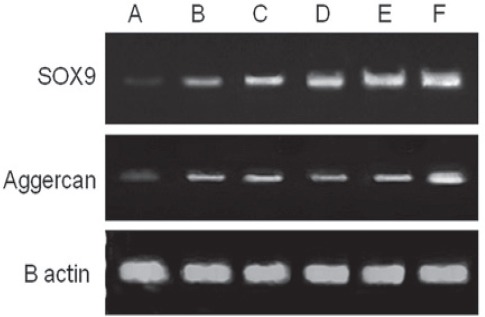
The quality checking of RT-PCR for SOX9 and aggrecan from the regenerated cartilage of defects in the trochlear groove at four weeks after surgery. Lane A: Control group where transplantation was not performed; Lane B: PRP group; Lane C: PRF group; Lane D: Gel+SDF1 group; Lane E: PRP+SDF1 group, and Lane F: PRF+SDF1 group.
Discussion
This study considered the effects of PRP and PRF with or without SDF1 in full-thickness lesions in rabbits’ knees. A literature review indicated that the effects of PRF+SDF1 and PRP+SDF1 were not studied so far. We demonstrated that the implantation of PRF+SDF1 led to the greatest yield scores of full-thickness lesions in rabbits’ knee. Therefore, it can be concluded that the scaffolds containing SDF1 have a better result on cartilage repair. Zhang W. et al.12 implanted a combination of collagen І scaffold to mimic the subchondral bone matrix environment containing SDF1 in order to repair partial-thickness defects in rabbits’ knee. The results demonstrated that collagen І scaffold containing SDF1 improved the repairing process by a better support for C-MSCs and SM-MSCs migration and adhesion six weeks after transplantation. Some researchers used ultra-purified alginate gel containing SDF1 to improve osteochondral lesions in rabbits’ knee. According to their studies, the repairing effect of osteochondral defects is elevated, because SDF1 increases the migration of host cells (mainly BMSCs) to the site of injury, and this makes SDF1 as a potential factor in cartilage repair.15 Wei L. et al. used SDF1 to induce chondrocyte hypertrophy. They studied the length of the hypertrophic zone by immunohistochemical analysis of tibia cartilaginous growth plates of chicken embryos, which were incubated with SDF1 for 2, 4, and 6 days in organ culture. The results showed the length of the hypertrophic zone was significantly elevated after 4 and 6 days.3 In injured tissue with the permissive matrix environment, endogenous stem cells can participate in corrective process. Some studies have introduced populations of stem/progenitor cells in the synovial membrane16,17 and articular cartilage,18-20 which show characteristic of stem cells and have the potential for cartilage repair.21,22 The findings of the above studies are in agreement with our results that show SDF1 can facilitate the migration of C-MSCs and SM-MSCs to full-thickness defect sites. Although SDF1 has been notified to promote inflammatory cell migration,23 we could not find inflammatory cells in the newly formed tissue in RPF, PRP and Gel containing SDF1 groups. In parallel with our data, a study on tendon repair by exogenous SDF1 showed improvement of injury site and diminishing accumulation of inflammatory cells.9 In PRF, the fibrin formation meshes, which are similar to biochemical architectures of the natural one,8 can trap a significant amount of circulating cytokines. This is what renders PRF as a distinctive treatment in injury repair. This method increases the lifespan of these cytokines (as a long-term effect), without which, it will be only released and used at the time of the primary phase of matrix remodeling. As a result, when the cells begin extracellular matrix remodeling to reconstruct the injured site, the cytokines will be available in the affected area for the required longer period. Cytokines are important factors in the delicate balance of tissue homeostasis.9
In a pilot study, Haleem A.M. et al. showed that all patients with osteochondral defects who received BM-MSCs transplanted on platelet-rich fibrin glue (PR-FG) had a significant improvement in their functional knee scores and MRI findings after 6 months and remained stable 12 months post operation.1 In agreement with this clinical study, Choukroun J. et al. revealed that filling a tooth socket by PRF can enhance the healing process without any complication.24 In a supplementary study, they reported that adding PRF to bone allograft in sinus elevation surgery can lead to 4-month reduction in healing time by increasing the speed of bone allograft maturation.25 However, in contrast to our results, an in-vivo study by Shao X.X. et al. on osteochondral defects in rabbits’ knee showed that BMSCs seeded in fibrin glue matrix forms a poor-quality cartilage-like tissue.26 It can be concluded that the reason for varying results can be due to the differences in biomechanical conditions of defect types. Kelly D.J. et al. reported that differentiation of bone-marrow derived stem cells can be affected by mechanical signals.27 Moreover, suitable recurring compressive stress significantly increases chondrocyte proliferation in addition to aggrecan and collagen synthesis by chondrocytes.28 Some researchers reported that cell adhesion and migration can facilitate by some elements such as different cell type and substrate stiffness.29,30 Most importantly, in a separate study, Wang H. et al. had compared the amount of bounded bovine chondrocytes on various surfaces of middle deep zone bone or calcified cartilage or hyaline cartilage. They reported that different contact surface stiffness give different ability to cells for migration and attachment.31
Opinions about autologous PRP usage in terms of its composition is controversial.32,33 Activated PRP can be used in different forms like gel as a scaffold, liquid form injection or can be infiltrated in the tissue thickness.34 The selection of each form relies upon the type of tissue and the existing problem.35,36 By degradation of the fibrin skeleton of PRP, it can release endogenous growth factors without modifying genes or cytokine delivery technologies. Growth factors play a significant role in tissue repair, especially in the regenerative process of tissues with low cellular density and vascularization, such as cartilage.6,37,38 A study showed that if PRP was applied in full-thickness chondral lesions, the signs of healing will be observed, but it did not show healthy or functional behavior.4 Wu W. et al. reported cartilage formation into the PRP scaffold after implanting PRP gel as a carrier for cultured autologous chondrocytes in the subcutaneous tissue of rabbits’ knees.39 Another study suggested that transplantation of PRP gel along with synovial membrane derived mesenchymal stem cells (SDSCs) can facilitate the repair of osteochondral lesions in rabbits’ knees.11 Perhaps the underlying reason for different results is various compositions of this plasma fraction in different species, including rabbits.32,34,40
It should be noted that our study had a limitation. PRF was harvested from an autologous blood sample that had a limited volume and PRF tissue banks were not achievable.
Conclusion
In the present study, in addition to using PRP and PRF as scaffolds for cell seeding, we used a mixture of SDF1 and PRP and PRF scaffolds to enhance the migration of host cells. In our research, the effects of PRP and PRF alone on the full-thickness defects were also studied separately. We found that the added SDF1 stimulated cell or the combination of PRP, PRF, and SDF1 stimulated the observed improvement.
Acknowledgment
This work was supported by Shiraz University of Medical Sciences and Tehran University of Medical Sciences. The authors would like to thank Maryam Hatami for her assistance in developing the animal model. The present article was extracted from the thesis written by Maryam Ghasemi and was financially supported by Shiraz University of Medical Sciences, under grant number 93-7010.
Conflict of Interest: None declared.
References
- 1.Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, et al. The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage. 2010;1:253–61. doi: 10.1177/1947603510366027. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 3.Wei L, Kanbe K, Lee M, Wei X, Pei M, Sun X, et al. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol. 2010;341:236–45. doi: 10.1016/j.ydbio.2010.02.033. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serra CI, Soler C, Carrillo JM, Sopena JJ, Redondo JI, Cugat R. Effect of autologous platelet-rich plasma on the repair of full-thickness articular defects in rabbits. Knee Surg Sports Traumatol Arthrosc. 2013;21:1730–6. doi: 10.1007/s00167-012-2141-0. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed TA, Giulivi A, Griffith M, Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng Part A. 2011;17:323–35. doi: 10.1089/ten.TEA.2009.0773. [DOI] [PubMed] [Google Scholar]
- 6.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–15. doi: 10.1007/s11999-011-1857-3. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34:589–97. doi: 10.1007/s00264-009-0793-2. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Shen W, Chen X, Chen J, Yin Z, Heng BC, Chen W, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–49. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Fermas S, Gonnet F, Sutton A, Charnaux N, Mulloy B, Du Y, et al. Sulfated oligosaccharides (heparin and fucoidan) binding and dimerization of stromal cell-derived factor-1 (SDF-1/CXCL 12) are coupled as evidenced by affinity CE-MS analysis. Glycobiology. 2008;18:1054–64. doi: 10.1093/glycob/cwn088. [DOI] [PubMed] [Google Scholar]
- 11.Lee JC, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 2013;29:1034–46. doi: 10.1016/j.arthro.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Chen J, Tao J, Jiang Y, Hu C, Huang L, et al. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34:713–23. doi: 10.1016/j.biomaterials.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda H, Kitamura N, Kurokawa T, Arakaki K, Gong JP, Kanaya F, et al. Influence of the gel thickness on in vivo hyaline cartilage regeneration induced by double-network gel implanted at the bottom of a large osteochondral defect: short-term results. BMC Musculoskelet Disord. 2013;14:50. doi: 10.1186/1471-2474-14-50. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A. 2012;18:934–45. doi: 10.1089/ten.TEA.2011.0380. [DOI] [PubMed] [Google Scholar]
- 16.Fickert S, Fiedler J, Brenner RE. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthritis Cartilage. 2003;11:790–800. doi: 10.1016/S1063-4584(03)00167-5. [DOI] [PubMed] [Google Scholar]
- 17.De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–18. doi: 10.1083/jcb.200212064. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13:R64. doi: 10.1186/ar3320. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–35. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Shimomura K, Ando W, Tateishi K, Nansai R, Fujie H, Hart DA, et al. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 2010;31:8004–11. doi: 10.1016/j.biomaterials.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Sekiya I, Muneta T, Koga H, Nimura A, Morito T, Shimaya M, et al. [Articular cartilage regeneration with synovial mesenchymal stem cells] Clin Calcium. 2011;21:879–89. doi: CliCa1106879889. [PubMed] [Google Scholar]
- 23.de Vries-van Melle ML, Mandl EW, Kops N, Koevoet WJ, Verhaar JA, van Osch GJ. An osteochondral culture model to study mechanisms involved in articular cartilage repair. Tissue Eng Part C Methods. 2012;18:45–53. doi: 10.1089/ten.TEC.2011.0339. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Shao XX, Hutmacher DW, Ho ST, Goh JC, Lee EH. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials. 2006;27:1071–80. doi: 10.1016/j.biomaterials.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 27.Kelly DJ, Jacobs CR. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90:75–85. doi: 10.1002/bdrc.20173. [DOI] [PubMed] [Google Scholar]
- 28.Smith RL, Carter DR, Schurman DJ. Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Relat Res. 2004:S89–95. [PubMed] [Google Scholar]
- 29.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 30.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Kandel RA. Chondrocytes attach to hyaline or calcified cartilage and bone. Osteoarthritis Cartilage. 2004;12:56–64. doi: 10.1016/j.joca.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 33.Milano G, Sanna Passino E, Deriu L, Careddu G, Manunta L, Manunta A, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18:971–80. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Anitua E, Sanchez M, Orive G, Andia I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28:4551–60. doi: 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Azofra SM, Anitua E, Andía I, Padilla S, Mújica I. Use of autologous plasma rich in growth factors in the treatment of a large, non-traumatic avulsion of articular cartilage a case report. Med Sci Sport Exer. 2002 doi: 10.1249/01.MSS.0000089344.44434.50. [DOI] [PubMed] [Google Scholar]
- 36.Mizuta H, Kudo S, Nakamura E, Otsuka Y, Takagi K, Hiraki Y. Active proliferation of mesenchymal cells prior to the chondrogenic repair response in rabbit full-thickness defects of articular cartilage. Osteoarthritis Cartilage. 2004;12:586–96. doi: 10.1016/j.joca.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Freyria AM, Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43:259–65. doi: 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307–14. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65:1951–7. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 40.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]


