Abstract
Aims
The globalization of clinical trials has highlighted geographic variations in patient characteristics, event rates, and treatment effects. We investigated these further in PARADIGM-HF, the largest and most globally representative trial in heart failure (HF) to date.
Methods and results
We looked at five regions: North America (NA) 602 (8%), Western Europe (WE) 1680 (20%), Central/Eastern Europe/Russia (CEER) 2762 (33%), Latin America (LA) 1433 (17%), and Asia-Pacific (AP) 1487 (18%). Notable differences included: WE patients (mean age 68 years) and NA (65 years) were older than AP (58 years) and LA (63 years) and had more coronary disease; NA and CEER patients had the worst signs, symptoms, and functional status. North American patients were the most likely to have a defibrillating-device (54 vs. 2% AP) and least likely prescribed a mineralocorticoid receptor antagonist (36 vs. 65% LA). Other evidence-based therapies were used most frequently in NA and WE. Rates of the primary composite outcome of cardiovascular (CV) death or HF hospitalization (per 100 patient-years) varied among regions: NA 13.6 (95% CI 11.7–15.7) WE 9.6 (8.6–10.6), CEER 12.3 (11.4–13.2), LA 11.2 (10.0–12.5), and AP 12.5 (11.3–13.8). After adjustment for prognostic variables, relative to NA, the risk of CV death was higher in LA and AP and the risk of HF hospitalization lower in WE. The benefit of sacubitril/valsartan was consistent across regions.
Conclusion
There were many regional differences in PARADIGM-HF, including in age, symptoms, comorbidity, background therapy, and event-rates, although these did not modify the benefit of sacubitril/valsartan.
Clinical trial registration URL
http://www.clinicaltrials.gov. Unique identifier: NCT01035255.
Keywords: Heart failure, Treatment outcome, Geographical variation, Clinical trial, Prognosis
Introduction
The declining risk of adverse outcomes in patients with heart failure (HF) and reduced ejection fraction (HF-REF), resulting from the cumulative benefit of treatments over time, has meant that contemporary randomized controlled clinical trials require greater numbers of participants and longer follow-up to accrue the number of events needed to test the effect of new therapies.1 In order to recruit a sufficient number of patients in a timely manner, and to improve generalizability of results, these trials now include participants from many different regions of the world.2 On the other hand, geographical differences in demographics, race, ethnicity, other patient characteristics, aetiology of HF, co-morbidity, health care systems, physician-practice, and especially background therapy can raise questions about the applicability of the results of the trial in certain regions of the world.2–14 In particular, there has been controversy about the effect of certain treatments in patients from the USA compared with those from the rest of the world.3,15–17
The prospective comparison of Angiotensin Receptor-neprilysin inhibitor with Angiotensin-converting–enzyme inhibitor to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) is the largest trial in patients with HF and HF-REF to date and it is the most globally representative, with ∼8400 patients enrolled in 47 countries on six continents.18 Our aim was to evaluate geographical differences in patient characteristics (age, sex, background pharmacotherapy, and comorbidity) and event rates. We also wanted to examine the effect of sacubitril/valsartan (formerly known as LCZ696) according to geographical region. Our hypothesis was that patient characteristics and outcomes would vary by geographic region in PARADIGM-HF but that the effect of sacubitril/valsartan, compared with enalapril, would not.
Methods
The design, baseline characteristics, and results of PARADIGM-HF have been published.18–20 The Ethics Committee of each of the 1043 participating institutions (in 47 countries) approved the protocol, and all patients gave written, informed consent.
Study patients
Briefly, patients had New York Heart Association (NYHA) classes II–IV symptoms, a left ventricular ejection fraction (LVEF) ≤40% and modestly elevated plasma B-type natriuretic peptides. Patients were required to be taking an angiotensin converting enzyme inhibitor or angiotensin receptor blocker in a dose equivalent to enalapril 10 mg daily for at least 4 weeks before screening, along with a stable dose of a β-blocker (unless contraindicated or not tolerated) and a mineralocorticoid antagonist (MRA), if indicated. Key exclusion criteria included a systolic blood pressure (SBP) <95 mmHg, a serum potassium >5.4 mmol/L, or an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 at randomization.
Trial outcomes
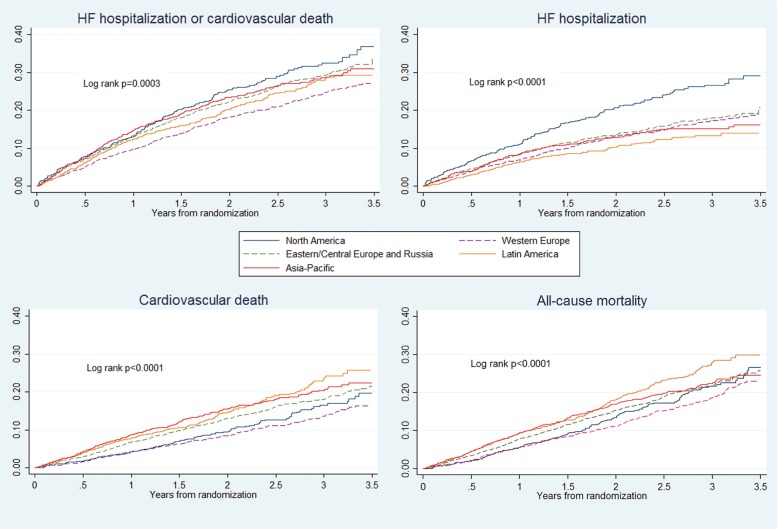
The primary outcome was a composite of death from CV causes or a first hospitalization for HF. The secondary outcomes included death from any cause and change from baseline to 8 months in the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS). In the present study, we examined the primary composite outcome and its components, as well as all-cause death. We further determined the proportion of patients experiencing a five or greater points reduction in their KCCQ-CSS as this is considered to be a clinically important deterioration in health-related quality of life (HRQL).21 Safety outcomes included hypotension, elevation of serum creatinine, hyperkalaemia, cough, and angioedema, as previously reported.18 We also reported total number of adverse, and serious adverse events according to geographic region. Furthermore, we assessed length of stay for all-cause hospitalizations during the study, and total days alive and out of hospital in the first year (Figure 1).
Figure 1.
Kaplan–Meier plots of endpoints according to region.
Geographic regions
The following geographic regions were examined: North America (NA), Western Europe (WE), Central/Eastern Europe/Russia (CEER), Latin America (LA), and the Asia-Pacific (AP) region. The countries in each of these regions are listed in Appendix.
Statistical analysis
Data are reported as mean ± SD or median and interquartile ranges and as frequencies and percentages for categorical variables. Student's t-tests were used to compare baseline variables between regions. Cox proportional hazard models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the time-to-event endpoints, and logistic regression analysis for the binomial endpoint of ≥5 point decline in KCCQ-CSS at 8 months follow-up. In analyses of treatment effect, the primary variable of interest was the interaction P-value for randomized treatment × geographic region. For analyses comparing risk according to region, we did unadjusted analyses and analyses including multivariable adjustment for treatment effect and other variables known to be predictors of risk in patients with HF-REF (see Supplementary material online, List). A two-tailed P-value of <0.05 was considered significant. Statistical analyses were performed using STATA, College Station, Texas, version 14.0.
Results
Of 8399 patients in PARADIGM-HF, 602 (7%) were randomized in NA, 1680 (20%) in WE, 2762 (33%) in CEER, 1433 (17%) in LA, and 1487 (18%) in the AP region.
Baseline characteristics
The majority of characteristics differed across regions (Table 1 and see Supplementary material online, Tables A and B).
Table 1.
Baseline characteristics according to region
| North America | Western Europe | Central/Eastern Europe/Russia | Latin America | Asia-Pacific | P-value | |
|---|---|---|---|---|---|---|
| Number of patients | 602 (7%) | 1680 (20%) | 2762 (33%) | 1433 (17%) | 1487 (18%) | |
| Age (years) | 65.1 ± 11.4 | 68.3 ± 9.9 | 65.1 ± 10.0 | 63.0 ± 11.6 | 57.8 ± 11.9 | <0.0001 |
| Female sex, n (%) | 104 (17%) | 297 (18%) | 641 (23%) | 391 (27%) | 292 (20%) | <0.0001 |
| Caucasian, n (%) | 466 (77%) | 1645 (98%) | 2712 (98%) | 485 (34%) | 11 (1%) | <0.0001 |
| BMI (kg/m2) | 31 ± 7 | 29 ± 5 | 30 ± 5 | 27 ± 5 | 24 ± 4 | <0.0001 |
| Heart rate (b.p.m.) | 70 ± 11 | 69 ± 11 | 74 ± 12 | 71 ± 11 | 75 ± 11 | <0.0001 |
| Syst. BP (mmHg) | 118 ± 15 | 121 ± 16 | 126 ± 14 | 118 ± 14 | 117 ± 15 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 59 (48, 71) | 61 (50, 74) | 67 (55, 80) | 67 (55, 80) | 72 (59, 85) | <0.0001 |
| LVEF | 0.27 ± 0.07 | 0.30 ± 0.06 | 0.32 ± 0.05 | 0.28 ± 0.06 | 0.28 ± 0.06 | <0.0001 |
| NYHA class, n (%) | <0.0001 | |||||
| I | 23 (4%) | 58 (4%) | 33 (1%) | 107 (7%) | 119 (8%) | |
| II | 456 (76%) | 1292 (77%) | 1512 (55%) | 1168 (82%) | 1180 (79%) | |
| III | 117 (19%) | 319 (19%) | 1169 (42%) | 156 (11%) | 184 (13%) | |
| IV | 4 (1%) | 7 (0%) | 45 (2%) | 0 (0%) | 3 (0%) | |
| KCCQ score | 71 ± 21 | 73 ± 20 | 68 ± 19 | 79 ± 18 | 79 ± 16 | <0.0001 |
| NT-proBNP pg/mLa (IQR) | 1582 (897, 3080) | 1517 (857, 2888) | 1599 (877, 3134) | 1760 (917, 3645) | 1714 (911, 3677) | 0.0003 |
| Medical history, n (%) | ||||||
| Ischaemic aetiology | 381 (63%) | 980 (58%) | 1942 (70%) | 617 (43%) | 863 (58%) | <0.0001 |
| HF hospitalization | 394 (65%) | 948 (56%) | 1961 (71%) | 773 (54%) | 894 (60%) | <0.0001 |
| Hypertension | 503 (84%) | 1050 (63%) | 2395 (87%) | 975 (68%) | 719 (48%) | <0.0001 |
| Atrial fibrillation | 239 (40%) | 744 (44%) | 1428 (52%) | 340 (24%) | 249 (17%) | <0.0001 |
| Diabetes | 293 (49%) | 597 (36%) | 936 (34%) | 391 (27%) | 515 (35%) | <0.0001 |
| CKD (eGFR < 60b) | 311 (52%) | 759 (45%) | 950 (34%) | 494 (35%) | 397 (27%) | <0.0001 |
aNT-proBNP data are from the screening visit. Information on KCCQ score was only available for 7623 (92%) patients.
bmL/min/1.73 m2.
Age, sex, and race/ethnicity
Patients in WE, who were the most elderly (with a mean age 68.3 years), were an average of 10.5 years older than those enrolled in AP. The proportion of women was highest in LA (27%) and lowest in NA (17%). The proportion of patients who were black was highest in NA (19%).
Social physiological measures
Body mass index varied by region, being highest in NA (31 Kg/m2) and lowest in AP (24 kg/m2) (Table 1). Heart rate in patients without atrial fibrillation (AF) was lowest in NA (70 b.p.m.) and WE (68 b.p.m.) and highest in AP (75 b.p.m.), consistent with the geographical variation in β-blocker use but inversely related to digoxin use (see below). Baseline SBP was highest in CEER (mean 126 mmHg) compared with 117 mmHg in AP and 118 mmHg in NA and LA, consistent with geographical variation in hypertension history (see below).
Coronary heart disease
An ischaemic aetiology was most common in CEER (70%) and NA (63%) and least common in LA (43%). Consistent with this, history of myocardial infarction was most frequent in CEER (50%) and NA (55%) and least frequent in LA (32%) and AP (34%). The proportions of patients with any manifestation of coronary disease and prior coronary revascularization varied in a similar way (Table 1).
Other co-morbidity
Co-morbidity was generally most common in NA (except a history of hypertension and AF which was highest in CEER). Co-morbidity was less common in LA and AP. The proportion of patients with chronic kidney disease (CKD) was lowest in AP (27%) and mean eGFR highest in that region (72 mL/min/1.73 m2); mean eGFR was lowest in NA (59 mL/min/1.73 m2), where there was also the greatest proportion with CKD (52%).
Drug, device, and other treatments
Although β-blocker use was mandated by protocol (unless contraindicated/not tolerated), there was still variability with the highest rate in NA (97%) and lowest in AP (89%) (Table 2). There was more striking variation in the use of mineralocorticoid receptor antagonists—prescribed least commonly in NA (36%) and most frequently in LA (65%). Digoxin use also varied greatly in patients with no history of AF—from 6% in WE to 42% in AP. There was less variation in digoxin use among patients with a history of AF (range 33% WE to 55% AP).
Table 2.
Pharmacological and device treatment
| North America | Western Europe | Central/Eastern Europe/Russia | Latin America | Asia-Pacific | P-value | |
|---|---|---|---|---|---|---|
| Medication, n (%) | ||||||
| Loop-diuretic | 488 (81%) | 1362 (81%) | 2292 (83%) | 1116 (78%) | 1091 (73%) | <0.0001 |
| β-Blocker | 586 (97%) | 1570 (94%) | 2612 (95%) | 1322 (92%) | 1320 (89%) | <0.0001 |
| MRA | 217 (36%) | 742 (44%) | 1695 (61%) | 924 (65%) | 830 (56%) | <0.0001 |
| Digoxin | ||||||
| All | 163 (27%) | 307 (18%) | 805 (29%) | 499 (35%) | 660 (44%) | <0.0001 |
| History of AF | 96 (40%) | 248 (33%) | 647 (45%) | 174 (51%) | 136 (55%) | <0.0001 |
| No history of AF | 67 (19%) | 58 (6%) | 155 (12%) | 325 (30%) | 523 (42%) | <0.0001 |
| Oral anticoagulants | ||||||
| All | 199 (33%) | 792 (47%) | 1181 (43%) | 280 (20%) | 143 (10%) | <0.0001 |
| History of AF | 149 (62%) | 606 (82%) | 964 (68%) | 185 (54%) | 87 (35%) | <0.0001 |
| No history of AF | 50 (14%) | 186 (20%) | 217 (16%) | 95 (9%) | 56 (5%) | <0.0001 |
| Devices | ||||||
| Any CRT | 130 (22%) | 207 (12%) | 113 (4%) | 29 (2%) | 42 (3%) | <0.0001 |
| ICD or CRT-D | 327 (54%) | 559 (33%) | 193 (7%) | 61 (4%) | 26 (2%) | 0.0033 |
MRA, mineralocorticoid receptor antagonist; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; AF, atrial fibrillation.
The use of non-HF medications also varied geographically. The highest rates of prescription of oral anticoagulants in patients with an AF history were in Europe (WE 82%, CEER 68%) and NA (63%) and lowest in AP (35%).
Device therapy varied substantially by region, being highest in NA and lowest in CEER, LA, and AP. For example, a defibrillating-device (ICD or CRT-D) had been implanted in 54% in NA but in only 7, 4, and 2% of patients in CEER, LA, and AP, respectively. Similarly, other guideline-recommended interventions varied by region. Influenza vaccination in the past year was most frequently reported in NA (53%) and WE (53%) but much less commonly elsewhere. A quarter to a third of patients in NA and WE were enrolled in a structured disease management programme but few patients were enrolled elsewhere. Prescription of an exercise regimen was provided in between a fifth and a quarter of patients in NA, WE, and CEER but was uncommon elsewhere. These non-drug/device interventions were rarely used in AP.
Heart failure outcomes
Cardiovascular death or heart failure hospitalization
The unadjusted rate of the primary endpoint varied less between regions than the rate of HF hospitalization, with the following ranking from lowest to highest: WE (9.6 per 100 patient-years), LA, CEER, AP, and NA (13.6 per 100 patient-years). Using NA as the reference region, and after adjustment, WE exhibited a lower risk (adjusted HR 0.85. 95% CI 0.70, 1.02) but AP a higher risk (adjusted HR 0.85. 95% CI 0.70, 1.02). A supplementary analysis including South Africa, Israel, and Turkey in alternative geographical regions gave similar results (see Supplementary material online, Table D). We also compared Eastern/Central Europe and Russia (see Supplementary material online, Table E); the crude rates of the primary endpoint were similar but mortality rates were slightly higher and hospitalization rates lower, in Russia.
Mortality
The unadjusted rate of death from any cause varied modestly among regions with the following ranking from lowest to highest: WE (6.7 per 100 patient-years), NA, CEER, AP, and LA (10.1 per 100 patient-years) (Table 3). Using NA as the reference and adjusting for differences between regions in prognostic variables, patients in LA (adjusted HR 1.67, 95% CI 1.28, 2.19) and AP (HR 1.40, 95% CI 1.03, 1.91) exhibited a higher risk of death. The findings for CV death were similar.
Table 3.
Event rate (per 100 patient-years) and risk of study endpoints according to region (North America reference region)
| North America | Western Europe | Central/Eastern Europe and Russia | Latin America | Asia-Pacific | |
|---|---|---|---|---|---|
| No. of patients | 602 | 1680 | 2762 | 1433 | 1487 |
| HF hospitalization or cardiovascular death | 180 | 358 | 694 | 314 | 369 |
| Event rates per 100 patient-years (95% CI) | 13.6 (11.7–15.7) | 9.6 (8.6–10.6) | 12.3 (11.4–13.2) | 11.2 (10.0–12.5) | 12.5 (11.3–13.8) |
| Unadjusted HR | 1.00 (ref.) | 0.70 (0.59–0.84) | 0.89 (0.76–1.05) | 0.81 (0.67–0.97) | 0.90 (0.76–1.08) |
| Adjusteda HR | 1.00 (ref.) | 0.85 (0.70–1.02) | 1.09 (0.90–1.32) | 1.17 (0.93–1.49) | 1.39 (1.06–1.80) |
| HF hospitalization | 142 | 244 | 402 | 148 | 192 |
| Event rates per 100 patient-years (95% CI) | 10.7 (9.1–12.6) | 6.5 (5.7–7.4) | 7.1 (6.5–7.8) | 5.3 (4.5–6.2) | 6.5 (5.6–7.5) |
| Unadjusted HR | 1.00 (ref.) | 0.61 (0.49–0.75) | 0.65 (0.54–0.79) | 0.47 (0.38–0.60) | 0.59 (0.47–0.73) |
| Adjusteda HR | 1.00 (ref.) | 0.79 (0.63–0.98) | 0.94 (0.75–1.17) | 0.80 (0.59–1.08) | 0.99 (0.71–1.39) |
| Cardiovascular death | 86 | 192 | 419 | 240 | 252 |
| Event rates per 100 patient-years (95% CI) | 5.7 (4.6–7.1) | 4.8 (4.1–5.5) | 6.8 (6.2–7.5) | 8.2 (7.2–9.3) | 8.0 (7.1–9.1) |
| Unadjusted HR | 1.00 (ref.) | 0.84 (0.65–1.08) | 1.20 (0.95–1.51) | 1.44 (1.12–1.84) | 1.40 (1.10–1.79) |
| Adjusteda HR | 1.00 (ref.) | 0.95 (0.73–1.23) | 1.30 (0.99–1.68) | 1.84 (1.34–2.51) | 1.81 (1.28–2.55) |
| All-cause mortality (no. of events) | 119 | 271 | 510 | 295 | 279 |
| Event rates per 100 patient-years (95% CI) | 7.9 (6.6–9.5) | 6.7 (6.0–7.6) | 8.3 (7.6–9.1) | 10.1 (9.0–11.3) | 8.9 (7.9–10.0) |
| Unadjusted HR | 1.00 (ref.) | 0.85 (0.69–1.06) | 1.06 (0.87–1.29) | 1.29 (1.04–1.60) | 1.14 (0.91–1.40) |
| Adjusteda HR | 1.00 (ref.) | 0.94 (0.75–1.17) | 1.14 (0.91–1.42) | 1.67 (1.28–2.19) | 1.40 (1.03–1.91) |
| Significant worsening in KCCQ clinical score (≥5) at 8 monthsb | 205 (34%) | 564 (34%) | 870 (32%) | 341 (24%) | 264 (18%) |
| Unadjusted OR | 1.00 (ref.) | 0.98 (0.80–1.19) | 0.89 (0.74–1.07) | 0.60 (0.49–0.74) | 0.42 (0.34–0.52) |
| Adjusteda OR | 1.00 (ref.) | 1.06 (0.86–1.30) | 0.95 (0.77–1.17) | 0.83 (0.64–1.07) | 0.94 (0.70–1.25) |
Information on KCCQ score was only available for 7623 (92%) patients.
ICD, ischaemic aetiology and history of myocardial infarction, diabetes, AF, and stroke.
aModel adjusted for age, sex, treatment arm, race, HF duration, heart rate, SBP, body mass index, NYHA class, ejection fraction, KCCQ score, and glomerular filtration rate.
bScores on the Kansas City Cardiomyopathy Questionnaire (KCCQ) range from 0 to 100 (higher scores indicating fewer symptoms).
Hospitalization
The unadjusted rate of HF hospitalization varied more among regions with the following ranking from lowest to highest: LA (5.3 per 100 patient-years), AP, WE, CEER, and NA (10.7 per 100 patient-years). Using NA as the reference, all other regions showed a significantly lower unadjusted risk of hospitalization. After adjustment, this difference persisted only in WE (adjusted 0.79, 95% CI 0.63, 0.98). Length of stay for hospitalization was shortest in NA and WE and longest in CEER (see Supplementary material online, Table F). Days alive and out of hospital in first year of study, varied between 345 and 351 days, with highest number in NA.
Health-related quality of life
The proportion of patients exhibiting a decrease of ≥5 points in KCCQ-CSS at 8 months was largest in NA, WE, and CEER (where around a third of patients deteriorated) and smaller in LA (24%) and AP (18%). The odds of deteriorating were significantly less in these latter regions in unadjusted analyses, but not after multivariable adjustment.
Consistency of effect of sacubitril/valsartan on clinical outcomes by region
As shown in Table 4, the effect of sacubitril/valsartan was consistent across regions for the primary composite endpoint, its components and for all-cause mortality. The same was true for the effect of sacubitril/valsartan on deterioration in KCCQ.
Table 4.
Treatment effect according to region (sacubitril/valsartan vs enalapril hazard ratio and 95% confidence interval)
| Overall HR (95% CI) | North America HR (95% CI) | Western Europe HR (95% CI) | Central/Eastern Europe and Russia HR (95% CI) | Latin America HR (95% CI) | Asia-Pacific HR (95% CI) | P for interaction | |
|---|---|---|---|---|---|---|---|
| HF hospitalization or cardiovascular death | 0.80 (0.73–0.87) | 0.67 (0.50–0.90) | 0.88 (0.71–1.08) | 0.78 (0.67–0.91) | 0.71 (0.56–0.88) | 0.85 (0.69–1.04) | 0.4954 |
| HF hospitalization | 0.79 (0.71–0.89) | 0.70 (0.50–0.97) | 0.89 (0.69–1.15) | 0.80 (0.66–0.98) | 0.69 (0.50–0.96) | 0.85 (0.64–1.12) | 0.6855 |
| Cardiovascular death | 0.80 (0.71–0.89) | 0.76 (0.49–1.16) | 0.84 (0.63–1.11) | 0.80 (0.66–0.97) | 0.71 (0.55–0.92) | 0.79 (0.62–1.01) | 0.9315 |
| All-cause mortality | 0.84 (0.76–0.93) | 0.82 (0.57–1.18) | 0.99 (0.78–1.26) | 0.80 (0.67–0.95) | 0.70 (0.63–1.00) | 0.81 (0.64–1.03) | 0.6310 |
| Significant worsening in KCCQ clinical score (≥5) at 8 monthsa | 0.83 (0.75–0.92)b | 0.86 (0.61–1.21)b | 0.85 (0.69–1.04)b | 0.83 (0.70–0.97)b | 0.82 (0.64–1.05) | 0.79 (0.61–1.03)b | 0.708 |
Information on KCCQ score was only available for 7623 (92%) patients.
aScores on the Kansas City Cardiomyopathy Questionnaire (KCCQ) range from 0 to 100 (higher scores indicating fewer symptoms).
bEffect of sacubitril/valsartan on worsening KCCQ clinical score (≥5) at 8 months was estimated by logistic regression, and is shown as odds ratios (ORs). Information on KCCQ score was only available for 7623 (92%) patients.
Study drug tolerability
Adverse events varied considerably by region (see Supplementary material online, Table C). In the enalapril group, hypotension and renal impairment were most common in NA, hyperkalaemia was most common in CEER and cough most common in AP. The differences between sacubitril/valsartan and enalapril (more hypotension and less renal impairment and hyperkalaemia with the former) were consistent across regions. Study drug discontinuation for reasons other than death varied significantly by geographical region, from 32% in NA to 10% in AP.
Discussion
Although there are a few analyses of geographic variation among patients with acute HF, there is only one report from a trial, the Assessment of Treatment with Lisinopril and Survival study (ATLAS), in ambulatory patients with chronic HF-REF, published in 1998.3,7,8,10,11 No acute or chronic HF trial has been as globally diverse as PARADIGM-HF which included patients from geographical areas under-represented in prior studies including LA and, especially, AP. More data were collected on baseline characteristics and treatment in PARADIGM-HF than in prior trials, including information on non-pharmacolgical/non-device recommendations, e.g. enrolment in disease management programmes.
Baseline characteristics
We found some inevitable differences between regions (e.g. in race and ethnicity), some possibly related to genetic background (e.g. low prevalence of AF in AP22). It is harder to explain other differences. Compared with NA and WE, where clinical trials were initially conducted, the average patient age was lower (especially in AP) and the proportion of women larger (especially in LA). Ischaemic aetiology was least frequent in LA, which was also reported in two acute HF trials, presumably reflecting different causes of HF on that continent, including Chagas cardiomyopathy.7,8
Regarding HF status, we found that the greatest differences were between CEER and the rest of the World. Specifically, CEER patients had worse symptoms and more severe functional limitation as evaluated by the investigators (NYHA class) and reported by patients (KCCQ-CSS). Consistent with this, signs (dyspnoea, oedema, and rales) and prior HF hospitalization were more common. However, patients from CEER had the highest average LVEF and lowest NT-proBNP and, overall, examination of regional patterns showed no clear relationship between objective measurements of cardiac dysfunction and symptom frequency and physical limitation.
As observed previously, the rate of prior coronary intervention was highest in NA,7,8,11 followed closely by WE; patients in CEER had less prior coronary intervention but the rates in this region were still considerably higher than in LA and AP. Despite similar recommendations regarding ICD and CRT use in all major guidelines, an even more extreme pattern of disparity of use was seen, with a 30-fold difference in use of a defibrillating-device between NA and AP.7,8,11 Of all regional variation, use of procedures, devices, and surgery showed the greatest differences, probably, in part, reflecting economic differences.
Variation in HF medication use was less pronounced but was still substantial, at least for MRAs. Prescription of MRAs showed exactly the opposite pattern to use of devices i.e. was least common in NA and WE and most frequent in the other regions with lower device use. Patients in NA and WE had the lowest eGFR (and highest proportion of patients with CKD) which may have led to reluctance to prescribe, although the entry eGFR in PARADIGM-HF would not have precluded safe use of a MRA, and other explanations may exist.23 Among patients without a history of AF, there was also an almost seven-fold difference in digoxin prescription, with the highest use in AP (42%) and lowest use in WE (6%), illustrating the dramatic decline in digitalis glycoside use in certain regions of the world (in ATLAS 80% of sinus rhythm patients in NA and 57% in continental Europe were treated with digoxin).3
Of non-HF drugs, the greatest variation was in use of oral anticoagulants which were much less frequently prescribed in AP, where under-treatment of AF patients with these agents has been highlighted recently.24
The use of other guideline recommendations, including influenza vaccination, exercise prescription, and enrolment in disease management programmes, varied greatly between regions. For example, influenza vaccination was used in around half of patients in NA and WE but rarely outside these regions. Similar patterns were observed for the other non-drug/non-device recommendations.
Collectively, our data show the use of guideline-recommended therapies is highest in NA and WE, with less application in CEER and LA and least use in AP. The one striking exception was use of MRAs, as described above.
Clinical outcomes
Despite the considerable heterogeneity in baseline characteristics and treatment, the crude rate of all-cause mortality varied modestly and was only clearly higher in one region—LA—compared with the others. However, after adjustment the risk of all-cause and cardiovascular death was found to be higher in LA and in AP, compared with NA. Both of the former regions also had the highest average NT-proBNP levels and lower LVEF (mean 28%) compared with the others (except NA which had the lowest LVEF–mean 27%). However, the higher risk of death persisted (or even increased) after adjusting for these and additional differences in known prognostic variables suggesting other biological factors, social, and environmental influences or aspects of health care provision in these regions contribute to the observed differences in survival. There may also be geographical differences in the treatment of comorbidities such as coronary artery disease, CKD, and AF which might also affect outcome.
Efficacy of Vasopressin Antagonism in hEart failuRE: Outcome Study With Tolvaptan (EVEREST), the only other large trial reporting long-term outcomes according to region (in patients with acute HF), observed similar crude mortality rates in NA, LA, and WE (no patients from AP were included).7 The adjusted risk of death was highest in LA. The Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT), another acute HF trial, is the only study to have reported outcomes in AP as well as the Americas and Europe.11 In that study, all-cause mortality at 12 months was highest in AP (and high in LA). Interestingly, however, these other two studies reported quite different findings about EE with the lowest risk of death in that region in EVEREST but the second highest (after AP) in ASTRONAUT. There has been recent controversy about a low event rate in CEER17 and it is interesting that both PARADIGM-HF and ASTRONAUT did not observe low event rates in this region. Notably, unlike EVEREST, both PARADIGM-HF and ASTRONAUT required elevated natriuretic peptides for inclusion, perhaps helping ensure correct diagnosis and selection of patients at risk of events.
Greater variation in HF hospitalization rates, compared with mortality, might have been predicted, given the likely differences in decision making about admission, community programmes to prevent hospitalization, thresholds for admission and even provision and availability of hospital beds between regions. However, again, only one region was clearly different from the others and that was NA, which had almost twice the crude rate of HF hospitalization compared with LA, although the differences between regions were greatly attenuated by adjustment, with only WE having a significantly lower risk. There was also no convincing evidence of ‘competing risk’ influencing our findings, i.e. regions with higher mortality did not consistently have lower admission rates. In ASTRONAUT, NA patients also had a much higher unadjusted HF hospitalization rate than elsewhere, although in EVEREST the crude re-admission rates did not vary greatly between regions.7,11
Health-related quality of life
We are not aware of prior reports of geographic variation in patient-reported outcomes. A clinically important deterioration in KCCQ-CSS after 8 months was significantly less likely in LA and AP (in unadjusted analyses) than elsewhere and it is notable that these two regions had the highest baseline KCCQ-CSS (i.e. the best HRQL). This finding illustrates the clear discrepancy between patient-reported outcomes and clinical events, given that these two regions had the highest all-cause and cardiovascular mortality rates.
Treatment effect
Despite the differences highlighted, we did not identify any modification of the effect of sacubitril/valsartan by geographical region—the benefit over enalapril was consistent for all outcomes across all regions, contrasting with controversy about consistency of benefit of β-blockers in HF-REF and spironolactone in HF with preserved ejection fraction (HF-PEF).15–17
Implications
Globalization of clinical trials is a reality that has highlighted regional disparities of many types.2,9,13 Some are demographic and biologically determined and unavoidable whereas others reflect variation in clinical practice and economic influences. Despite this, we found that the key outcomes constituting trial endpoints varied less than has been suggested previously. This may reflect more standardized inclusion and exclusion criteria in HF-REF trials compared with trials in HF-PEF and acute HF, and, in PARADIGM-HF in particular, the use of a natriuretic peptide inclusion criterion. The latter may be an even more important consideration in trials in patients with (HF-PEF).12 Although we found no variation in treatment effect by region, inevitably questions will also remain about the applicability of the findings of trials enrolling a large proportion of patients from regions where certain evidence-based therapies are substantially underused (e.g. MRAs in NA and ICDs in the rest of the world).
Limitations
Our study has a number of limitations. Although PARADIGM-HF is the largest HF trial to date, it is a single study. The analyses conducted were retrospective and PARADIGM-HF was not designed to evaluate geographical differences in HF. Because our analyses involved comparisons of many different variables across several regions, statistically significant differences may have arisen through the play of chance. Although we performed multivariable adjustments, we may not have captured the effect of all predictors influencing the outcomes studied. While we examined recognized geographical regions, differences may also exist between countries within these regions and these may be more marked in some regions than others, e.g. AP compared with NA. The number of patients recruited in NA was limited (n = 622), although analyses showed similar benefit for these patients compared with other regions. The low number of black patients (n = 6) in WE probably reflects selection bias in trial enrolment. As with any trial, the generalizability of the findings to ‘real-world’ patients may be limited.
Conclusion
There were many regional differences in PARADIGM-HF, including in age, symptoms, comorbidity, background therapy, and event-rates, although these did not modify the benefit of sacubitril/valsartan.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
S.L.K., P.J., J.J.V.M. performed statistical analysis. J.J.V.M., M.P., M.P.L., A.R. handled funding and supervision. J.J.V.M., M.P., M.P.L., A.R.R., J.L.R., V.C.S., S.D.S., K.S., M.R.Z. acquired the data. S.L.K., P.J., J.J.V.M., M.P. conceived and designed the research. S.L.K., J.J.V.M. drafted the manuscript. F.M., J.L.A., J.B., S.B., W.C., E.G. A.A.H., J.H., S.K., K.-S.K., I.M., M.S., I.B.S.D.V., R.C.-C.W., J.G., M.P.L., A.R.R., J.L.R., V.C.S., S.D.S., K.S., M.R.Z. made critical revision of the manuscript for key intellectual content.
Funding
The study was funded by Novartis. S.L.K. is supported by a Postdoctoral grant from the Danish Independent Research Council, and a Research Fellowship from the Heart Failure Association of the European Society of Cardiology (ESC).
Conflict of interest: All authors except S.L.K. have consulted for or received research support from Novartis, sponsor of the PARADIGM-HF trial. M.P. has consulted for Novartis, Cytokinetics, Cardiokinetix, BioControl, Janssen, Amgen, CardioMEMS, and Cardiorentis. Prof McMurray's employer, University of Glasgow, was paid by Novartis for Prof McMurray's time spent as cochairman of the PARADIGM-HF trial. Dr Desai consulted for Novartis, Relypsa, and St. Jude Medical. M.P.L. and V.C.S. are employees of Novartis. K.S. and F.M. is on the speaker's bureau of Novartis. I.B.S. received honoraria from Novartis for participating in various activities.
Supplementary Material
References
- 1. Rush CJ, Campbell RT, Jhund PS, Connolly EC, Preiss D, Gardner RS, Petrie MC, McMurray JJ. Falling cardiovascular mortality in heart failure with reduced ejection fraction and implications for clinical trials. JACC Heart Fail 2015;3:603–614. [DOI] [PubMed] [Google Scholar]
- 2. O'Connor CM. The globalization of heart failure research. JACC Heart Fail 2015;3:657–658. [DOI] [PubMed] [Google Scholar]
- 3. Wedel H, Demets D, Deedwania P, Fagerberg B, Goldstein S, Gottlieb S, Hjalmarson A, Kjekshus J, Waagstein F, Wikstrand J, MERIT-HF Study Group. Challenges of subgroup analyses in multinational clinical trials: experiences from the MERIT-HF trial. Am Heart J 2001;142:502–511. [DOI] [PubMed] [Google Scholar]
- 4. Massie BM, Cleland JG, Armstrong PW, Horowitz JD, Packer M, Poole-Wilson PA, Ryden L. Regional differences in the characteristics and treatment of patients participating in an international heart failure trial. The Assessment of Treatment with Lisinopril and Survival (ATLAS) Trial Investigators. J Card Fail 1998;4:3–8. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG, Cohen-Solal A, Aguilar JC, Dietz R, Eastaugh J, Follath F, Freemantle N, Gavazzi A, van Gilst WH, Hobbs FD, Korewicki J, Madeira HC, Preda I, Swedberg K, Widimsky J, IMPROVEMENT of Heart Failure Programme Committees and Investigators. Improvement programme in evaluation and management; Study Group on Diagnosis of the Working Group on Heart Failure of The European Society of Cardiology. Management of heart failure in primary care (the IMPROVEMENT of Heart Failure Programme): an international survey. Lancet 2002;360:1631–1639. [DOI] [PubMed] [Google Scholar]
- 6. Sturm HB, van Gilst WH, Veeger N, Haaijer-Ruskamp FM. Prescribing for chronic heart failure in Europe: does the country make the difference? A European survey. Pharmacoepidemiol Drug Saf 2007;16:96–103. [DOI] [PubMed] [Google Scholar]
- 7. Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M, EVEREST Investigators. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol 2008;52:1640–1648. [DOI] [PubMed] [Google Scholar]
- 8. Howlett JG, Ezekowitz JA, Podder M, Hernandez AF, Diaz R, Dickstein K, Dunlap ME, Corbalán R, Armstrong PW, Starling RC, O'Connor CM, Califf RM, Fonarow GC, ASCEND-HF Investigators. Global variation in quality of care among patients hospitalized with acute heart failure in an international trial: findings from the acute study clinical effectiveness of nesiritide in decompensated heart failure trial (ASCEND-HF). Circ Cardiovasc Qual Outcomes 2013;6:534–542. [DOI] [PubMed] [Google Scholar]
- 9. Pitt B, Gheorghiade M. Geographic variation in heart failure trials: time for scepticism? Eur J Heart Fail 2014;16:601–602. [DOI] [PubMed] [Google Scholar]
- 10. Mentz RJ, Cotter G, Cleland JG, Stevens SR, Chiswell K, Davison BA, Teerlink JR, Metra M, Voors AA, Grinfeld L, Ruda M, Mareev V, Lotan C, Bloomfield DM, Fiuzat M, Givertz MM, Ponikowski P, Massie BM, O'Connor CM. International differences in clinical characteristics, management, and outcomes in acute heart failure patients: better short-term outcomes in patients enrolled in Eastern Europe and Russia in the PROTECT trial. Eur J Heart Fail 2014;16:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greene SJ, Fonarow GC, Solomon SD, Subacius H, Maggioni AP, Böhm M, Lewis EF, Zannad F, Gheorghiade M, ASTRONAUT Investigators and Coordinators. Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail 2015;17:591–600. [DOI] [PubMed] [Google Scholar]
- 12. Kristensen SL, Køber L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, Zile MR, Granger CB, Wikstrand J, Komajda M, Carson PE, Pfeffer MA, Swedberg K, Wedel H, Yusuf S, McMurray JJ. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015;131:43–53. [DOI] [PubMed] [Google Scholar]
- 13. Ferreira JP, Girerd N, Rossignol P, Zannad F. Geographic differences in heart failure trials. Eur J Heart Fail 2015;9:893–905. [DOI] [PubMed] [Google Scholar]
- 14. Poole-Wilson PA. Global differences in the outcome of heart failure: implications for clinical practice. J Am Coll Cardiol 2008;52:1649–1651. [DOI] [PubMed] [Google Scholar]
- 15. O'Connor CM, Fiuzat M, Swedberg K, Caron M, Koch B, Carson PE, Gattis-Stough W, Davis GW, Bristow MR. Influence of global region on outcomes in heart failure β-blocker trials. J Am Coll Cardiol 2011;58:915–922. [DOI] [PubMed] [Google Scholar]
- 16. Chatterjee S, Udell JA, Sardar P, Lichstein E, Ryan JJ. Comparable benefit of β-blocker therapy in heart failure across regions of the world: meta-analysis of randomized clinical trials. Can J Cardiol 2014;30:898–903. [DOI] [PubMed] [Google Scholar]
- 17. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013;15:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Committees Investigators. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail 2014;16:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular Outcomes Research Consortium. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 22. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 23. Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, Pitt B, EMPHASIS-HF Investigators. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization and SurvIval Study in Heart Failure). J Am Coll Cardiol 2013;62:1585–1593. [DOI] [PubMed] [Google Scholar]
- 24. Chen CH, Chen MC, Gibbs H, Kwon SU, Lo S, On YK, Rosman A, Suwanwela NC, Tan RS, Tirador LS, Zirlik A. Antithrombotic treatment for stroke prevention in atrial fibrillation: The Asian agenda. Int J Cardiol 2015;191:244–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.