Expression of C-reactive protein and serum amyloid A acute-phase reactants was significantly increased in early Lyme disease but not in the later stages of active infection. Post-treatment Lyme disease syndrome was associated with elevated C-reactive protein, suggesting ongoing enhanced inflammation.
Keywords: Lyme disease, Borrelia burgdorferi, acute-phase response, inflammation, post-treatment Lyme disease syndrome
Abstract
Background. Infection with Borrelia burgdorferi, the causative agent of Lyme disease, triggers host immune responses that affect the clinical outcome and are a source of biomarkers with diagnostic utility. Although adaptive immunity to B. burgdorferi has been extensively characterized, considerably less information is available about the development of innate acute-phase responses in Lyme disease. Our aim in this study was to evaluate the expression of C-reactive protein (CRP) and serum amyloid A (SAA), the prototype acute-phase response proteins, in the context of the varying manifestations associated with Lyme borreliosis.
Methods. Circulating concentrations of CRP and SAA in patients with a range of early to late objective manifestations of Lyme disease and in individuals with post-treatment Lyme disease syndrome were compared with those in healthy control groups.
Results. CRP and SAA levels were significantly elevated in early localized and early disseminated Lyme disease but not in the later stages of active infection. Levels of CRP, but not SAA, were also found to be significantly increased in patients with antibiotic-refractory Lyme arthritis and in those with post-treatment Lyme disease syndrome.
Conclusions. These findings indicate that circulating CRP and SAA levels are highest when the concentration of spirochetes is greatest in skin and/or blood and that levels decline after the dissemination of the organism to extracutaneous sites in subsequent stages of infection. The data also suggest that antibiotic-refractory Lyme arthritis and post-treatment Lyme disease syndrome are associated with elevated CRP responses that are driven by inflammatory mechanisms distinct from those in active infection.
Lyme disease, a multisystemic infection caused by Borrelia burgdorferi spirochetes and transmitted by ticks, is often described as occurring in 3 stages: early localized, early disseminated, and late disease [1]. Early localized Lyme disease occurs days after the tick bite and is typically associated with a single erythema migrans (EM) skin lesion. Early disseminated disease occurs days to weeks following the tick bite, when the bacteria have spread through circulation, and is associated with multiple EM lesions and certain extracutaneous manifestations, such as neurologic abnormalities. Late disease occurs months to years after the initial exposure and generally presents as arthritis or late neuroborreliosis [1, 2]. Antibiotics resolve clinical symptoms in most cases, but approximately 10% of patients with Lyme arthritis continue to experience persistent joint inflammation after treatment [2]. Known as antibiotic-refractory Lyme arthritis, this manifestation is believed to involve a mechanism other than active infection and usually responds to anti-inflammatory therapies [2]. In addition, some Lyme disease patients experience persistent symptoms of pain, fatigue, and/or cognitive difficulties despite standard antibiotic treatment and in the absence of evidence for active infection [3]. The causes of these symptoms, collectively referred to as post-treatment Lyme disease syndrome (PTLDS) when they last longer than 6 months, remain largely unknown, and no biomarkers or effective therapies are available.
Host immunologic reaction to infection with B. burgdorferi includes a robust antibody response to many of the pathogen's proteins and glycolipids. Serologic assays that detect the antibody response to borrelial proteins are used extensively to aid in the diagnosis of Lyme disease [4]. As with other bacterial infections, B. burgdorferi is also expected to trigger an acute-phase innate immune reaction that is likely to precede the adaptive B-cell response. However, in contrast to the antibody response, the innate acute-phase reaction in Lyme borreliosis has not been systematically analyzed, and its evolution throughout the course of disease is unclear.
C-reactive protein (CRP), the prototype acute-phase response protein, is a highly sensitive marker of infection and inflammation. As a pattern recognition molecule, it binds a variety of ligands that are displayed on the surface of pathogens or become exposed during autologous cell stress, injury, or death [5]. Once bound, the effects of CRP can resemble some of the key properties of antibodies, including opsonin deposition and activation of the classical complement pathway, as well as direct interaction with phagocytic cells through Fcγ receptors [6]. Circulating CRP concentrations increase in response to many forms of infection, inflammation, tissue trauma, and malignancy. Levels greater than 10 mg/L are recognized to indicate a clinically significant inflammatory state or “macro-inflammation”, whereas levels of 3–10 mg/L are generally considered to represent “low-grade inflammation”, possibly driven by mild cellular stress or injury [7, 8]. Because CRP decreases rapidly following elimination of the stimulatory ligands, its measurement is considered to be a reliable indicator of acute infection and/or inflammation [5, 6]. Serum amyloid A (SAA), an apolipoprotein associated with high-density lipoprotein particles, is another highly sensitive acute-phase reactant. Circulating concentrations of SAA can increase up to 1000 fold in response to an acute stimulus, while mildly elevated levels are associated with ongoing chronic inflammation [9, 10]. SAA has significant immunologic activity, such as triggering the expression of several cytokines and acting as a chemoattractant for various immune cells [9]. The cytokine-mediated regulation of SAA release is distinct from that for CRP, and the two acute-phase reactants are expressed in response to different stimuli [10]. Here, we examine CRP and SAA levels in well-characterized cohorts of patients representing the various stages and manifestations of Lyme disease.
METHODS
Patients and Controls
Lyme disease and control serum samples were obtained with written informed consent under institutional review board-approved protocols at the National Institute of Allergy and Infectious Diseases (NIAID) and New York Medical College. Sera from individuals with PTLDS were obtained as part of a previous clinical trial study [11] and provided through a National Institutes of Health (NIH)–supported biorepository (contract N01-AI-65308). Sera were maintained at −80°C. This study was approved by the institutional review board of Columbia University Medical Center.
Serum samples were from 90 individuals with a range of early to late objective manifestations of Lyme disease, including single EM, multiple EM, early neurologic, late neurologic, antibiotic-responsive arthritis, and antibiotic-refractory arthritis, collected at the time the clinical manifestations listed were present. The cohort did not include patients with cardiac manifestations. Patients with single EM, multiple EM, early neurologic, late neurologic, and antibiotic-responsive arthritis manifestations are referred to as having active Lyme disease. All patients met the Centers for Disease Control and Prevention (CDC) case definition for Lyme disease [12]. Patients with EM had culture evidence of B. burgdorferi infection. Early neurologic Lyme disease was defined as the presence of compatible objective clinical findings (eg, cranial nerve palsy, lymphocytic meningitis, and/or radiculoneuritis) in conjunction with current or recent EM and/or serologic evidence of the infection. Late neurologic Lyme disease was defined based on the presence of a compatible objective clinical finding (eg, encephalopathy, polyneuropathy, or encephalomyelitis) in association with serologic evidence of borrelial infection. Lyme arthritis was defined as the presence of clinically compatible joint swelling in conjunction with serologic evidence of the infection. Antibiotic-responsive Lyme arthritis was defined as the resolution of symptoms within 3 months after the start of treatment with up to 4 weeks of intravenous (IV) antibiotics or up to 8 weeks of oral antibiotics. Antibiotic-refractory Lyme arthritis was defined as persistent joint swelling for more than 3 months after the initiation of treatment with at least 4 weeks of IV antibiotics or at least 8 weeks of oral antibiotics. Seventy-one active Lyme disease patients and all patients with antibiotic-refractory Lyme arthritis were seropositive for immunoglobulin (Ig) G to B. burgdorferi by a commercial enzyme-linked immunosorbent assay (ELISA) [13]. Sera from 67 healthy individuals without a history or serologic evidence of Lyme disease who resided in Mid-Atlantic and southern New England states were included as controls. Screening questionnaires were used to evaluate the general health of these individuals.
In addition, serum samples from 74 individuals with PTLDS (mean [standard deviation] elapsed time since the original diagnosis of Lyme disease, 4.8 [3.0] years) were included. The source of samples and case definition of PTLDS have been previously described [11, 14, 15]. Patients had at least 1 of the following: a history of EM skin lesion, early neurologic or cardiac symptoms attributed to Lyme disease, late neurologic manifestations, or Lyme arthritis. Documentation of previous treatment of active Lyme disease with a recommended antibiotic regimen was required. Patients had 1 or more of the following symptoms at the time of enrollment: widespread musculoskeletal pain, cognitive impairment, radicular pain, paresthesias, or dysesthesias. Fatigue often accompanied 1 or more of these symptoms. Fifty-two patients had a history of EM. Forty-six patients were seropositive for IgG antibodies to B. burgdorferi [13]. Control sera for the PTLDS cohort were collected from 68 individuals with a history of Lyme disease who had no residual post-treatment symptoms at ≥1 year of follow-up (mean elapsed time since the original diagnosis of Lyme disease, 5.1 [3.5] years); these individuals are referred to as post-treatment Lyme disease healthy (PTLDH). Forty were ELISA seropositive for IgG to B. burgdorferi [13]. All had met the CDC surveillance criteria for Lyme disease at the time of initial diagnosis [16], including 40 who had EM.
Assays
Serum levels of IgG antibody reactivity to B. burgdorferi (whole cell lysate supplemented with recombinant VlsE protein; Euroimmun), CRP (high sensitivity; Alpco), SAA (R&D Systems), and leptin (R&D Systems) were measured by ELISA, according to assay manufacturers' protocols. Samples were tested in duplicate. The utilized CRP ELISA was compared with a high-sensitivity CRP assay on a Cobas 6000 analyzer (Roche Diagnostics) and found to yield consistently similar results.
Data Analysis
Group differences for continuous data were assessed by the analysis of covariance, using the general linear model to account for the potential confounding effect of differences in age, sex, and leptin level. We considered the frequencies of CRP concentration above 3 or 10 mg/L, as discussed in the introduction [5, 7]. Frequency data were analyzed by multiple logistic regression, and the potential confounding effect of differences in the above variables were taken into account. All P values were 2-sided, and differences were considered statistically significant at P < .05. Correlation analysis was performed using Spearman r. Statistical analyses were done with Prism 6 (GraphPad) and Minitab 17 (Minitab) software.
RESULTS
Patients and Controls
The demographic and clinical characteristics of the study cohorts are included in Table 1. Patients with active Lyme disease as a group (as well as each of the constituent single EM, multiple EM, early neurologic, late neurologic, and antibiotic-responsive arthritis subgroups) or patients with antibiotic-refractory arthritis did not differ significantly in terms of age or sex from individuals in the healthy control group. Furthermore, the differences between the PTLDS and PTLDH cohorts in age, sex, elapsed time since the original diagnosis of Lyme disease, history of EM, or seropositivity for anti-B. burgdorferi antibodies were not statistically significant.
Table 1.
Demographic and Clinical Characteristics of Study Cohorts
| Participant Group | No. of Participants | Female Sex—No. (%) | Mean Age—Years ± Standard Deviation |
|---|---|---|---|
| Healthy control | 67 | 28 (41.8) | 47.6 ± 12.1 |
| Active Lyme disease | 79 | 34 (43.0) | 47.8 ± 14.6 |
| Early localized (single EM) | 18 | 7 (38.9) | 46.5 ± 14.3 |
| Early disseminated (multiple EM) | 17 | 8 (47.1) | 47.1 ± 16.4 |
| Early neurologic | 16 | 5 (31.2) | 46.3 ± 9.6 |
| Late neurologic | 16 | 6 (37.5) | 52.5 ± 16.9 |
| Antibiotic-responsive arthritis | 12 | 4 (33.3) | 45.5 ± 15.5 |
| Antibiotic-refractory Lyme arthritis | 11 | 4 (36.4) | 52.5 ± 20.9 |
| Post-treatment Lyme disease healthy | 68 | 24 (35.3) | 53.0 ± 14.9 |
| Post-treatment Lyme disease syndrome | 74 | 36 (48.6) | 56.0 ± 12.6 |
Abbreviation: EM, erythema migrans.
Antibody Response
The anti-B. burgdorferi IgG antibody response was significantly higher in the active Lyme disease cohort in comparison with healthy controls (P < .0001). Within the active Lyme disease group, the anti-B. burgdorferi IgG levels were significantly elevated in the multiple EM, early neurologic, late neurologic, and antibiotic-responsive arthritis patient subgroups (P < .001, P < .0001, P < .0001, and P < .0001, respectively), but not in the single EM subgroup, when compared with healthy controls (Figure 1A). The anti-B. burgdorferi IgG antibody levels were also significantly greater in the antibiotic-refractory arthritis group than in healthy controls (P < .0001; Figure 1A). There was no significant difference in the anti-B. burgdorferi IgG antibody response between the PTLDS and PTLDH cohorts (Figure 1B).
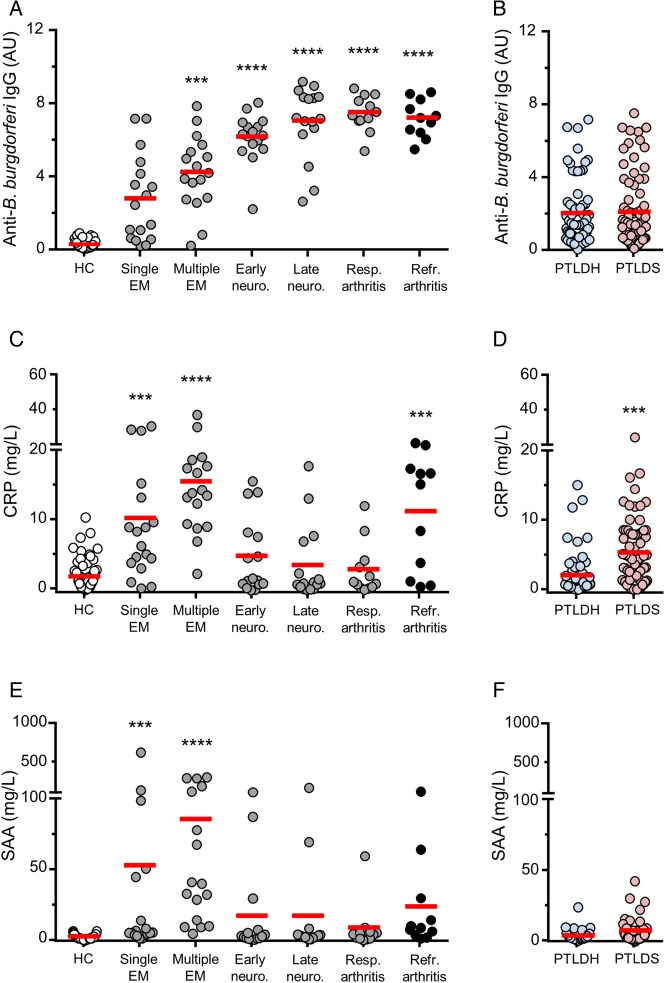
Figure 1.
Anti-Borrelia burgdorferi antibody, C-reactive protein (CRP), and serum amyloid A (SAA) responses in patients and controls. A, Immunoglobulin G (IgG) antibody reactivity to B. burgdorferi in healthy controls without a history or serologic evidence of past Lyme disease (HC) (n = 67) and in patients with objective manifestations of Lyme disease, including early to late stages of active infection (n = 79) (single erythema migrans [EM] (n = 18), multiple EM (n = 17), early neurologic (n = 16), late neurologic (n = 16), and antibiotic-responsive arthritis (n = 12)), as well as antibiotic-refractory Lyme arthritis (n = 11). In comparison with the healthy control group, the active Lyme disease cohort had significantly increased serum levels of CRP (P < .0001). Among the active Lyme disease patients, anti-B. burgdorferi antibody levels were significantly increased in each of the multiple EM (P < .001), early neurologic (P < .0001), late neurologic (P < .0001), and antibiotic-responsive arthritis (P < .0001) subgroups, but not in the single EM subgroup. IgG antibody reactivity was also significantly greater in the antibiotic-refractory Lyme arthritis group in comparison with healthy controls (P < .0001). B, IgG antibody reactivity to B. burgdorferi in post-treatment Lyme disease healthy individuals (PTLDH) (n = 68) and patients with post-treatment Lyme disease syndrome (PTLDS; n = 74). There was no significant difference between the 2 groups. C, CRP concentrations in the same cohorts of patients and controls as in panel A. In comparison with the healthy control group, the active Lyme disease cohort had significantly increased serum levels of CRP (P < .0001). Among the active Lyme disease patients, CRP levels were greater in each of the single EM (P < .001) and multiple EM (P < .0001) subgroups, but not in the early neurologic, late neurologic, or arthritis subgroups. CRP levels were significantly increased in the antibiotic-refractory Lyme arthritis group in comparison with healthy controls (P < .001). D, CRP levels in the same cohorts as in panel B. CRP concentrations were significantly higher in the PTLDS group compared with the PTLDH group (P < .001). E, SAA concentrations in the same cohorts of patients and controls as in panel A. In comparison with healthy controls, the active Lyme disease patients had significantly higher SAA levels (P < .001). Among the active Lyme disease patients, SAA levels were significantly elevated only in the single EM (P < .001) and multiple EM (P < .0001) subgroups, but not in later stages. The difference in SAA levels between the antibiotic-refractory Lyme arthritis and the healthy control groups was not significant. F, SAA concentrations in the same cohorts as in panel B. The difference between the PTLDS and PTLDH cohorts was not significant. Horizontal red bars represent the mean for each group. All P values were calculated by analysis of covariance. ***P < .001; ****P < .0001. Abbreviations: HC, healthy control; PTLDH, post-treatment Lyme disease healthy; PTLDS, post-treatment Lyme disease syndrome.
CRP Response
In comparison with healthy controls, the active Lyme disease cohort had higher serum levels of CRP, as well as higher frequency of CRP concentrations greater than both 3 and 10 mg/L (P < .0001 for each comparison). Within the active Lyme disease group, however, CRP levels and frequencies of concentrations above both cutoff values were increased only in the single EM (P < .0001 to P < .001) and multiple EM subgroups (P < .0001 for all comparisons), but not in other subgroups of active Lyme disease, when compared with healthy controls (Figure 1C, Table 2). In contrast with the antibiotic-responsive arthritis group, CRP levels and frequencies of concentrations above both cutoff values were greater in the antibiotic-refractory arthritis group than in healthy controls (P < .0001 to P < .001; Figure 1C; Table 2). Furthermore, CRP levels were significantly higher in the PTLDS cohort when compared with the PTLDH group (P < .001; Figure 1D). The frequency of CRP values above 3 mg/L, but not 10 mg/L, was also significantly greater in the PTLDS cohort when compared with the PTLDH group (P < .0001; Table 2).
Table 2.
Frequencies of Serum C-Reactive Protein Concentration Above 3 or 10 mg/L in Patient and Control Cohorts
| Participant Group | CRP > 3 mg/La | CRP > 10 mg/La |
|---|---|---|
| Healthy control (n = 67) | 13 (19%) | 1 (1%) |
| Active Lyme disease (n = 79) | 44 (56%)****b | 24 (30%)****b |
| Early localized (single EM) (n = 18) | 15 (83%)****b | 6 (33%)***b |
| Early disseminated (multiple EM) (n = 17) | 16 (94%)****b | 12 (71%)****b |
| Early neurologic (n = 16) | 6 (38%) | 3 (19%) |
| Late neurologic (n = 16) | 4 (25%) | 2 (13%) |
| Antibiotic-responsive arthritis (n = 12) | 4 (33%) | 1 (8%) |
| Antibiotic-refractory Lyme arthritis (n = 11) | 8 (73%)***b | 6 (55%)****b |
| Post-treatment Lyme disease healthy (n = 68) | 12 (18%) | 3 (4%) |
| Post-treatment Lyme disease syndrome (n = 74) | 41 (55%)****c | 11 (15%) |
Abbreviations: CRP, C-reactive protein; EM, erythema migrans.
a Frequencies are shown as number (%).
b P value is for comparison to the healthy control group, adjusted for baseline variables.
c P value is for comparison to the post-treatment Lyme disease healthy group, adjusted for baseline variables.
***P < .001; ****P < .0001.
SAA Response
In comparison with healthy controls, the active Lyme disease patients had significantly higher SAA levels (P < .001). Within the active Lyme disease cohort, SAA levels were greater only in the single EM (P < .001) and multiple EM (P < .0001) subgroups in comparison to healthy controls (Figure 1E). Differences in SAA levels between the antibiotic-refractory arthritis and healthy control groups (P = .056) or between the PTLDS and PTLDH groups (P = .061) did not reach statistical significance (Figure 1E and 1F). There was a significant correlation between CRP and SAA concentrations in the active Lyme disease (r = 0.840, P < .0001), antibiotic-refractory Lyme arthritis (r = 0.918, P < .001), and PTLDS (r = 0.369, P = .001) groups.
Relationship Between Antibody Seropositivity and Acute-Phase Reactants
CRP and SAA responses were significantly higher in individuals who were seronegative for IgG anti-B. burgdorferi antibody than seropositive individuals within the active Lyme disease group (P < .05 for each).This was driven by the high CRP and SAA values in the single and multiple EM subgroups. Such a relationship did not exist in the PTLDS group.
Leptin Levels
Because greater body fat is associated with increased CRP expression [17], we considered whether the elevated concentrations of CRP could be confounded by increased adiposity. However, there were no statistically significant differences in levels of leptin, an adipocyte hormone known to correlate strongly with measures of body fat [18], among the healthy control (mean [standard deviation], 5.0 [5.7] µg/L), active Lyme disease (8.1 [9.6] µg/L), antibiotic-refractory Lyme arthritis (6.0 [6.1] µg/L), PTLDH (6.8 [9.6] µg/L), and PTLDS (6.6 [8.5] µg/L) cohorts. The above intercohort differences in CRP and SAA levels remained at the same magnitudes of statistical significance after adjusting for leptin concentration.
DISCUSSION
We chose to examine the levels of CRP and SAA as acute-phase reactants due to their established utility and reproducibility in a large body of scientific literature, the availability of highly sensitive assays for accurate measurement in serum, and the lack of significant response to diurnal and seasonal variation or most drugs unless the underlying pathology is affected [5, 19]. The results of this study delineate the evolution of these major acute-phase reactants' expression during active infection and also establish their expected concentrations in the context of antibiotic-refractory Lyme arthritis and PTLDS.
Although we found CRP expression to be elevated in specific stages of active Lyme disease, as well as in antibiotic-refractory Lyme arthritis and PTLDS, the results are suggestive of distinct underlying mechanisms within the different manifestations. Our data indicate that the highest levels of CRP during active Lyme disease correspond to the early stage of infection, when the concentration of spirochetes is greatest in skin and/or blood [20]. The CRP response in early Lyme disease appeared to subside after the dissemination of the organism to extracutaneous sites and its clearance from blood, when a robust IgG response to B. burgdorferi has developed. This contrasts with the antibiotic-refractory Lyme arthritis and PTLDS groups in which, despite the observation of elevated CRP levels, no evidence for the presence of spirochetes in the skin or blood has been found, and no inverse relationship between CRP and antibody to B. burgdorferi was observed. Furthermore, unlike the early stage of active infection, SAA responses were not significantly elevated in antibiotic-refractory Lyme arthritis and PTLDS. Additionally, the PTLDS cohort contrasted with the active Lyme disease and refractory Lyme arthritis groups in that there was not a significantly increased rate of CRP concentrations above 10 mg/L, corroborating the observation that this condition is not associated with overt and clinically apparent inflammation.
It is important to emphasize that although CRP is a highly sensitive acute-phase reactant, it is expressed in response to many forms of infection, inflammation, and tissue injury. Therefore, our results should not be interpreted as implicating CRP in the pathogenesis of any manifestations of Lyme disease or identifying the responsible stimulus. In the case of PTLDS, our data are suggestive of increased inflammation in affected individuals but do not provide evidence to link the presumed inflammatory state to the preceding infection with B. burgdorferi. A potential limitation of this study was the lack of detailed information about lifestyle and additional comorbidities in patients and controls, which can affect the levels of acute-phase reactants.
Few studies have examined the levels of CRP or SAA in the context of Lyme disease. Two studies measured CRP concentration in Lyme arthritis patients in the context of comparison to non-Lyme arthritis, without incorporating healthy controls [21, 22], and one study examined CRP levels in 20 Lyme arthritis patients and 10 healthy volunteers [23]. All three pointed to a lack of substantially enhanced systemic CRP response in most patients with Lyme arthritis [21–23], in agreement with our results. A serum analysis in 20 patients with early neurologic Lyme disease reported a lack of increased CRP concentration in most [24], while a study of 44 Lyme disease patients with EM found both CRP and SAA to be significantly elevated in comparison to 23 healthy controls [25]. Our data confirm these findings as well.
In summary, using sera from cohorts of well-characterized patients and controls, our results document the association of increased levels of highly sensitive acute-phase reactants with specific stages and manifestations of Lyme disease. Further investigation is needed to determine whether these findings may be used to follow the course of infection, predict the outcome of patients with active Lyme disease, or monitor the response to treatment. In addition, the results offer a platform for further research to understand the underlying mechanism(s) responsible for the ongoing inflammatory processes in post-treatment manifestations of Lyme disease and devise therapies targeted at the identified patient subsets.
Notes
Acknowledgments. This work involved the use of specimens derived from a National Institutes of Health (NIH)–supported repository (contract N01-AI-65308). We are grateful to Professor Mark Pepys of University College London for insightful discussions and input during the course of this study. We thank Siu-Ping Turk, Carla Williams, Doreen Garabedian, Diane Holmgren, Donna McKenna, and Susan Bittker for their assistance with specimen collection and organization.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of NIH (grants R56AI093763 to A. A., and U54AI057158 to A. A.; principal investigator of the Center of Excellence Grant, W. I. Lipkin), the Global Lyme Alliance (A. A.), and the Intramural Research Program of NIAID (A. R. M.).
Potential conflicts of interest. G. P. W. reports receiving research grants from Immunetics, Inc., Institute for Systems Biology, Rarecyte, Inc., and Quidel Corporation. He owns equity in Abbott; has been an expert witness in malpractice cases involving Lyme disease and babesiosis; and is an unpaid board member of the American Lyme Disease Foundation. A. R. M. is a coinventor on a US patent that uses the Luciferase Immunoprecipitation Systems assay for profiling antibody responses to a panel of B. burgdorferi proteins. A. A. reports receiving grants from the NIH and the Global Lyme Alliance for research related to Lyme disease. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hu LT. In the clinic. Lyme disease. Ann Intern Med 2012; 157:ITC2–ITC-16. [DOI] [PubMed] [Google Scholar]
- 2.Wormser GP, Dattwyler RJ, Shapiro ED et al. . The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 3.Feder HM Jr, Johnson BJ, O'Connell S et al. . A critical appraisal of “chronic Lyme disease.” N Engl J Med 2007; 357:1422–30. [DOI] [PubMed] [Google Scholar]
- 4.Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep 2010; 10:13–20. [DOI] [PubMed] [Google Scholar]
- 5.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol 2008; 4:379–90. [DOI] [PubMed] [Google Scholar]
- 7.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med 2006; 119:166.e17–28. [DOI] [PubMed] [Google Scholar]
- 8.Pepys MB. CRP or not CRP? That is the question. Arterioscler Thromb Vasc Biol 2005; 25:1091–4. [DOI] [PubMed] [Google Scholar]
- 9.Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol 2012; 32:335–48. [DOI] [PubMed] [Google Scholar]
- 10.Poole S, Walker D, Gaines Das RE, Gallimore JR, Pepys MB. The first international standard for serum amyloid A protein (SAA). Evaluation in an international collaborative study. J Immunol Methods 1998; 214:1–10. [DOI] [PubMed] [Google Scholar]
- 11.Klempner MS, Hu LT, Evans J et al. . Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 2001; 345:85–92. [DOI] [PubMed] [Google Scholar]
- 12.Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep 1990; 39:1–43. [PubMed] [Google Scholar]
- 13.Ajamian M, Kosofsky BE, Wormser GP, Rajadhyaksha AM, Alaedini A. Serologic markers of Lyme disease in children with autism. JAMA 2013; 309:1771–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra A, Wormser GP, Klempner MS et al. . Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun 2010; 6:1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang KS, Klempner MS, Wormser GP, Marques A, Alaedini A. Association of immune response to endothelial cell growth factor with early disseminated and late manifestations of Lyme disease but not post-treatment Lyme disease syndrome. Clin Infect Dis 2015; 61:1703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease—United States, 1992–2006. MMWR Surveill Summ 2008; 57:1–9. [PubMed] [Google Scholar]
- 17.Pannacciulli N, Cantatore FP, Minenna A, Bellacicco M, Giorgino R, De Pergola G. C-reactive protein is independently associated with total body fat, central fat, and insulin resistance in adult women. Int J Obes Relat Metab Disord 2001; 25:1416–20. [DOI] [PubMed] [Google Scholar]
- 18.Ronnemaa T, Karonen SL, Rissanen A, Koskenvuo M, Koivisto VA. Relation between plasma leptin levels and measures of body fat in identical twins discordant for obesity. Ann Intern Med 1997; 126:26–31. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med 1999; 37:381–8. [DOI] [PubMed] [Google Scholar]
- 20.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 2005; 18:484–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson A, Mannix R, Bachur R. Acute pediatric monoarticular arthritis: distinguishing Lyme arthritis from other etiologies. Pediatrics 2009; 123:959–65. [DOI] [PubMed] [Google Scholar]
- 22.Milewski MD, Cruz AI Jr, Miller CP, Peterson AT, Smith BG. Lyme arthritis in children presenting with joint effusions. J Bone Joint Surg Am 2011; 93:252–60. [DOI] [PubMed] [Google Scholar]
- 23.Pietruczuk A, Swierzbinska R, Pancewicz S, Pietruczuk M, Hermanowska-Szpakowicz T. Serum levels of interleukin-18 (IL-18), interleukin-1beta (IL-1beta), its soluble receptor sIL-1RII and C-reactive protein (CRP) in patients with Lyme arthritis. Infection 2006; 34:158–62. [DOI] [PubMed] [Google Scholar]
- 24.Pietruczuk A, Swierzbinska R, Pietruczuk M et al. . Concentration of interleukin-18, interleukin-1beta, soluble receptor for interleukin-1 (sIL-1RII) and C-reactive protein in patients with neuroborreliosis. Neurol Neurochir Pol 2005; 39:33–9. [PubMed] [Google Scholar]
- 25.Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, Aucott JN. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS One 2014; 9:e93243. [DOI] [PMC free article] [PubMed] [Google Scholar]