Abstract
Leukemia is known as the world’s fifth most prevalent cancer. New cytotoxic drugs have created considerable progress in the treatment, but side effects are still the important cause of mortality. Plant derivatives have been recently considered as important sources for the treatment of various diseases, including cancer. Gallic acid (GA) is a polyhydroxyphenolic compound with a wide range of biological functions. The aim of the present study was to evaluate the effect of GA on proliferation inhibition and apoptosis induction of a lymphoblastic leukemia cell line. Jurkat cell (C121) line was cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) with different concentrations of GA (10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 μM) for 24, 48 and 72 hours. The effect of GA on cell viability was measured using MTS assay. Induction of apoptosis was evaluated with Annexin V-FITC/PI kit and flow cytometry. Data were analyzed by SPSS version 20 using Kruskal-Wallis and Dunn’s multiple comparison tests. Decline of cell viability to less than 50% was observed at 60.3±1.6, 50.9±1.5, and 30.9±2.8 μM concentration after 24, 48, and 72 hours incubation, respectively. All concentrations of GA (10, 30, 50 and 80 μM) enhanced apoptosis compared to the control (P<0.05). The results demonstrate that the polyphenolic compound, GA, is effective in inhibition of proliferation and induction of apoptosis in Jurkat cell line. It is recommended to study the mechanism of apoptosis induction in future investigations.
Keywords: Gallic acid, Apoptosis, Jurkat cells, Precursor cell lymphoblastic leukemia-lymphoma
What’s Known
Gallic acid (GA) and its analogs reportedly have many biological activities, including antioxidant, antimutagenic, and anticarcinogenic.
Major interest in GA and its analogs is related to its antitumor activity.
GA-induced apoptosis is associated with oxidative stress.
Anticancer activity of GA has been reported in some cancer cell lines such as prostate, lung, gastric, colon, breast, cervical, and esophageal cancer.
What’s New
GA can inhibit the proliferation and induction of apoptosis in a lymphoblastic leukemia Jurkat cell line.
Effect of GA on the Jurkat cell line is dose- and time-dependent.
IC50 of GA on Jurkat cells was 50.9 ± 1.5 after 48 hours’ incubation time, which is slightly different from other cell lines.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a neoplastic disturbance of the lymphoblast devoted to the T-cell line.1 Acute lymphoblastic leukemia (ALL) is the most common malignancy of infantile. The majority (about 80%) of infantile leukemia cases is ALL and it is usually more prevalent in males than females.2
Plants are an important source of natural products and have a wide variety of structural and biological attributes. They have played a significant role in traditional medicine of various countries.Plant derivatives have been recently considered as important sources for the treatment of various diseases, including cancer.3 Induction of apoptosis in cells is one of the targets of anti-cancer therapy.4
Gallic acid (GA) is a polyhydroxyphenolic compound (figure 1) which is widely distributed in natural plants, fruits, and food as well as having a wide range of biological functions.5 Many foods such as gallnuts, sumac, grape, green tea, oak bark, strawberry, lemon, banana, pineapple, witch hazel, and apple peel are known to be rich in GA.6 This polyhydroxyphenolic compound has been seen in human blood plasma after intake of GA-rich food.GA and its analogs have been reported to have many biological activities, including antioxidant, anti-tumor, anti-mutagenic, and anti-carcinogenic.6 However, the main interest in gallic acid and its analogs is related to its antitumor activity. It has been shown that propyl, lauryl, methyl gallate induces apoptosis and inhibits proliferation in tumor cell lines.7 GA-induced apoptosis is associated with oxidative stress.6 However, GA showed no cytotoxicity against endothelial cells and usual fibroblast.8 Some studies have shown that GA causes DNA fragmentation7 and is also responsible for the suppression of tumor angiogenesis, leading to inhibition of tumor metastasis.9 In fact, anti-cancer activity of GA has been reported in various cancer cells, such as prostate, lung, gastric, colon, breast, cervical and esophageal cancer.10
Figure 1.
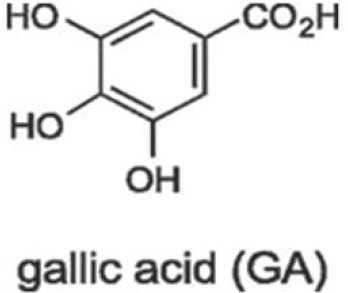
Gallic acid chemical structure.
In the absence of any reports on the effect of GA on lymphoblastic leukemia cell line, we aimed to evaluate the effect of GA on proliferation inhibition and apoptosis induction of this cell line.
Materials and Methods
Reagents
Jurkat cells were purchased from Pasteur Institute (Iran). RPMI 1640 medium, pen/strep, trypan blue, GA, and MTS were obtained from Sigma-Aldrich (Germany). Fetus bovine serum (FBS) was from GIBCO (USA) and Annexin V-FITC/PI apoptosis kit from BD (USA).
Cell Culture and Treatment
Lymphoblastic leukemia cell line (Jurkat cell (C121)) was cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/mL penicillin, 0.1 mg/mL streptomycin (PS) and 0.3 mg/ml L-glutamine. The cells were incubated at 37°C in a humidified incubator with 5% carbon dioxide. The cells were maintained at the concentration of 2×105 per ml and then were transferred to 96 and/or 6-well plates for experiments. To determine viable cells, the culture was harvested and the cells were counted by the trypan blue staining and a standard hemocytometer. Different concentrations (μM) of GA were prepared in the RPMI 1640 medium and untreated cells were considered as a control group.
Jurkat Cell Growth Curve
Jurkat cells were cultured in a 24-well plate between passages 5-7 at a concentration of 2×105 per well. The culture medium was replaced every 3 days and the cell growth rate was calculated for each triplicate wells using trypan blue and counting live cells every 24 hours for 8 days (192 h).
Assessment of Cell Viability by MTS Assay
This chromogenic assay involves biological reduction by the viable cells of tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium (MTS).
Briefly, Jurkat cells were seeded in the above complete media (at the concentration of 1×104 cells per well in 96-well plates) and allowed to grow overnight. Cells were treated with different concentrations (10-100 μM) of GA. After incubation for 48 h, 20 μl of MTS (5 mg/mL in phosphate buffered saline) was added to each well and incubated for 3 hours in the darkness. The absorbance was measured at 490-620 nm by an ELISA reader (Stat Fax 2100, Awareness).
Annexin V-FITC/PI Flow Cytometric Analysis
Apoptosis was measured using Annexin V-FITC/PI kit and flow cytometry analysis according to manufacturer’s instruction. Jurkat cells (2×105) were cultured in 6-well plates for 24 hours and treated with different concentrations of GA (10, 30, 50, and 80 μM) for 48 h. The cells were then washed twice in PBS and resuspended in 100 μl binding buffer. The cells were added to 10 μl of PI and 2 μl of Annexin V-FITC and incubated for 20 minutes in the darkness. The cells were then analyzed by flow cytometry.
Statistical Analysis
Data from at least three independent experiments are presented as the mean±SD (standard deviation) and were analyzed by SPSS version 20 using Kruskal-Wallis and Dunn’s multiple comparison tests. The difference was considered significant if P<0.05. IC50 was obtained from Probit analysis.
Results
Cell Growth Curve
The number of cells increased in the first 24 h after passage. From 24 to 120 h, Jurkat cells showed normal exponential growth and then entered stationary phase. As shown in figure 2, the peak growth rate of the cells occurred on the fifth day and they entered death phase after 168 h.
Figure 2.
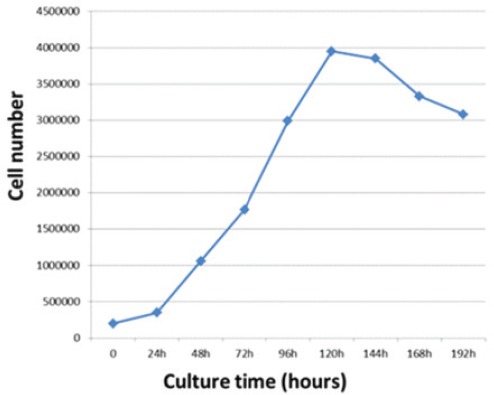
Growth curves of Jurkat cells during 8 days. The growth curve shows exponential growth phase at 24-120 h, stationary phase at 120-144 h, and death phase after 168 h. The peak growth rate of the cells is on the fifth day.
Decrease in Cell Viability by GA
The data obtained from the absorption at 490 nm was converted into percentages. The results showed a dose- and time-dependent behavior (figure 3). Cell viability was about 93%, 87%, and 65% at a concentration of 20 μM after 24, 48 and 72 hours, respectively. However, viability declined to 13%, 8%, and 5% at a concentration of 100 μM after the above incubation periods. IC50 (inhibition concentration) was 60.3±1.6 μM, 50.9±1.5 μM and 30.9±2.8 μM after a period of 24, 48 and 72 hours, respectively (figure 4).
Figure 3.
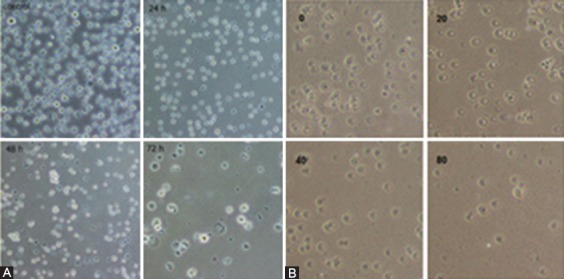
A) Decreased cell viability in the presence of 50 μM concentration of GA after 24, 48 and 72 hours (time-dependent). B) Decreased cell viability in Jurkat cells incubated with various concentrations (0, 20, 40, and 80) after 48 hours (dose-dependent) (magnification 40x).
Figure 4.
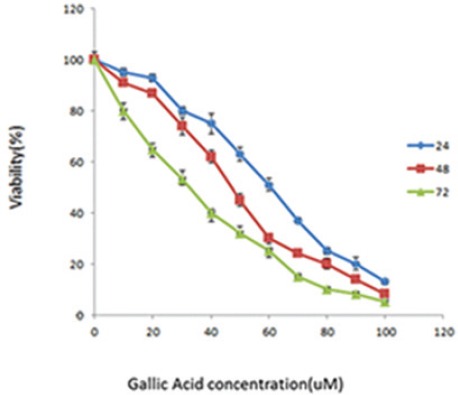
Jurkat cell viability by MTS assay. Cells were treated with different concentrations of GA; 0 (control), 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μM. Cell viability was determined by measuring the mitochondrial metabolism of MTS after 24, 48 and 72 h. IC50 was 60.3±1.6 μM in 24 h, 50.9±1.5 μM in 48 h and 30.9±2.8 μM in 72 h. Data are presented as mean±SD.
Induction of Apoptosis
Apoptosis was induced by GA as described in the “materials and methods” section. The Annexin V positive cells show the apoptosis (figure 5A) and the percentages of apoptotic cells increased markedly in GA treated cells compared with the control group (without treatment) (figure 5B). The mean of apoptosis was 25%, 35%, 50%, and 86% at the concentration of 10, 30, 50, and 80 μM, respectively, compared to 3.80% in the control group.
Figure 5.
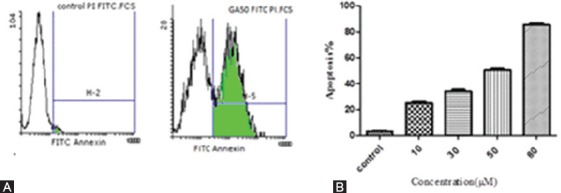
Flow cytometric analysis of cell apoptosis. Cells were cultured for 48 h and then treated with different concentrations of GA for 48 h. The cell apoptosis was detected by flow cytometry using Annexin V-FITC/PI staining. A) An example of flow cytometric analysis of cell apoptosis. Cells were treated with 50 μM of GA. The apoptosis was observed in 50.73% of treated cells compared to 3.80% in control. B) The rate of apoptosis at different concentrations of GA was compared to the control. Each data shows the mean of three independent experiments (P=0.009).
Discussion
New cytotoxic drugs have made considerable progress in the treatment but side effects such as heart and kidney problems, suppression of the immune and hematopoietic systems are still the important causes of mortality.3 Several studies have shown the anti-cancer activity of GA, inhibition of proliferation, and induction of apoptosis in some tumor cell lines.7 It has also been reported that GA induces apoptosis in cancer cells (TE2) but not in non-cancerous cells with the same origin.11 In the process of cancer development, existed mutations allow cells to dispose of apoptosis; therefore, induction of apoptosis could be an approach for prevention and treatment.12
The results of our study showed that GA in a dose- and time-dependent manner decreased cell viability and increased the induction of apoptosis in Jurkat cells. The results showed that GA caused a remarkable decrease in the cell growth in 72 hours and an IC50 of about 60 μM in 24 h, about 50 μM in 48 h and about 30 μM in 72 h. We found the concentration of drug that was associated with a high percentage of apoptosis by flow cytometry analysis caused a significant decrease in cell viability in the MTS assay.
GA inhibitory effect on cell proliferation has been studied in several cancer cell lines in various studies. Larry et al. showed that GA could induce apoptosis depending on reactive oxygen species (ROS) in prostate cancer cells. In their study, 80 μg/ml GA has induced the maximum inhibitory effect in 24 hours, which is slightly higher than our finding. The cells showed the activation of caspases 3, 8, and 9. They also found that cells could be protected against GA cytotoxicity when pretreated with increasing levels of superoxide dismutase/catalase mixture, NAC, or GSH.13 The ROS-dependent apoptotic mechanism of GA killed these malignant cells effectively.
You et al. studied the effect of GA in two types of lung cancer cells (A549 and Calu-6). The drug inhibits the growth of lung cancer cells and induces cell death, which is related to glutathione depletion as well as ROS level changes.14 As GA may affect various cells differently, the mechanism of cell death and the required dose could be varied.
Lu et al. studied the anti-proliferative, anti-invasion, and angiogenesis and induction of cell death by GA in human glioma cells. They showed that the inhibition of cell viability by GA is dose-dependent, which is consistent with our data.9
Chia et al. studied the anticancer effects of GA in oral cancer cell lines including UM1, UM2, SCC-4, and SEC-9. They showed that the required concentration and the time of exposure of GA for the induction of apoptosis are different in various cell lines. The compound caused cell death at 48 hours in SCC-4, SCC-9, and UM1 cells, but not in UM2 cells.15 This finding can be explained by the fact that different pathways of apoptosis may cooperate in different cells as it has been reported in different studies.16 It is well known that apoptosis plays an important role in responses to chemotherapy agents and can be activated through extrinsic and intrinsic pathways. It is proposed that in a type of melanoma cell line (A375.S2), GA triggers apoptosis through the regulation of FAS/FASL, Bax, BCL2, and through the activation of caspase cascade.16
You et al. studied the effect of GA in Hela cervical cancer cells. They showed that GA inhibits the growth of Hela cells and induces apoptosis and GA-induced Hela cell death is accompanied by ROS increase and glutathione depletion.6 Hwang et al. showed that GA exerts a strong antiproliferative activity against breast cancer cells.17
Our results from flow cytometric assays indicated that the apoptosis in Jurkat cells is dose-dependent and about 80% apoptosis was observed after 48 hours in the treatment with 80 μM concentration. This value is higher than that found in HL-60 promyelocytic leukemia cells.18 GA has demonstrated early apoptosis in some oral squamous carcinoma cell lines and late apoptosis in some others.15 The effect ofGA on the ratio of pro-apoptotic/anti-apoptotic molecules and signaling pathways in Jurkat cells need to be investigated. Anti-proliferative effect of GA is not just by the induction of apoptosis through the classic pathways, since some studies have reported delays in DNA repair and cell cycle arrest. Human prostate PC3 cells after exposure to GA, have demonstrated a decrease of cell viability in a time- and dose-dependent manner, more DNA damage, and also inhibition of the expression of P53 mRNA and other DNA repair genes.19 In fact, the inhibitory effect of cancer cell growth is mediated by the modulation of genes involved in cell cycle, metastasis angiogenesis, and apoptosis.20
Madlener et al. studied the effect of GA in promyelocytic leukemia cells (HL-60).18 They showed that apoptosis induced by GA is dose-dependent, which is again in agreement with our findings. However, they found 39% apoptosis in the presence of 80 μM GA, which is lower compared to our data at the same concentration. This finding can be related to different origins of the cell line. They also found dose-dependent cell cycle arrest in their study. Other studies have demonstrated the effect of GA on gastric cancer cells, leukemia cells (k562) and L1210,4 which is consistent with our study. Although our study showed that GA could decrease cell viability and induced apoptosis in Jurkat cells, which is consistent with the studies of other cell lines, the mechanism of apoptosis may be different and needs to be addressed in future studies. GA might be a potential anti-cancer compound against lymphoblastic leukemia; however, these effects on ALL patient lymphoblasts need to be investigated.
Conclusion
It seems that the decrease in Jurkat cell numbers in the presence of GA was dependent on the level of apoptosis, which is a favorable effect of anti-cancer treatments. GA could be promising in T-ALL combination chemotherapy, but it needs to be investigated in other lymphoblastic cell lines, ALL lymphoblasts, followed by in vivo experimental models.
Acknowledgement
This work was supported by the Deputy of Research and Technology of Shahrekord University of Medical Sciences. We also thank the Medical Plants Research Center of this university.
Conflict of Interest: None declared.
References
- 1.Graux C, Cools J, Michaux L, Vandenberghe P, Hagemeijer A. Cytogenetics and molecular genetics of T-cell acute lymphoblastic leukemia: from thymocyte to lymphoblast. Leukemia. 2006;20:1496–510. doi: 10.1038/sj.leu.2404302. [DOI] [PubMed] [Google Scholar]
- 2.Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012;122:3398–406. doi: 10.1172/JCI61269. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shashi B, Jaswant S, Madhusudana RJ, Kumar SA, Nabi QG. A novel lignan composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide. 2006;14:72–88. doi: 10.1016/j.niox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Denicourt C, Dowdy SF. Medicine. Targeting apoptotic pathways in cancer cells. Science. 2004;305:1411–3. doi: 10.1126/science.1102974. [DOI] [PubMed] [Google Scholar]
- 5.Abdelwahed A, Bouhlel I, Skandrani I, Valenti K, Kadri M, Guiraud P, et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem Biol Interact. 2007;165:1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.You BR, Moon HJ, Han YH, Park WH. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem Toxicol. 2010;48:1334–40. doi: 10.1016/j.fct.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Fiuza SM, Gomes C, Teixeira LJ, Girao da Cruz MT, Cordeiro MN, Milhazes N, et al. Phenolic acid derivatives with potential anticancer properties--a structure-activity relationship study. Part 1: methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg Med Chem. 2004;12:3581–9. doi: 10.1016/j.bmc.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Sohi KK, Mittal N, Hundal MK, Khanduja KL. Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: A Bcl-2 independent mechanism. J Nutr Sci Vitaminol (Tokyo) 2003;49:221–7. doi: 10.3177/jnsv.49.221. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai Y, et al. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol. 2010;641:102–7. doi: 10.1016/j.ejphar.2010.05.043. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, et al. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.). Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605–13. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CL, Lo WH, Yen GC. Gallic acid induces apoptosis in 3T3-L1 pre-adipocytes via a Fas- and mitochondrial-mediated pathway. J Agric Food Chem. 2007;55:7359–65. doi: 10.1021/jf071223c. [DOI] [PubMed] [Google Scholar]
- 12.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 13.Russell LH, Jr, Mazzio E, Badisa RB, Zhu ZP, Agharahimi M, Oriaku ET, et al. Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res. 2012;32:1595–602. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 14.You BR, Park WH. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol In Vitro. 2010;24:1356–62. doi: 10.1016/j.tiv.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Chia YC, Rajbanshi R, Calhoun C, Chiu RH. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules. 2010;15:8377–89. doi: 10.3390/molecules15118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo C, Lai TY, Yang JH, Yang JS, Ma YS, Weng SW, et al. Gallic acid induces apoptosis in A375.S2 human melanoma cells through caspase-dependent and -independent pathways. Int J Oncol. 2010;37:377–85. doi: 10.3892/ijo_00000686. [DOI] [PubMed] [Google Scholar]
- 17.Hwang EY, Huh JW, Choi MM, Choi SY, Hong HN, Cho SW. Inhibitory effects of gallic acid and quercetin on UDP-glucose dehydrogenase activity. FEBS Lett. 2008;582:3793–7. doi: 10.1016/j.febslet.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Madlener S, Illmer C, Horvath Z, Saiko P, Losert A, Herbacek I, et al. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007;245:156–62. doi: 10.1016/j.canlet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Liu KC, Ho HC, Huang AC, Ji BC, Lin HY, Chueh FS, et al. Gallic acid provokes DNA damage and suppresses DNA repair gene expression in human prostate cancer PC-3 cells. Environ Toxicol. 2013;28:579–87. doi: 10.1002/tox.20752. [DOI] [PubMed] [Google Scholar]
- 20.Verma S, Singh A, Mishra A. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35:473–85. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]


