Six human immunodeficiency virus (HIV) care metrics predicted acute myocardial infarction (AMI) and mortality among HIV-infected individuals. Time-updated Veterans Aging Cohort Study Index provided the best prediction for both AMI and mortality.
Keywords: acute myocardial infarction, HIV, mortality, VACS Index
Abstract
Background. After adjustment for cardiovascular risk factors and despite higher mortality, those with human immunodeficiency virus (HIV+) have a greater risk of acute myocardial infarction (AMI) than uninfected individuals.
Methods. We included HIV+ individuals who started combination antiretroviral therapy (cART) in the Veterans Aging Cohort Study (VACS) from 1996 to 2012. We fit multivariable proportional hazards models for baseline, time-updated and cumulative measures of HIV-1 RNA, CD4 counts, and the VACS Index. We used the trapezoidal rule to build the following cumulative measures: viremia copy-years, CD4-years, and VACS Index score-years, captured 180 days after cART initiation until AMI, death, last clinic visit, or 30 September 2012. The primary outcomes were incident AMI (Medicaid, Medicare, and Veterans Affairs International Classification of Diseases-9 codes) and death.
Results. A total of 8168 HIV+ individuals (53 861 person-years) were analyzed with 196 incident AMIs and 1710 deaths. Controlling for known cardiovascular risk factors, 6 of the 9 metrics predicted AMI and all metrics predicted mortality. Time-updated VACS Index had the lowest Akaike information criterion among all models for both outcomes. A time-updated VACS Index score of 55+ was associated with a hazard ratio (HR) of 3.31 (95% confidence interval [CI], 2.11–5.20) for AMI and a HR of 31.77 (95% CI, 26.17–38.57) for mortality.
Conclusions. Time-updated VACS Index provided better AMI and mortality prediction than CD4 count and HIV-1 RNA, suggesting that current health determines risk more accurately than prior history and that risk assessment can be improved by biomarkers of organ injury.
Once those with human immunodeficiency virus infection (HIV+) achieve viral suppression on combination antiretroviral therapy (cART), their life expectancy is dramatically extended [1], and morbidity and mortality due to non–AIDS-related events including cardiovascular disease become the predominant concern [2]. Accounting for established risk factors, HIV+ individuals have 50%–75% greater risk of acute myocardial infarction (AMI) than demographically similar uninfected individuals [3, 4]. Suggested underlying causes include a greater burden of chronic inflammation, immune suppression and dysfunction, anemia, renal disease, liver disease, and hepatitis C coinfection among those with HIV compared with uninfected individuals [4–6].
While many of these factors have been studied in a cross-sectional or time-updated manner, few have considered the association of cumulative HIV viral load (HIV-1 RNA), CD4 count, or organ injury measures with incident AMI among HIV+ individuals. Viremia copy-years, a measure of the amount of HIV-1 RNA exposure over time, has been used to predict mortality but not incident AMI [7]. Although chronic immunosuppression has also been postulated as a risk factor for the development of non-AIDS events [4], it has not been extensively studied in a cumulative fashion [8]. Notably, the Veterans Aging Cohort Study (VACS) Index incorporates HIV-specific measures (HIV-1 RNA and CD4 count), hepatitis C infection, and measures of organ system injury (anemia, renal disease, and liver disease). The Index has been shown to predict AIDS and non-AIDS morbidity and mortality in multiple settings [9–16] but has not been evaluated as a cumulative measure. Further, when studying associations between biomarkers and clinical events, mortality can act as a competing risk in which those with advanced disease die before they experience the clinical event of interest. To compare our findings with previous work and to determine whether competing risk of death might explain a lack of association for some measures, we felt it important to consider AMI and mortality in parallel analyses. Here, we compare the associations of baseline, time-updated, and cumulative measures of HIV-1 RNA, CD4 counts, and VACS Index scores with incident AMI and mortality in a cohort of HIIV+ individuals.
METHODS
The VACS study has been well described [17, 18]. This analysis included all HIV+ individuals who initiated cART with at least 3 unique antiretrovirals in VACS between 1 July 1996 and 30 September 2012. The analysis excluded patients with previous mono or dual ART history, defined as having used at least 1 antiretroviral drug; those with HIV-1 RNA <500 copies/mL at the time of cART initiation; those without baseline and with fewer than 2 HIV-1 RNA, CD4, or VACS Index values during the study period; and patients with known coronary heart disease prior to cART initiation using International Classification of Diseases-9 (ICD-9) codes 410.xx–414.xx from Medicaid, Medicare, and Veterans Affairs (VA) data.
We began follow-up 180 days after cART initiation to allow sufficient time for virologic suppression and for ICD-9 codes to be updated after qualifying events [7]. Patients were followed through incident AMI, last known follow-up, or censor date (30 September 2012). An inpatient ICD-9 code of 410.xx was used to determine the presence of an AMI (Supplementary Table 1). When ICD-9–based outcomes were compared with a smaller validated VACS dataset of AMI outcomes, the ICD-9 classification had a sensitivity of 86%, specificity of 100%, positive predictive value of 82%, and negative predictive value of 100% (Supplementary Table 2). We built baseline, time-updated, and cumulative time-updated measures for HIV-1 RNA (in copies/mL), CD4 values (in cells/mm3), and VACS Index scores (totaling 9 measures). The VACS Index score is calculated using age, gender, race, HIV-1 RNA, CD4 count, aspartate and alanine transaminases, hemoglobin, platelet count, creatinine, and known hepatitis C infection (Supplementary Table 3). Baseline laboratory values were the closest to cART initiation date within a range of 180 days prior to and 7 days after cART initiation date. The time-updated measures were calculated daily using the date that new laboratory data were available. The cumulative measures of viremia copy-years (in copy-years/mL), CD4-years (in cells-years/ mm3), and VACS Index score-years were created using the trapezoidal method [7]. To be consistent with current viremia copy-years literature, all extreme HIV-1 RNA values (>1 000 000 copies/mL) were set at 1 000 000 copies/mL [19]. Additionally, since there had been varying levels of HIV-1 RNA assay sensitivity over time, all undetectable viral load values were set to 200 copies/mL (half of the highest limit of detection during the study period). Our proposed cumulative measures (viremia copy-years, CD4-years, and VACS Index score-years) have not been previously assessed for AMI incidence prediction. Of them, viremia copy-years has been previously used to predict mortality, and we validated our method by assessing its predictive value in mortality incidence.
We created age-adjusted and fully adjusted Cox proportional hazards models for risk of AMI and mortality using baseline, time-updated, and cumulative time-updated versions for each exposure of interest (HIV-1 RNA, CD4, and the VACS Index score). The cut-points used to categorize each measure were derived by distributing the number of incident AMIs equally over the categories and then rounding to the nearest clinically relevant threshold. The fully adjusted models controlled for age, diabetes, total cholesterol, low-density lipoprotein, high-density lipoprotein, smoking, and hypertension at baseline as well as time-updated calendar year. Models for the investigation of HIV-1 RNA as the predictor were adjusted for baseline CD4 count. Conversely, models for the investigation of CD4 as the predictor were adjusted for HIV-1 RNA. We examined interactions between each exposure of interest and calendar year in the fully adjusted models.
The Akaike information criterion (AIC) has been used as a means of model selection, and lower AICs represent better model fit [20]. In this analysis, we used the AIC in conjunction with the magnitude and precision of the main effect estimates to determine which exposure best predicted incident AMI.
VACS has been approved by the institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine, granted a waiver of informed consent, and deemed Health Insurance Portability and Accountability Act compliant. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
During the time period of interest, 47 805 HIV+ patients were in VACS and 35 300 (74%) initiated cART. Of the 35 300 initiators, 13 924 (39%) were exposed to mono or dual therapy prior to receiving more effective 3 or more antiretroviral cART, 7.379 (21%) had a baseline HIV-1 RNA <500 copies/mL, 6303 (18%) did not have the required laboratory results, 3523 (10%) had coronary heart disease prior to baseline, and 1736 (5%) had less than 6 months of follow-up time after cART. After applying exclusions, 8168 (23%) patients remained eligible for the study.
We analyzed data on 8168 individuals (53 861 person-years) from VACS who initiated cART for the first time during the time period and experienced 196 AMIs and 1710 deaths (Table 1). The median age was 46 years (interquartile range = 40–53 years), most were male (96.9%) and African-American (54.8%). Those who experienced AMIs were older, more likely to be white, and more likely to have hypertension and metabolic disease (all P < .002). They did not differ substantially at baseline by CD4 count (P = .08) or HIV-1 RNA (P = .74). Similarly, hemoglobin and fibrosis-4 were not significantly different (P = .27 and P = .06, respectively), but those who experienced AMI were less likely to have an elevated estimated glomerular filtration rate (P < .0001) and more likely to have hepatitis C coinfection (P = .04). Further, their VACS Index scores were more likely to be high (55+; 43% vs 31%; P = .0005).
Table 1.
Baseline Characteristics of 8168 Human Immunodeficiency Virus–Infected Veterans
| Characteristic | No AMI (n = 7972) | AMI (n = 196) | P Valuea |
|---|---|---|---|
| Age, y | 46 (40–52) | 51 (46–57) | <.0001 |
| Male sex | 7726 (96.9) | 192 (98.0) | .4014 |
| Race/ethnicity | |||
| Black/African-American | 4388 (55.0) | 90 (45.9) | .0008 |
| White | 2651 (33.3) | 89 (45.4) | |
| Hispanic | 573 (7.2) | 15 (7.7) | |
| Other | 360 (4.5) | 2 (1.0) | |
| Diabetes | 532 (6.7) | 28 (14.3) | <.0001 |
| Hypertension | 1386 (17.4) | 51 (26.0) | .0017 |
| Composite LDL and TC | |||
| LDL <130 or TC <200 | 5141 (64.5) | 93 (47.5) | <.0001 |
| LDL 130–160 or TC 200–240 | 634 (8.0) | 19 (9.7) | |
| LDL >160 or TC >240 | 209 (2.6) | 8 (4.1) | |
| Other or missing | 1988 (24.9) | 76 (38.8) | |
| HDL | |||
| HDL <40 | 3184 (39.9) | 65 (33.2) | .1442 |
| HDL ≥60 | 417 (5.2) | 10 (5.1) | |
| Other or missing | 4371 (54.8) | 121 (61.7) | |
| Smoking | |||
| Current | 4873 (61.1) | 128 (65.3) | .0098 |
| Former | 980 (12.3) | 33 (16.8) | |
| Never | 2119 (26.6) | 35 (17.9) | |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | |||
| ≥60 | 6054 (75.9) | 119 (60.7) | <.0001 |
| 30–59 | 284 (3.6) | 13 (6.6) | |
| <30 | 86 (1.1) | 5 (2.6) | |
| Missing | 1548 (19.4) | 59 (30.1) | |
| Veterans Aging Cohort Study Index | |||
| <20 | 1297 (16.3) | 20 (10.2) | .0005 |
| 20–34 | 2016 (25.3) | 35 (17.9) | |
| 35–54 | 2173 (27.3) | 56 (28.6) | |
| 55+ | 2486 (31.2) | 85 (43.4) | |
| CD4 count, cells/mm3 | |||
| ≥500 | 703 (8.8) | 20 (10.2) | .0804 |
| 350–499 | 1194 (15.0) | 29 (14.8) | |
| 200–349 | 2403 (30.1) | 43 (21.9) | |
| <200 | 3672 (46.1) | 104 (53.1) | |
| Human immunodeficiency virus viral load, copies/mL | |||
| 501–999 | 216 (2.7) | 7 (3.6) | .7352 |
| 1000–9999 | 1173 (14.7) | 30 (15.3) | |
| 10 000+ | 6583 (82.6) | 159 (81.1) | |
| Hemoglobin, g/dL | |||
| ≥14 | 3049 (38.3) | 66 (33.7) | .2700 |
| 12–13.9 | 3020 (37.9) | 73 (37.2) | |
| 10–11.9 | 1383 (17.4) | 44 (22.5) | |
| <10 | 520 (6.5) | 13 (6.6) | |
| Fibrosis-4 | |||
| <1.45 | 4508 (56.6) | 98 (50.0) | .0612 |
| 1.45–3.25 | 2683 (33.7) | 70 (35.7) | |
| >3.25 | 781 (9.8) | 28 (14.3) | |
| Hepatitis C coinfection | 1742 (21.9) | 55 (28.1) | .0381 |
| AIDSb | 662 (8.3) | 18 (9.2) | .6597 |
Statistics given in median (interquartile range) or n (%).
Abbreviations: AMI, acute myocardial infarction; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol.
a Tested for significance with 2-sided Wilcoxon rank-sum and χ2 tests.
b Includes diagnosis of Kaposi's sarcoma or Pneumocystis pneumonia.
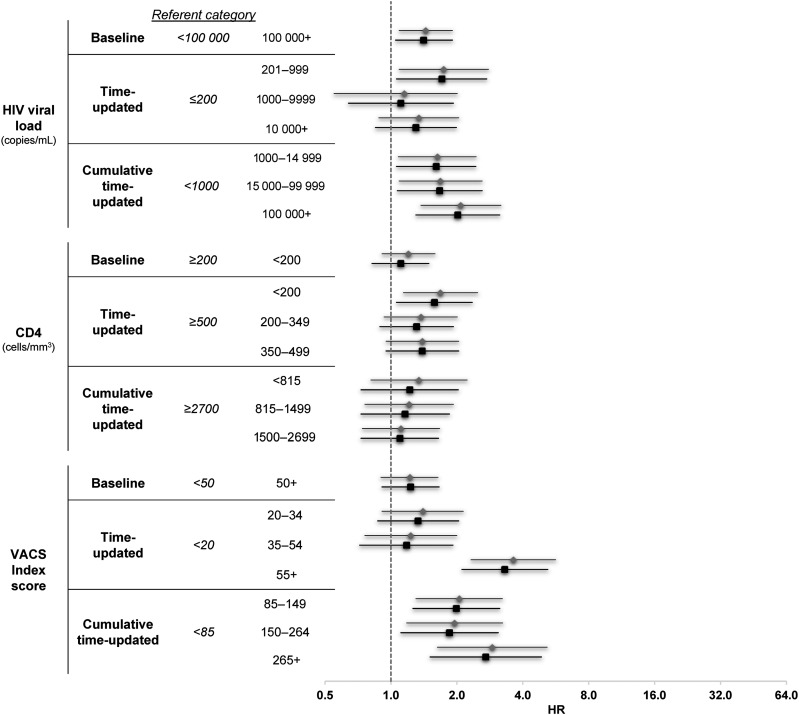
Six of 9 metrics were significantly associated with risk of AMI. In the fully adjusted HIV-1 RNA models (Table 2 and Figure 1), individuals with baseline HIV-1 RNA ≥100 000 copies/mL had 41% higher risk of AMI in age-adjusted and fully adjusted models (hazard ratio [HR], 1.41; 95% confidence interval [CI], 1.05–1.91) compared with those with HIV-1 RNA <100 000 copied/mL. At any time during the study period (time-updated HIV-1 RNA), patients with HIV-1 RNA = 201–999 copies/mL had a 71% increased risk of AMI than those who had a HIV-1 RNA ≤200 copies/mL (HR, 1.71; 95% CI, 1.06–2.74). However, time-updated HIV-1 RNA was not predictive of AMI at higher levels of viremia: HIV-1 RNA = 1000–9999 copies/mL (HR, 1.11; 95% CI, .64–1.93) or HIV-1 RNA ≥10 000 copies/mL (HR, 1.30; 95% CI, .85–1.99). The cumulative measure, viremia copy-years, demonstrated a significant association with AMI at all levels: viremia copy-years = 1000–14 999 (HR, 1.61; 95% CI, 1.06–2.44), 15 000–99 999 (HR, 1.67; 95% CI, 1.07–2.61), and ≥100 000 (HR, 2.02; 95% CI, 1.30–3.14) all compared with <1000 copy-years/mL.
Table 2.
Crude and Adjusted Hazard Ratios and 95% Confidence Intervals by Exposure of Interest and Outcome, n = 8168
| HIV Care Metrics | AMI Models |
Mortality Models |
||||||
|---|---|---|---|---|---|---|---|---|
| PY | Events | Age-Adjusted HR (95% CI) | Fully Adjusted HR (95% CI) | PY | Events | Age-Adjusted HR (95% CI) | Fully Adjusted HR (95% CI) | |
| HIV-1 RNA measuresa | ||||||||
| Baseline HIV-1 RNA (copies/mL) | ||||||||
| <100 000 | 32 253 | 99 | 1 | 1 | 32 587 | 930 | 1 | 1 |
| 100 000+ | 21 608 | 97 | 1.44 (1.09, 1.91) | 1.41 (1.05, 1.91) | 21 881 | 780 | 1.24 (1.13, 1.37) | 1.17 (1.06, 1.30) |
| Time-updated HIV-1 RNA (copies/mL) | ||||||||
| ≤200 | 42 258 | 135 | 1 | 1 | 42 769 | 842 | 1 | 1 |
| 201–999 | 2660 | 20 | 1.74 (1.09, 2.79) | 1.71 (1.06, 2.74) | 2617 | 108 | 1.44 (1.18, 1.76) | 1.40 (1.15, 1.72) |
| 1000–9999 | 2710 | 14 | 1.15 (.66, 2.01) | 1.11 (.64, 1.93) | 2737 | 159 | 1.98 (1.67, 2.35) | 1.88 (1.58, 2.23) |
| 10 000+ | 6233 | 27 | 1.34 (.88, 2.04) | 1.30 (.85, 1.99) | 6345 | 601 | 4.32 (3.88, 4.81) | 4.00 (3.59, 4.47) |
| Cumulative time-updated viremia copy-years (VCY) (copy-years/mL) | ||||||||
| <1000 | 10 914 | 49 | 1 | 1 | 10 895 | 360 | 1 | 1 |
| 1000–14 999 | 15 657 | 56 | 1.63 (1.08, 2.45) | 1.61 (1.06, 2.44) | 15 855 | 303 | 1.39 (1.18, 1.63) | 1.36 (1.16, 1.59) |
| 15 000–99 999 | 10 205 | 39 | 1.68 (1.09, 2.61) | 1.67 (1.07, 2.61) | 10 341 | 305 | 1.97 (1.68, 2.31) | 1.89 (1.61, 2.21) |
| 100 000+ | 17 085 | 52 | 2.08 (1.37, 3.18) | 2.02 (1.30, 3.14) | 17 377 | 742 | 4.38 (3.82, 5.03) | 4.09 (3.55, 4.70) |
| CD4 measuresb | ||||||||
| Baseline CD4 (cells/mm3) | ||||||||
| <200 | 25 192 | 104 | 1.20 (.91, 1.59) | 1.11 (.82, 1.49) | 25 461 | 962 | 1.40 (1.27, 1.54) | 1.34 (1.21, 1.49) |
| 200+ | 28 669 | 92 | 1 | 1 | 29 007 | 748 | 1 | 1 |
| Time-updated CD4 (cells/mm3) | ||||||||
| <200 | 9356 | 43 | 1.68 (1.14, 2.49) | 1.58 (1.06, 2.35) | 9522 | 881 | 7.63 (6.65, 8.75) | 6.92 (6.03, 7.96) |
| 200–349 | 9483 | 46 | 1.37 (.93, 2.01) | 1.31 (.89, 1.93) | 9540 | 336 | 2.31 (1.97, 2.72) | 2.21 (1.88, 2.60) |
| 350–499 | 10 053 | 46 | 1.39 (.95, 2.04) | 1.39 (.95, 2.04) | 10 137 | 223 | 1.54 (1.29, 1.84) | 1.55 (1.30, 1.85) |
| 500+ | 24 968 | 61 | 1 | 1 | 25 269 | 270 | 1 | 1 |
| Cumulative time-updated CD4-years (cell-years/mm3) | ||||||||
| <815 | 4106 | 42 | 1.34 (.81, 2.23) | 1.22 (.73, 2.03) | 4067 | 665 | 5.44 (4.62, 6.41) | 4.66 (3.94, 5.50) |
| 815–1499 | 5635 | 40 | 1.21 (.76, 1.93) | 1.16 (.73, 1.85) | 5640 | 329 | 2.50 (2.11, 2.96) | 2.32 (1.96, 2.74) |
| 1500–2699 | 10 714 | 48 | 1.11 (.74, 1.67) | 1.10 (.73, 1.65) | 10 744 | 343 | 1.76 (1.51, 2.06) | 1.71 (1.46, 2.00) |
| 2700+ | 33 406 | 66 | 1 | 1 | 34 018 | 373 | 1 | 1 |
| VACS Index measuresc,d | ||||||||
| Baseline VACS Index score | ||||||||
| <50 | 34 319 | 97 | 1 | 1 | 34 678 | 729 | 1 | 1 |
| 50+ | 19 542 | 99 | 1.22 (.90, 1.64) | 1.23 (.91, 1.66) | 19 790 | 981 | 1.98 (1.79, 2.19) | 1.91 (1.73, 2.12) |
| Time-updated VACS Index score | ||||||||
| <20 | 18 082 | 38 | 1 | 1 | 18 120 | 125 | 1 | 1 |
| 20–34 | 15 272 | 56 | 1.40 (.91, 2.14) | 1.33 (.87, 2.04) | 15 408 | 198 | 2.24 (1.79, 2.81) | 2.14 (1.71, 2.69) |
| 35–54 | 10 281 | 38 | 1.23 (.76, 2.00) | 1.18 (.72, 1.92) | 10 509 | 306 | 5.56 (4.49, 6.89) | 5.28 (4.26, 6.55) |
| 55+ | 10 226 | 64 | 3.62 (2.32, 5.65) | 3.31 (2.11, 5.20) | 10 432 | 1081 | 34.19 (28.22, 41.43) | 31.77 (26.17, 38.57) |
| Cumulative time-updated VACS Index score-years | ||||||||
| <85 | 10 405 | 37 | 1 | 1 | 10 354 | 246 | 1 | 1 |
| 85–149 | 8197 | 51 | 2.05 (1.30, 3.23) | 1.99 (1.26, 3.14) | 8153 | 315 | 4.17 (3.48, 5.00) | 3.92 (3.27, 4.70) |
| 150–264 | 13 994 | 50 | 1.95 (1.18, 3.24) | 1.85 (1.11, 3.09) | 14 092 | 496 | 10.74 (8.87, 13.00) | 9.82 (8.10, 11.91) |
| 265+ | 21 265 | 58 | 2.90 (1.63, 5.17) | 2.71 (1.51, 4.87) | 21 868 | 653 | 30.10 (24.37, 37.18) | 26.95 (21.75, 33.39) |
All undetectable human immunodeficiency virus type 1 RNA values were set to 200 copies/mL, which is half of the largest lower detection limit.
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; HIV-1 RNA, human immunodeficiency virus viral load; HR, hazard ratio; PY, person-years; VACS, Veterans Aging Cohort Study.
a Adjusted factors include age, CD4, diabetes, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), smoking, hypertension, and calendar year.
b Adjusted factors include age, HIV viral load, diabetes, cholesterol, LDL, HDL, smoking, hypertension, and calendar year.
c VACS Index scores include age, indicators of HIV disease, and indicators of organ system injury.
d Adjusted factors include age, diabetes, cholesterol, LDL, HDL, smoking, hypertension, and calendar year.
Figure 1.
Crude and adjusted hazard ratios (HRs) and 95% confidence interval (CI) for the risk of acute myocardial infarction. HRs and 95% CIs are presented on a log2 scale, gray diamonds denote age-adjusted measures, black squares denote fully adjusted measures. Adjusting factors: all models age, diabetes, cholesterol, low- and high-density lipoprotein, smoking, hypertension; human immunodeficiency virus (HIV) viral load models additionally adjusted for CD4; CD4 models additionally adjusted for HIV viral load. Abbreviation: VACS, Veterans Aging Cohort Study.
In the fully adjusted CD4 models, there was no evidence of increased risk of AMI among HIV+ individuals with a baseline CD4 <200 cells/mm3 (HR, 1.11; 95% CI, .82–1.49) compared with those with a baseline CD4 ≥200 cells/mm3. The time-updated CD4 model demonstrated an association with AMI incidence only at the lowest CD4 levels: CD4 <200 cells/mm3 (HR, 1.58; 95% CI, 1.06–2.35) compared with CD4 ≥500 cells/mm3. The cumulative immunosuppression measure (CD4-years) was not significantly associated with AMI risk in any of the studied categories (P > .05).
In the fully adjusted VACS Index models, baseline VACS Index scores ≥50 were not associated with AMI incidence when compared with those with scores <50 (HR, 1.23; 95% CI, .91–1.66), but time-updated VACS Index scores ≥55 were associated with incident AMI (HR, 3.31; 95% CI, 2.11–5.20) when compared with those with VACS Index scores <20. Values of cumulative VACS Index score-years significantly predicted AMI incidence at all levels: VACS Index score-years = 85–149 (HR, 1.99; 95% CI, 1.26–3.14), VACS Index score-years 150–264 (HR, 1.85; 95% CI, 1.11–3.09), and VACS Index score-years ≥265 (HR, 2.71; 95% CI, 1.51–4.87) when compared with VACS Index score-years <85. Finally, there was no evidence that time modified the relationship between any exposure of interest and incident AMI (all P > .05).
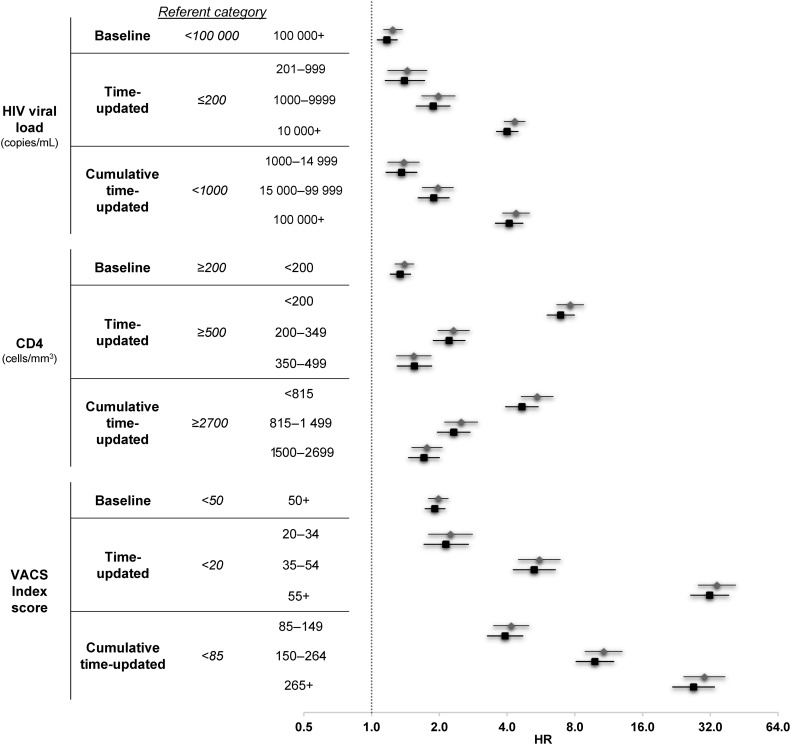
All 9 metrics were associated with mortality in the age-adjusted and fully adjusted models (Table 2 and Figure 2). The strongest associations were seen for time-updated HIV-1 RNA and viremia copy-years demonstrating a 4-fold higher risk of mortality when comparing the highest HIV-1 RNA levels with the lowest for each respective measure, for time-updated CD4 count <200 cells/mm3 demonstrating a nearly 7-fold higher risk of mortality compared with ≥500 cells/mm3 (HR, 6.92; 95% CI, 6.03, 7.96), and for time-updated VACS Index of >55 compared with <20 demonstrating a 32-fold increased risk of mortality (HR, 31.8; 95% CI, 26.2, 38.6).
Figure 2.
Crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of all-cause mortality. HRs and 95% CIs are presented on a log2 scale, gray diamonds denote age-adjusted measures, black squares denote fully adjusted measures. Adjusting factors: all models age, diabetes, cholesterol, low- and high-density lipoprotein, smoking, hypertension; human immunodeficiency virus (HIV) viral load models additionally adjusted for CD4; CD4 models additionally adjusted for HIV viral load. Abbreviation: VACS, Veterans Aging Cohort Study.
Based on AIC measures (Table 3) of fully adjusted models, among HIV-1 RNA models, viremia copy-years provided more information regarding the risk of AMI, and time-updated viremia provided more information regarding mortality among the HIV-1 RNA models. Time-updated CD4 provided the most information among the CD4 models for both AMI and mortality. Among VACS Index models, the time-updated VACS Index provided more information regarding risk of AMI and mortality. Based on AICs, the fully adjusted time-updated VACS Index model was preferred over any HIV-1 RNA or CD4 count models for both AMI and mortality.
Table 3.
Akaike Information Criterion Values for Crude and Adjusted Cox Regression Models by Exposure of Interest and Outcome, n = 8168
| HIV Care Metrics | Acute Myocardial Infarction Models |
Mortality Models |
||
|---|---|---|---|---|
| Age-Adjusted | Fully Adjusted | Age-Adjusted | Fully Adjusted | |
| HIV-1 RNA measures | ||||
| Baseline HIV-1 RNA (copies/mL) | 3154 | 3141 | 28 339 | 28 007 |
| Time-updated HIV-1 RNA (copies/mL) | 3158 | 3145 | 27 728 | 27 466 |
| Cumulative time-updated viremia (copy-years/mL) | 3152 | 3139 | 27 820 | 27 552 |
| CD4 measures | ||||
| Baseline CD4 (cells/mm3) | 3159 | 3140 | 28 312 | 27 953 |
| Time-updated CD4 (cells/mm3) | 3157 | 3139 | 27 222 | 26 987 |
| Cumulative time-updated CD4-years (cell-years/mm3) | 3163 | 3144 | 27 939 | 27 650 |
| VACS Index measures | ||||
| Baseline VACS Index score | 3158 | 3145 | 28 178 | 27 905 |
| Time-updated VACS Index score | 3122 | 3114 | 25 608 | 25 507 |
| Cumulative time-updated VACS Index score-years | 3149 | 3138 | 27 189 | 27 004 |
Abbreviations: HIV-1 RNA, human immunodeficiency virus viral load; VACS, Veterans Aging Cohort Study.
DISCUSSION
Ongoing HIV viral replication and inflammation, immunosuppression, anemia, renal disease, and liver disease have been postulated in the pathogenesis of coronary heart disease in HIV+ individuals [4]. After adjusting for traditional AMI risk factors, we present a comparison of the ability to predict AMI and mortality using baseline, time-updated, and cumulative measures of 3 HIV care parameters (HIV-1 RNA, CD4 count, and the VACS Index). The VACS Index provided substantially more information than either HIV-1 RNA or CD4 counts alone. Specifically, the time-updated VACS Index best predicted both AMI incidence and all-cause mortality; a score of 55+ was associated with a HR of 3.31 (95% CI, 2.11–5.20) for AMI and a HR of 31.8 (95% CI, 26.2–38.6) for mortality.
Most previous studies have focused on time-updated and cumulative measures of HIV-1 RNA and CD4 count. Some studies demonstrated an association of uncontrolled viremia with mortality [2] and AMIs [21]. Cumulative HIV viremia is more predictive of mortality over single cross-sectional measures of HIV viremia [7, 22]. Our findings show that baseline HIV-1 RNA and cumulative viremia copy-years were associated with AMI while time-updated viremia was not. SMART [23] also found “no clear evidence that … time-updated viral load is … associated with CVD [cardiovascular] risk.” Similarly, there is no clear consensus regarding the association of immunosuppression with AMI. Studies have shown conflicting results for CD4 measures at baseline, nadir, last value, duration of immunosuppression, and time-updated values [2, 23]. A recent study [24] showed a protective effect of higher CD4 values; individuals with recent or nadir CD4 ≥500 cells/mm3 had risk of AMI comparable to that of an HIV-uninfected population. Another study that assessed immunosuppression and cardiovascular outcomes found a small association of immunosuppression with strokes but not with AMI [25]. Our findings did not support an advantage of measuring cumulative CD4 counts, but there did appear to be a trend toward protection for individuals with time-updated CD4 counts ≥500 cells/mm3.
We found stronger associations between more extreme values of time-updated HIV-1 RNA and CD4 count and mortality than with AMI. It is tempting to attribute the weaker association with AMI to competing risk from mortality. If this were true, we would have expected the VACS Index to have an even weaker association with AMI since its association with mortality was stronger than that for HIV-1 RNA or CD4 count. Instead, we found that the time-updated VACS Index was a better predictor of both outcomes.
The VACS Index is a validated score capable of predicting all-cause mortality [16], cardiovascular mortality [26], and an array of morbidity measures [10, 15]. It can be constructed with basic clinical information available in most settings. An online calculator is available (https://vacs-apps2.med.yale.edu/calculator/IC; Accessed 30 August 2016). Both time-updated and cumulative measures of VACS Index were more strongly associated with incident AMI than CD4 or HIV-1 RNA measures alone. This may not be surprising since, in addition to HIV-RNA and CD4 counts, the VACS Index also accounts for anemia, chronic kidney diseases, liver disease, and hepatitis C coinfection, giving a more comprehensive overview of the nontraditional factors associated with cardiovascular disease. Of note, all components of the VACS Index are correlated with measures of chronic inflammation, including interleukin-6, soluble CD4, and D-dimer [27], which may explain the strength of this association. The time-updated VACS Index may be complementary to traditional risk factors in AMI risk assessment.
Our finding that time-updated VACS Index provides superior prediction of risk of AMI and mortality compared with all cumulative measures considered is clinically convenient since past measures may not always be obtained. Additionally, cumulative measures are highly dependent upon the period of observation, making them less generalizable. Further, it suggests that a patient's current status is much more important than how they got there or the duration of time they spent in a particular state. Future studies are required to assess how the VACS Index might enhance AMI risk estimation beyond currently proposed indices such as DAD [28] and/or Framingham [29, 30]. Further, because mortality can act as a competing risk in which those with advanced disease die before they experience the clinical event of interest, our findings are more conclusive. Had one measure been superior for mortality and another for AMI, we might have been concerned about competing risk. Fortunately, time-updated VACS Index was the best predictor for both events, thus we can be confident that competing risk of death did not distort our comparison.
Our study has limitations. Findings may not generalize to women since only a small proportion of our sample was female. An in-depth analysis of cART regimen was beyond the scope of this study. Some reports have suggested that protease inhibitors as a class and abacavir as a specific agent may be associated with risk of AMI [31, 32]. We see no reason why the relative prognostic importance of the biomarkers and index we report should depend upon regimen. We used administrative data (ICD-9 coding), which may limit the accuracy of the outcome. To address this issue, we validated the use of ICD-9 coding with data from chart review with an acceptable positive predictive value. Additionally, the use of ICD-9 codes to identify AMI precludes our ability to further differentiate AMI into type 1 and type 2 classifications. Our study measured and compared 9 clinical metrics, but we were unable to compare them with established risk estimators such as the Framingham risk score calculator or the ASVCD risk estimator [29, 30]. We did, however, adjust at baseline for important components of the Framingham calculator, including diabetes, hypertension, cholesterol levels, and smoking. Given the observational nature of our study, we can only postulate associations, not causality. Despite these limitations, we were able to compare the predictive ability of novel cumulative measures for AMI incidence and mortality using one of the largest cohorts of aging HIV+ individuals in the United States.
In conclusion, we determined that the time-updated VACS Index was the best predictor of AMI incidence and all-cause mortality compared with 8 other HIV care metrics included in this study of US veterans. Future studies seeking to refine cardiovascular risk in HIV+ individuals should consider the time-updated VACS Index as it has the potential to improve currently available cardiovascular risk assessment strategies.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author contributions. All authors contributed to study design. A. C. J., J. L. S., C. R., and J. T. contributed to data collection; J. L. S., C. R., V. C. M., J. T., A. C. J., and D. R. contributed to data quality and analysis; all authors contributed to manuscript development and have critically reviewed the manuscript and approved the final version.
Acknowledgments. We are grateful to the patients and clinical staff at the Veteran Affairs clinical sites that were part of the Veterans Aging Cohort Study.
Financial support. This work was supported by the following: Agency for Healthcare Research and Quality (R01-HS018372); National Institute on Alcohol Abuse and Alcoholism (U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799, U24-AA022001, U24 AA022007, U10 AA013566-completed); National Heart, Lung, and Blood Institute (R01-HL095136, R01-HL090342); National Institute of Allergy and Infectious Diseases (U01-A1069918); Fogarty International Center (R25TW009337); National Institute of Mental Health (P30-MH062294); National Institute on Drug Abuse (R01DA035616); National Cancer Institute (R01 CA173754), Veterans Health Administration Office of Research and Development (VA REA 08-266, VA IRR Merit Award), and Office of Academic Affiliations (Medical Informatics Fellowship).
Potential conflicts of interest. A. A. B. has received investigator-initiated research grants (to the institution, unrelated to current work) from Gilead Sciences and AbbVie. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sabin CA. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med 2013; 11:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin B, Thiebaut R, Bucher HC et al. . Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 2009; 23:1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Post WS, Budoff M, Kingsley L et al. . Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Kuller LH et al. . HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med 2010; 11:462–8. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mugavero MJ, Napravnik S, Cole SR et al. . Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis 2011; 53:927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanik EL, Napravnik S, Cole SR et al. . Relationship of immunologic response to antiretroviral therapy with non-AIDS defining cancer incidence. AIDS 2014; 28:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justice AC, Modur SP, Tate JP et al. . Predictive accuracy of the Veterans Aging Cohort Study Index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013; 62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womack JA, Goulet JL, Gibert C et al. . Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 2013; 56:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akgun KM, Gordon K, Pisani M et al. . Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV infected veterans. J Acquir Immune Defic Syndr 2013; 62:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akgun KM, Tate JP, Crothers K et al. . An adapted frailty-related phenotype and the VACS Index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr 2014; 67:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin D, Heaton R, Woods S et al. . Veterans Aging Cohort Study index score is associated with neurocognitive and functional impairment: a CHARTER study. In: 20th Conference on Retroviruses and Opportunistic Infections (CROI) Atlanta, Georgia, 2013. [Google Scholar]
- 14.Escota G, Patel P, Brooks JT et al. . The VACS Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses 2015; 31:313–7. [DOI] [PubMed] [Google Scholar]
- 15.Marquine MJ, Umlauf A, Rooney AS et al. . The Veterans Aging Cohort Study Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr 2014; 65:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tate JP, Justice AC, Hughes MD et al. . An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013; 27:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fultz SL, Skanderson M, Mole LA et al. . Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006; 44(8 suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 18.Justice AC, Dombrowski E, Conigliaro J et al. . Veterans Aging Cohort Study (VACS): overview and description. Med Care 2006; 44(8 suppl 2):S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugavero MJ, Amico KR, Westfall AO et al. . Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr 2012; 59:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rentsch C, Bebu I, Guest JL, Rimland D, Agan BK, Marconi V. Combining epidemiologic and biostatistical tools to enhance variable selection in HIV cohort analyses. PLoS One 2014; 9:e87352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010; 55:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol 2010; 171:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips AN, Carr A, Neuhaus J et al. . Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther 2008; 13:177–87. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg MJ, Leyden WA, Xu L et al. . Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014; 65:160–6. [DOI] [PubMed] [Google Scholar]
- 25.Sabin CA, Ryom L, De Wit S et al. . Associations between immune depression and cardiovascular events in HIV infection. AIDS 2013; 27:2735–48. [DOI] [PubMed] [Google Scholar]
- 26.Justice AC, Tate JP, Freiberg MS, Rodriguez-Barradas MC, Tracy R. Reply to Chow et al. Clin Infect Dis 2012; 55:751–2. [Google Scholar]
- 27.Justice AC, Freiberg MS, Tracy R et al. . Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 2012; 54:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friis-Moller N, Ryom L, Smith C et al. . An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol 2016; 23:214–23. [DOI] [PubMed] [Google Scholar]
- 29.Goff DC Jr, Lloyd-Jones DM, Bennett G et al. . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63(25 Pt B):2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Agostino RB Sr, Vasan RS, Pencina MJ et al. . General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–53. [DOI] [PubMed] [Google Scholar]
- 31.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS 2003; 17:2479–86. [DOI] [PubMed] [Google Scholar]
- 32.Llibre JM, Hill A. Abacavir and cardiovascular disease: a critical look at the data. Antiviral Res 2016; 132:116–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.