This study provides strong, novel evidence that humans may introduce methicillin-resistant Staphylococcus aureus CC398 into closed pig populations; it also demonstrates that stringent control and eradication measures were effective and prevented dissemination from pig farms to the general human population.
Keywords: LA-MRSA, humans, pigs, epidemiology, control
Abstract
Background. Emerging livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) persist in livestock populations and represent a reservoir for transmission to humans. Understanding the routes of introduction and further transmission is crucial to control this threat to human health.
Methods. All reported cases of livestock-associated MRSA (CC398) in humans and pigs in Norway between 2008 and 2014 were included. Data were collected during an extensive outbreak investigation, including contact tracing and stringent surveillance. Whole-genome sequencing of isolates from all human cases and pig farms was performed to support and expand the epidemiological findings. The national strategy furthermore included a “search-and-destroy” policy at the pig farm level.
Results. Three outbreak clusters were identified, including 26 pig farms, 2 slaughterhouses, and 36 humans. Primary introductions likely occurred by human transmission to 3 sow farms with secondary transmission to other pig farms, mainly through animal trade and to a lesser extent via humans or livestock trucks. All MRSA CC398 isolated from humans without an epidemiological link to the outbreaks were genetically distinct from isolates within the outbreak clusters indicating limited dissemination to the general population.
Conclusions. This study identified preventable routes of MRSA CC398 introduction and transmission: human occupational exposure, trade of pigs and livestock transport vehicles. These findings are essential for keeping pig populations MRSA free and, from a “One Health” perspective, preventing pig farms from becoming reservoirs for MRSA transmission to humans.
Staphylococcus aureus is one of the main causes of nosocomial and community-acquired infections, and methicillin-resistant S. aureus (MRSA) infections are associated with increased morbidity and mortality rates and costs [1, 2]. For a long time, MRSA was almost exclusively a healthcare-associated problem, but the epidemiology has changed significantly since the late 1990s with dissemination of community-associated MRSA, and with further changes since the mid-2000s caused by emerging MRSA strains with a primary reservoir in livestock [3, 4].
Livestock-associated MRSA (LA-MRSA) has now spread worldwide, especially in pig farms where it is transmitted to humans mainly by occupational exposure [5–7]. In countries with a low overall prevalence of MRSA in humans, such as Denmark and the Netherlands, LA-MRSA has greatly affected the notification rate of MRSA in humans and is increasingly found in persons without livestock contact [8–10].
LA-MRSA in pig holdings in Europe most commonly belong to the clonal complex (CC) 398, but the prevalence varies greatly among European countries, with up to 70% of all farms positive in Denmark and the Netherlands [8, 11]. In contrast, several surveillance programs conducted in Norway, including the 2008 European Union baseline study and 2 more recent nationwide population surveillance programs, found either an absence or a very low prevalence of LA-MRSA in pigs [12–14]. Trade in pigs has been identified as the major risk factor for interfarm transmission of LA-MRSA [15, 16], including transboundary transmission [17]. In the period from 2000 to 2015, <80 live pigs were imported into the Norwegian commercial pig population, most of these in 2 separate imports of 49 and 20 breeding animals from Finland and the Netherlands respectively [18]. In the latter import, the animals were tested and confirmed to be negative for MRSA. Thus, the Norwegian pig population is de facto a “closed” production system.
The objective of the present study was to describe the first known introductions and transmission of MRSA CC398 in pig herds and the subsequent spread to humans in Norway. As a “One Health” initiative, the study included all identified cases of MRSA CC398 in humans and pigs in Norway from 2008 to 2014.
MATERIALS AND METHODS
MRSA Investigations in Pigs
MRSA in the Norwegian pig population was first investigated in an European Union baseline study in 2008, which did not detect LA-MRSA [12]. In 2011 and 2012, anonymized prevalence studies demonstrated MRSA CC398 in a few samples from a single slaughterhouse and a pig herd [13, 14]. In early 2013, two independent identifiable findings of MRSA CC398 in the Norwegian pig population initiated a public health risk assessment concerning the possible impacts of an increasing prevalence of LA-MRSA in pigs. This prompted an investigation to identify and control the transmission of MRSA CC398 to pig farms and humans. Norwegian authorities implemented a strategy including a farm-level “search-and-destroy” policy to prevent the establishment of LA-MRSA in the Norwegian pig population. In 2014, a nationwide surveillance program of all sow farms (n = 986) was initiated to investigate the prevalence of MRSA in the pig population [19].
The outbreak-related investigation collected epidemiological data from farmers by questionnaires (Supplementary, Table 1) and included both human and animal contact tracing. Demographic information, farm characteristics, husbandry and production details were collected. In total, 74 pig farms and 5 slaughterhouses were included and sampled in the outbreak investigation during 2013 and 2014 (Supplementary Appendix 1).
MRSA Investigations in Humans
Human MRSA infections have been notifiable to the Norwegian surveillance system for communicable diseases (MSIS) since 1995 and MRSA carriage has been notifiable since 2005 [20]. Humans are investigated for MRSA based on clinical signs of infection, admission screening in healthcare facilities, contact tracing and outbreak investigations [21]. All human MRSA CC398 cases reported to MSIS were included.
Epidemiological data on all persons occupationally exposed to pigs in the current outbreaks were collected (Supplementary, Table 2). Household members were sampled if they were patients in healthcare institutions, worked as healthcare personnel, or if a farm or abattoir worker was found to be MRSA positive. MRSA screening samples from humans were collected from the vestibulum nasi, throat, and skin lesions (if present). In total, 272 persons were included.
Bacteriological Analyses
All samples from animals and environment were investigated for MRSA using the protocol described by the European Food Safety Authority [22]. Human MRSA samples taken as part of the outbreak investigation were analyzed at 7 medical microbiological laboratories using slightly different methods (Supplementary Appendix 2).
The national reference laboratory for MRSA confirmed presumptive MRSA isolates from human, animal and environmental samples with polymerase chain reaction detection of the mecA, spa, and Panton-Valentine leukocidin genes, using polymerase chain reaction protocols described elsewhere [23, 24]; spa typing was performed on all isolates (http://www.spaserver.ridom.de/). Multilocus sequence typing was performed on new spa types as described by Enright et al [25]. Whole-genome sequencing (WGS) was performed, along with detection of resistance and virulence markers and phylogenetic analysis of MRSA CC398 from all pig farms, all human cases reported in Norway, and selected MRSA CC398 isolates from other countries (Supplementary Appendix 3).
Statistical Analyses
The data were collected with the objective of prevention and control of transmission of MRSA and not as a part of a planned scientific study. Stata software (version 13; StataCorp) was used to calculate attack rates and odds ratios (ORs) of MRSA among persons distributed by occupational exposure and pig farms distributed by type of pig production.
RESULTS
Overview of MRSA CC398 in Norway
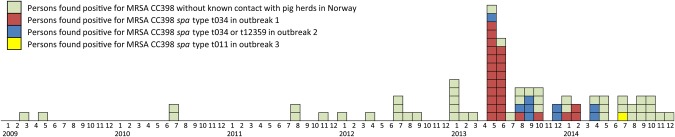
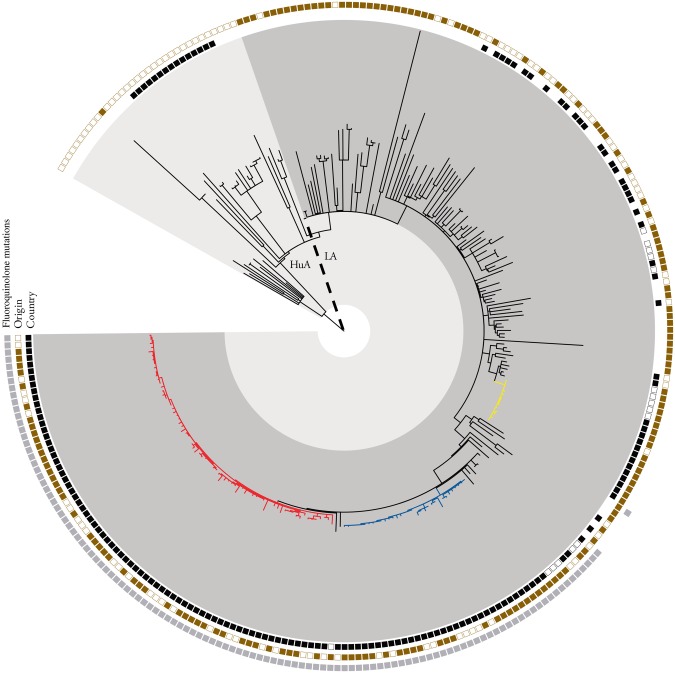
The first human case of MRSA CC398 was notified in March 2009, and by the end of 2014, a total of 84 human cases had been reported, including human cases identified through outbreak investigations (Figure 1). The first traceable finding of LA-MRSA in pigs occurred in February 2013, and by the end of 2014, outbreak investigations and surveillance had identified MRSA CC398 in 26 pig farms (Table 1), 2 slaughterhouses and 36 humans (Table 2). Epidemiological data placed these farms and persons in 3 clusters located in, or originating from, central eastern (outbreaks 1 and 3) and southwestern (outbreak 2) Norway (Figure 2). MRSA isolates from animals, environment and humans in these 3 clusters belonged to the following CC398-associated spa types: t034 in outbreak 1, t034 and t12359 in outbreak 2, and t011 in outbreak 3. The findings were further supported by WGS-based phylogenetic analysis (Figure 3 and Supplementary Appendix 4). MRSA CC398 detected in samples from a slaughterhouse in the anonymized survey of 2011 (NORM-VET 2011) was shown by WGS to be related to isolates in outbreak 1 (Figure 3). Most pig farms in outbreak 1 regularly supplied this slaughterhouse.
Figure 1.
Reported cases of human methicillin-resistant Staphylococcus aureus (MRSA) involving CC398 in Norway, from the first case in March 2009 through December 2014.
Table 1.
Pig Farms Sampled and Methicillin-Resistant Staphylococcus aureus Analysis Results by Farm Type and Outbreak
| Farm Type | Pig Farms MRSA Positive, No./Pig Farms Sampled, No. (AR, %)a |
OR (95% CI) | |||
|---|---|---|---|---|---|
| Outbreak 1 | Outbreak 2 | Outbreak 3 | Total | ||
| Sow | 3/7 (42.9) | 3/16 (18.8) | 1/1 (100.0) | 7/24 (28.0) | Reference |
| Finishing pig | 9/19 (47.4) | 8/28 (28.6) | 2/3 (66.7) | 18/49 (36.7) | 1.26 (.50–3.57) |
| Total | 12/26 (46.2) | 11/44 (25.0) | 3/4 (75.0) | 26/74 (35.1) | … |
Abbreviations: AR, attack rate; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio.
a One finishing pig farm sampled and found positive was included in both outbreaks 2 and 3.
Table 2.
Case Tracing in Human Cases, Distributed by Type of Known Exposure to Methicillin-Resistant Staphylococcus aureus
| Exposure | Persons MRSA Positive, No./Persons Sampled, No. (AR, %) |
OR (95% CI) | |||
|---|---|---|---|---|---|
| Outbreak 1 | Outbreak 2 | Outbreak 3 | Total | ||
| Working in sow pig farm | 10/19 (52.6) | 3/39 (7.7) | 1/4 (25.0) | 14/62 (22.6) | Reference |
| Working in finishing pig farm | 5/29 (17.2) | 4/34 (11.8) | … | 9/63 (14.3) | 0.63 (.24–1.57) |
| Veterinary practitioner | 2/11 (18.2) | 1/4 (25.0) | … | 3/15 (20.0) | 0.89 (.16–3.17) |
| Working in slaughterhouse | 9/107 (8.4) | 1/17 (5.9) | … | 10/124 (8.06) | 0.36 (.14–.86) |
| Household members | 0/5 (0.0) | 0/3 (0.0) | … | 0/8 (0.0) | … |
| Total | 26/171 (15.2) | 9/97 (9.3) | 1/4 (25.0) | 36/272 (13.2) | … |
Abbreviations: AR, attack rate; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio.
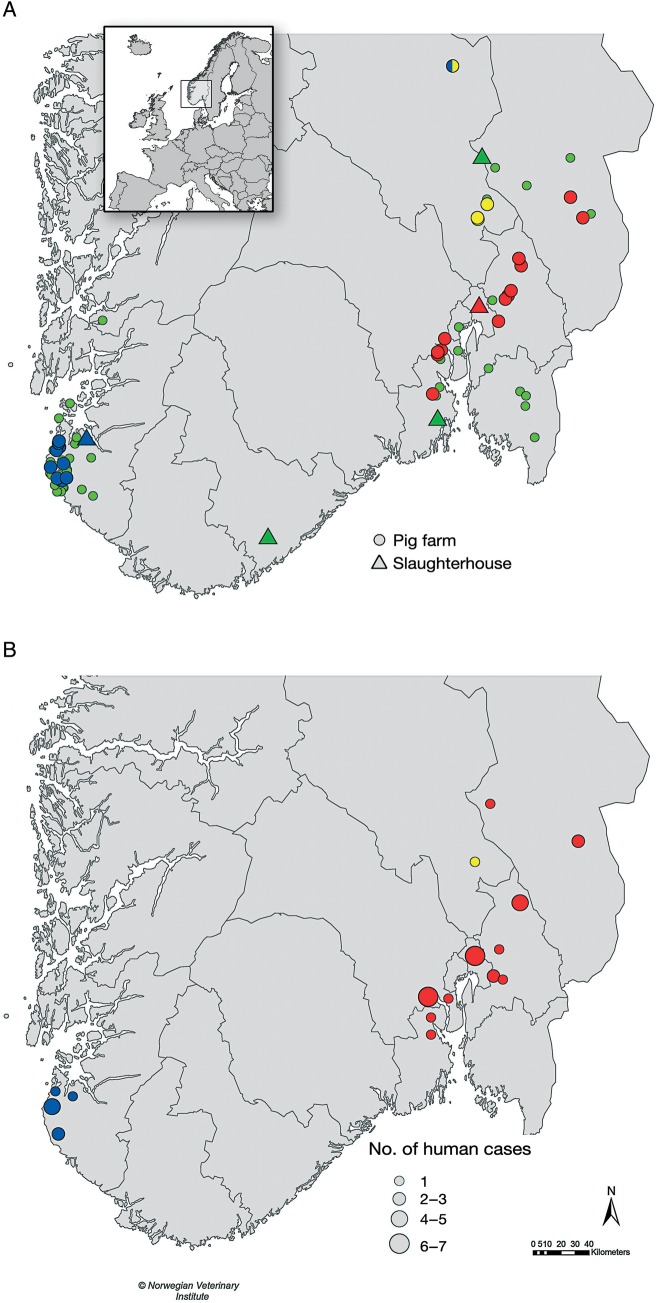
Figure 2.
Geographic distribution of methicillin-resistant Staphylococcus aureus (MRSA) CC398–positive farms (circles) and slaughterhouses (triangles) (A) and MRSA CC398–positive farm or slaughterhouse workers (B) in outbreaks 1 (red), 2 (blue), and 3 (yellow). In A, MRSA CC398–negative farms and slaughterhouses are shown in green. Insert in A depicts Norway in Europe, with box highlighting focus area in A and B.
Figure 3.
Phylogenetic analysis for understanding diversity and spread of methicillin-resistant Staphylococcus aureus (MRSA) CC398 isolates in Norway. The phylogenetic relationship was inferred using maximum likelihood based on 4854 single-nucleotide polymorphisms in 271 isolates. Human-adapted (HuA) and livestock-associated (LA) clades are highlighted. Identified outbreaks in relation to Norwegian livestock are highlighted, including outbreaks 1 (red), 2 (blue), and 3 (yellow). Genotypic and epidemiological data are represented, encircling the topology. Inner circle represents Norwegian isolates (filled squares), Danish pig production isolates (open squares), and other isolates (blank [no squares]). Middle circle represents sample environment with livestock, meat, and environmental samples (filled squares) and human isolates (open squares). Outer circle depicts occurrence of specific fluoroquinolone-associated resistance mutations in gyrA (Ser84Leu) and parC (Ser80Tyr).
A single pig isolate from the 2012 survey and 48 human isolates not epidemiologically linked to the 3 outbreaks described were all genetically distinct from the isolates in the outbreak clades (Figure 3 and Supplementary Appendix 4). Based on information reported to MSIS, 25 (52%) of the human cases not linked to the outbreak clusters had probably acquired MRSA CC398 abroad.
Introductions of MRSA CC398 to the Pig Population
The index cases in the 3 outbreaks were identified through samples collected from a postmortem examination of a fattening pig in February 2013 (outbreak 1), clinical infection in a farm worker in June 2013 (outbreak 2) and in a national surveillance program of sow farms in June 2014 (outbreak 3). Contact tracing identified 2 primary case sow farms having supplied the index case farms in outbreaks 1 and 2. The index case farm in outbreak 3 was considered the primary case farm. All primary case farms had farm workers and/or consultants originating from other European countries. The use of foreign labor was common; 24 of 62 (39%) of the sow farm workers and 4 of 63 (6%) of the finishing pig farm workers were of non-Norwegian nationality. The majority of foreign workers (25 of 28) were from Eastern Europe, and the remaining 3 were from Denmark (n = 2) or the Netherlands (n = 1). None of the farms investigated had imported pigs from abroad. WGS data from both human and pig isolates in outbreaks 1 and 2 demonstrated a close genetic relationship with isolates identified in Denmark, whereas isolates from outbreak 3 showed genetic relatedness to MRSA CC398 t011 strains from several European countries, including Denmark (Figure 3).
Further Transmission
The trade of pigs was identified as the main route of MRSA CC398 transmission from the 3 primary case farms. This was considered the most likely route of transmission to 19 farms. In 3 farms, the most probable explanation for transmission was through the mutual use of farm workers or veterinary practitioners.
One farm had 2 separate introductions of MRSA CC398 (t034 and t011), based on epidemiological information supported by WGS data, and was involved in both outbreaks 2 and 3. The trade of pigs or contact through personnel was excluded as the route of reintroduction to this farm. A livestock transport vehicle had twice transported pigs from a MRSA CC398–positive finishing farm to a slaughterhouse without subsequent disinfection shortly before transporting pigs to the farm involved and was considered the most likely transmission route. Pigs from MRSA CC398–positive farms were slaughtered at 5 slaughterhouses in southern Norway, and MRSA was detected in samples from pigs, personnel, or the environment in 2 of these (Figure 2).
In total, 48 of 74 farms sampled during outbreak investigations were identified as MRSA negative. Twelve farms were sampled because they had supplied pigs to MRSA CC398–positive farms, and 4 farms had contact through MRSA CC398–positive veterinary practitioners. Of the 51 farms that had received pigs from MRSA CC398–positive farms, 32 were MRSA negative. Of these 32 farms, 14 had received pigs from farms in which MRSA CC398 had most likely only been recently introduced, 12 had been only sporadically supplied, and 6 had changed suppliers and had washed and disinfected the premises before the change of supplying herd.
Of the 36 human cases included in the outbreaks, 33 were detected through contact tracing. Three were identified through notification to MSIS, and subsequently linked to the outbreaks by epidemiological data, supported by WGS results (Figure 3 and Supplementary Appendix 4). All 36 persons had direct and regular contact with positive pigs (Table 2). No differences in the MRSA prevalence between different types of occupational exposure were observed.
DISCUSSION
The present study encompasses all identified cases of MRSA CC398 in humans and pigs in Norway, between 2008 and 2014. All the traceable detections of MRSA CC398 in pig farms and slaughterhouses were clustered in 3 separate outbreaks. Furthermore, 43% (36 of 84) of all human MRSA CC398 cases in the period were related to these outbreaks. The study strongly suggests that the outbreaks were caused by human introduction of MRSA. Phylogenetic analysis revealed that the introduced MRSA strains were closely related to strains isolated in other European countries. The isolates from the primary case farms in outbreaks 1 and 2 showed close genetic relatedness to MRSA CC398 isolates from Denmark, and persons linked to the 2 farms had known contact with pig farms in Denmark. Furthermore, the primary case farm in outbreak 3 involved farm workers from abroad, although without confirmed livestock contact outside Norway.
To our knowledge, the present study is the first to describe the importance of the human introduction of MRSA CC398 to livestock populations. Because there is virtually no import of live pigs to Norway, human transmission of LA-MRSA should be regarded as the most important route of introduction into the Norwegian pig population. Our findings are therefore highly relevant for the future prevention of LA-MRSA introduction to pig populations, at both national and farm levels.
Based on other studies, the trade of pigs has been shown to be the predominant route of transmission of MRSA CC398 among pigs [15, 16], including transboundary transmission [17]. Domestic trade in pigs was found to be the main route of interfarm transmission of MRSA after primary introductions, indicating that limiting the number of farms connected through trade is important in preventing MRSA transmission. In addition, we found humans, and in one case a livestock truck, to be the most likely explanation for MRSA transmission to farms not connected through the trade of pigs. These transmission routes may further constitute routes of dissemination to other segments of the animal population.
Our results show that 32 of 51 pig farms that had purchased pigs from MRSA-positive suppliers were found to be MRSA negative at the time of sampling. This may be because the supplying farms were not MRSA positive when the animals were delivered or because management practices and hygiene routines prevented MRSA from becoming established in the recipient farms, the latter explanation being the most likely in at least 6 farms. This indicates that changing to a supplier with a MRSA-negative herd (all in–all out) combined with good routines for washing and disinfecting facilities may be effective measures to prevent MRSA establishment on finishing pig farms. These findings are supported by results from the Norwegian control strategy for LA-MRSA in the pig population and may also be relevant for pig farms in other countries [26].
Other studies have identified direct contact with positive animals as a major risk factor for MRSA CC398 in humans, and to a lesser extent indirect contact [5, 27, 28]. In addition, an increased incidence rate of MRSA CC398 in the general public without contact with pig farms has been described from areas with a high density of pig herds [8, 9, 29]. In the present study, we did not observe the transmission of MRSA CC398 from the outbreaks to the general public. This may be partly explained by the relatively short exposure times, because all pigs on MRSA CC398–positive farms were slaughtered, and the holding facilities thoroughly washed and disinfected.
Public health surveillance data from Norway show that more than one-third of all reported human MRSA cases have acquired MRSA abroad [12, 30]. An increased prevalence of MRSA on Norwegian pig farms could change this epidemiological situation by constituting a new domestic reservoir for MRSA, leading to an increase of the total public health burden of MRSA. Such a development has been described in Denmark, where the rapid spread of MRSA CC398 in the pig population has led this to be the dominant clone found in humans [8]. The rapid increase of MRSA CC398 in humans in other low-endemic countries, along with results from the present study, highlights the importance of control measures to prevent the introduction and further transmission of MRSA CC398 in pig populations. The present Norwegian control strategy includes targeted screening of personnel before working in pig herds, annual national surveillance of the pig population ,and contact tracing with eradication measures, resembling a search-and-destroy strategy. The preliminary results of testing in herds after the implementation of MRSA eradication measures show that this strategy has largely been effective [26].
Some of the data described herein were collected to control outbreaks and, although extensive, were not fully comprehensive. Only household contacts of occupationally exposed MRSA CC398–positive humans were screened, so bias may have been introduced regarding the detection of further spread. The WGS analysis was compared to available sequences primarily from Denmark; thus, the relatedness to isolates from other countries was explored to a lesser extent. The major strengths of the study are the extensive outbreak investigations and the active surveillance programs in the pig population, together with mandatory notification of all human MRSA diagnoses, giving a near-complete description of MRSA CC398 in Norway.
In conclusion, this study confirms that the trade of pigs and occupational exposure are the major risk factors for transmission of MRSA CC398 between humans and pigs. However, the primary introductions leading to the 3 outbreak clusters cannot be explained by the trade of animals. In these cases, both the epidemiological and the WGS data indicate that these introductions were the result of human-to-animal transmission. In addition, further transmission probably occurred via humans and livestock transport vehicles to farms not connected to MRSA CC398–positive farms through the trade of live animals. These findings have important implications for risk management to prevent the dissemination of MRSA CC398 among farms. In Norway, we believe that the prevention of human introduction of LA-MRSA is of the utmost importance if the current ambitious strategy to control LA-MRSA is to prove feasible and successful in the longer term.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We acknowledge the dedicated efforts of Bjørn Lium, DVM, Dr Sci, in the outbreak investigation and Berit Tafjord Heier, DVM, PhD, for preparing the maps. We thank the Norwegian Food Safety Authority, Public Health Officers, the Norwegian Pig Health Service, and the persons involved from the pig production sector for their contributions and cooperation in the outbreak investigations. The following laboratories provided samples and data: the Norwegian Veterinary Institute (Oslo), St Olav Hospital (Trondheim, Norway), Stavanger (Norway) University Hospital, Fürst Medical Laboratory (Oslo, Norway), Vestre Viken Hospital Trust (Drammen, Norway), Sørlandet Hospital HF (Kristiansand, Norway), Akershus University Hospital (Lørenskog, Norway), Vestfold Hospital Trust (Tønsberg, Norway), Sykehuset Innlandet Trust (Lillehammer, Norway), and Statens Serum Institute (Copenhagen, Denmark).
Author contributions. P. E., A. M. U., S. A., S. M. L., M. Sunde, and J. V. B. performed the outbreak investigations; C. A. G., P. E., K. W. L., A. M. U., O. A., J. L., S. A., S. M. L., M. Sunde, and J. V. B. collected the data; C. A. G., P. E., M. Stegger, R. L. S., P. S. A., K. W. L., A. M. U., O. A., J. L., M. Sunde, and J. V. B. analyzed and interpreted the data; C. A. G., P. E., and M. Stegger prepared the tables and figures; C. A. G. and P. E. contributed equally, with the primary responsibility of writing and revising the manuscript; M. Sunde and J. V. B. contributed equally; and all authors contributed to revising the manuscript and approved the final version.
Disclaimer. The funders of the study had no role in study design, data collection, data analysis and interpretation, or writing of the report. The corresponding author (C. A. G.) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Norwegian Food Safety Authority, the Norwegian Veterinary Institute, the Norwegian Institute of Public Health, Statens Serum Institut, and St. Olavs Hospital. J. L., M. Stegger, P. S. A., and R. L. S. report grants from the National Institute of Allergy and Infectious Diseases (Grant/Award Number 1R01AI101371-01A1), outside the submitted work.
Potential conflicts of interest. M. Stegger has a patent (US9,279,160) with royalties. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003; 36:53–9. [DOI] [PubMed] [Google Scholar]
- 2.Koeck R, Becker K, Cookson B et al. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 2010; 15:12–20. [DOI] [PubMed] [Google Scholar]
- 3.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009; 7:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis 2005; 11:1965–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graveland H, Duim B, Van Duijkeren E, Heederik D, Wagenaar JA. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int J Med Microbiol 2011; 301:630–4. [DOI] [PubMed] [Google Scholar]
- 6.Koeck R, Schaumburg F, Mellmann A et al. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS One 2013; 8:e55040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verkade E, Kluytmans J. Livestock-associated Staphylococcus aureus CC398: animal reservoirs and human infections. Infect Gen Evol 2014; 21:523–30. [DOI] [PubMed] [Google Scholar]
- 8.DANMAP 2014. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600-2032. Available at: http://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202014/Danmap_2014.ashx. Accessed 30 November 2015.
- 9.Larsen J, Petersen A, Sorum M et al. Meticillin-resistant Staphylococcus aureus CC398 is an increasing cause of disease in people with no livestock contact in Denmark, 1999 to 2011. Euro Surveill 2015; 20:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Cleef BA, Monnet DL, Voss A et al. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg Infect Dis 2011; 17:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broens EM, Graat EAM, Van Der Wolf PJ, Van De Giessen AW, De Jong MCM. Prevalence and risk factor analysis of livestock associated MRSA-positive pig herds in The Netherlands. Prev Vet Med 2011; 102:41–9. [DOI] [PubMed] [Google Scholar]
- 12.European Food Safety Authority. Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008 [1]. Part A: MRSA prevalence estimates. EFSA J; 2009; 7:1376 [82 pp.]. [Google Scholar]
- 13.NORM/NORM-VET 2011. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo: 2012. ISSN 1502-2307.Available at: http://wwweng.vetinst.no/eng/Publications/NORM-NORM-VET-Report/NORM-NORM-VET-2011.html. Accessed 5 April 2016. [Google Scholar]
- 14.NORM/NORM-VET 2012. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo: 2013. ISSN 1502-2307. Available at: http://wwweng.vetinst.no/eng/Publications/NORM-NORM-VET-Report/NORM-NORM-VET-2012.html. Accessed 5 April 2016. [Google Scholar]
- 15.Broens EM, Graat EAM, van der Wolf PJ et al. MRSA CC398 in the pig production chain. Prev Vet Med 2011; 98:182–9. [DOI] [PubMed] [Google Scholar]
- 16.Espinosa-Gongora C, Broens EM, Moodley A, Nielsen JP, Guardabassi L. Transmission of MRSA CC398 strains between pig farms related by trade of animals. Vet Rec 2012; 170:564. [DOI] [PubMed] [Google Scholar]
- 17.European Food Safety Authority. Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008. Part B: factors associated with MRSA contamination of holdings. EFSA J; 2010; 8:1597 [67 pp.]. [Google Scholar]
- 18.KOORIMP. Annual report 2014 (in Norwegian). KOORIMP and KIF: The Norwegian Livestock Industry's Biosecurity Unit (KOORIMP), 2014. Available at: http://www.animalia.no/upload/FIler%20til%20nedlasting/KOORIMP/KOORIMP%20%c3%a5rsmelding%202014%20web.pdf. Accessed 14 August 2015.
- 19.Urdahl AM, Bergsjø B, Hofshagen M, Nordstöm M, Lium B. The surveillance programme for methicillin resistant Staphylococcus aureus in pigs in Norway 2014. Annual report. Available at: http://wwweng.vetinst.no/eng/Publications/Surveillance-Programmes-annual-reports/2014/The-surveillance-programme-for-methicillinresistant-Staphylococcus-aureus-in-pigs-in-Norway-in-2014.html Accessed 30 November 2015.
- 20.Regulation on the Norwegian Surveillance System for Communicable Diseases (MSIS) (in Norwegian). Available at: https://lovdata.no/dokument/SF/forskrift/2003-06-20-740?q=MSIS2003 Accessed 15 February 2016.
- 21.Elstrom P, Kacelnik O, Bruun T, Iversen B, Hauge SH, Aavitsland P. Meticillin-resistant Staphylococcus aureus in Norway, a low-incidence country, 2006-2010. J Hosp Infect 2012; 80:36–40. [DOI] [PubMed] [Google Scholar]
- 22.European Food Safety Authority. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in methicillin-resistant Staphylococcus aureus in food-producing animals and food. EFSA J; 2012; 10:2742 [64 pp.]. [Google Scholar]
- 23.Lina G, Piemont Y, Godail-Gamot F et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 1999; 29:1128–32. [DOI] [PubMed] [Google Scholar]
- 24.Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. Identification of methicillin-resistant strains of Staphylococci by polymerase chain-reaction. J Clin Microbiol 1991; 29:2240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 2000; 38:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grøntvedt CA, Sunde M, Angen Ø et al. Control of LA-MRSA in swine—is it possible? lessons learned from outbreaks and eradication in Norway. Presented at: 4th ASM-ESCMID Conference on Methicillin-Resistant Staphylococci in Animals; 4 November 2015; Chicago, Illinois. [Google Scholar]
- 27.Garcia-Graells C, Antoine J, Larsen J, Catry B, Skov R, Denis O. Livestock veterinarians at high risk of acquiring methicillin-resistant Staphylococcus aureus ST398. Epidemiol Infect 2012; 140:383–9. [DOI] [PubMed] [Google Scholar]
- 28.Graveland H, Wagenaar JA, Bergs K, Heesterbeek H, Heederik D. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS One 2011; 6:e16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feingold BJ, Silbergeld EK, Curriero FC, van Cleef B, Heck M, Kluytmans J. Livestock density as risk factor for livestock-associated methicillin-resistant Staphylococcus aureus, the Netherlands. Emerg Infect Dis 2012; 18:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NORM/NORM-VET 2014. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo: 2015. ISSN 1502-2307. Available at: https://unn.no/Documents/Kompetansetjenester%2c%20-sentre%20og%20fagr%c3%a5d/NORM%20-%20Norsk%20overv%c3%a5kingssystem%20for%20antibiotikaresistens%20hos%20mikrober/Rapporter/NORM_NORM-VET_2014.pdf. Accessed 5 April 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.