Authors investigated the effect of subclinical cytomegalovirus (CMV) and Epstein-Barr virus replication on the CD4/CD8 ratio when antiretroviral therapy is started during early infection. Findings suggest that CMV may represent an attractive target for therapeutic intervention.
Keywords: cytomegalovirus, Epstein-Barr virus, CD4/CD8 ratio, ART, HIV
Abstract
Background. A low CD4/CD8 ratio in human immunodeficiency virus (HIV)–infected individuals is associated with inflammation and higher risk of non-AIDS morbidity and mortality. In this study, we investigated the effect of subclinical cytomegalovirus (CMV) and Epstein-Barr virus (EBV) replication on CD4+ and CD8+ T-cell dynamics when antiretroviral therapy (ART) is started during early infection.
Methods. We investigated 604 peripheral blood mononuclear cell samples from 108 CMV- and EBV-seropositive HIV-infected men who have sex with men, who started ART within a median of 4 months from their estimated date of infection and were followed for a median of 29.1 months thereafter. Levels of CMV and EBV DNA were measured at each timepoint. Mixed-effects asymptotic regression models were applied to characterize CD4+ and CD8+ T-cell dynamics, and Bayesian hierarchical models were used to quantify individual differences in CMV and EBV DNA replication.
Results. Higher levels of subclinical CMV replication were associated with lower predicted maximum levels of CD4/CD8 ratio (P < .05), which was driven by higher levels of CD8+ T-cell counts (P < .05), without affecting CD4+ T-cell counts (P > .1). Age was negatively associated with CD4/CD8 levels (P < .05), and this effect was independent of the CMV association (P < .05 for both CMV and age in a multivariate model).
Conclusions. Subclinical CMV replication in blood cells during early HIV infection and younger age were associated with lower CD4/CD8 ratios during suppressive ART. These findings suggest that active CMV infection in the setting of treated HIV may represent an attractive potential target for therapeutic intervention.
Antiretroviral therapy (ART) improves health, prolongs survival, and reduces human immunodeficiency virus (HIV) transmission [1]. Nevertheless, ART-treated individuals living with HIV have greater non-AIDS morbidity and mortality than the general population [2]. Increased morbidity and mortality are associated with immune dysfunction, which persists in some individuals despite suppressive ART [3].
Early in the HIV epidemic, it was recognized that a hallmark of immunosuppression during HIV infection was the loss of CD4+ T cells and an inverted ratio between CD4+ and CD8+ T cells [4]. Even in the setting of normal CD4+ counts among treated HIV-infected persons, low CD4/CD8 is associated with poor clinical outcomes [5, 6], T-cell dysfunction [6–8], and increased inflammation (eg, interleukin 6, C-reactive protein, soluble CD14) [6, 9]. These data suggest that CD8+ T-cell expansion might be a driver of increased morbidity and mortality [6, 10].
Similar to HIV, human cytomegalovirus (CMV) is a persistent pathogen associated with inflammation and increased morbidity [11, 12]. HIV-infected individuals are almost all coinfected with CMV, and in the setting of HIV, most individuals experience intermittent bursts of CMV replication during ART [11]. These bursts of replication are linked to persistent stimulation of the CD8+ T-cell population [13, 14]. Along these lines, positive CMV serology among persons with chronic HIV infection is associated with lower CD4/CD8 ratio during ART [7]. A recent study found elevated CD8+ T cells and low CD4/CD8 only in individuals coinfected with both viruses, but not in persons infected with HIV or CMV alone [13]. HIV-infected individuals who are seronegative for CMV show better immune recovery following ART initiation than do HIV-infected persons coinfected with CMV [15]. In persons with HIV and CMV coinfection, the presence of detectable CMV replication is associated with increased activation, proliferation, and exhaustion of T cells [16, 17], but the effect of subclinical CMV replication on CD4/CD8 dynamics after early initiation of ART is unknown. Similarly to CMV, Epstein-Barr virus (EBV) is another herpesvirus that establishes lifelong infection, has almost universal prevalence among HIV-infected people, and has recurrent reactivation; EBV coinfection has not been associated with lower CD4/CD8 ratio. Testing both viruses will help us to evaluate if observed effects are unique to CMV or are a generalized effect of persistent viral infections.
METHODS
Participants, Samples, and Clinical Laboratory Tests
Six hundred four blood samples were collected longitudinally from 108 recently HIV-infected participants from the San Diego Primary Infection Resource Consortium [18] between October 1996 and October 2012. All participants were CMV and EBV seropositive. The estimated date of infection (EDI) for HIV was calculated using established algorithms [19]. Stored samples were selected retrospectively from participants who started ART and achieved complete suppression of HIV RNA (defined as <50 or <200 copies/mL depending on assay sensitivity) during study follow-up. Most participants (81%) started ART within the first year of HIV infection. Participants maintained undetectable HIV RNA levels in blood plasma for a median of 28.6 months. The median number of HIV RNA measurements during the follow-up period was 4 (interquartile range [IQR], 1–7). Longitudinal blood samples (N = 511) were collected during ART at 6-month intervals. Pre-ART samples were available for 93 participants. Percentage and absolute counts of CD4+ T-lymphocytes were measured by flow cytometry (VA Flow Lab), and HIV RNA was quantified by Amplicor HIV Monitor Test (Roche Molecular Systems).
All participants provided written informed consent. No children were included in this study. The Office of Human Research Protections Program of the University of California approved the study.
Levels of CMV and EBV DNA in Peripheral Blood Mononuclear Cells
DNA was extracted from 5 million peripheral blood mononuclear cells (PBMCs) for each timepoint using the AllPrep DNA/RNA Mini Kit (Qiagen). Total CMV and EBV DNA was quantified by droplet digital polymerase chain reaction (PCR) as previously described [20, 21]. Copy numbers were calculated as the mean of replicate PCR measurements and normalized to 1 million cells, determined by RPP30 [22].
Statistical Analysis
To model nonlinear patterns of changes in the outcomes, we applied an asymptotic regression model with random effects, which is expressed as:
where Asym, Y0, and LRC are model parameters that represent the asymptote (ie, the predicted maximum level of CD4/CD8 ratio), the baseline value, and the natural logarithm of the rate of change constant, respectively. Yit is the observation for subject i at time t (ie, days since the onset of ART); u1i, u2i, and u3i represent random effects for subject I for Asym, Y0, and LRC, respectively. εit represents residuals. The random effects were allowed to be correlated, and their contributions to model fit were evaluated with likelihood ratio tests. To examine the influence of 1 or more predictors on the pattern of change, a fixed effect for the predictor(s) was added to each of the 3 parameters.
After identifying the best-fitting model for each outcome, we included a predictor or a covariate in an asymptotic regression model to examine the association of the predictor with the parameter change. We used 2 predictors (CMV and EBV DNA) and 3 covariates (age, peak HIV RNA, and time from EDI to ART initiation). To model participants' propensities to shed CMV, we used Bayesian hierarchical regression models [23]. Hierarchical models treat the participants as being selected from a population of HIV patients, and estimate the properties of the population and the participants at the same time. Specifically, hierarchical models estimate a single global intercept, then the varying intercepts for the participants as deviations from the global intercept. We used the negative binomial family with log link, as 65% (n = 339) of the CMV counts were undetectable (ie, 0). For sensitivity analysis, CMV measurements were also dichotomized as “detectable” or “undetectable,” and the binomial family with the logit link was used. Bayesian hierarchical models were applied to EBV counts using the negative binomial family with the log link (and the binomial family with the logit link for sensitivity analysis). For both variables a weakly informative prior distribution of N(0, 4) was used. Hierarchical models were evaluated using with R-hat values for model convergence (approximately 1.0 = convergence) and the leave-one-out information criterion (LOOIC) for model fit. Because peak HIV RNA and time from EDI to ART initiation were highly skewed, we log-transformed them before inclusion in an asymptotic regression model. Time over 1000 days was removed from analysis to improve distribution of the data (n = 72 [13%]). Whether a predictor or a covariate influenced a particular parameter was tested with a likelihood ratio test against a model without that parameter.
To examine robustness of the results, we repeated our asymptotic regression analysis as follows: (1) Outliers were removed before Bayesian hierarchical modeling (n = 1 for CMV and n = 2 for EBV); and (2) the binomial family with the logit link was applied to dichotomized data. Furthermore, the following sensitivity analyses were applied to all predictors and covariates: (1) removing an individual who produced residuals >6 standard deviations (SDs) away from the mean in CD8+ and the CD4/CD8 ratio; (2) weighing by the number of observations for each participant; and (3) weighing by 1/variance of the outcome for each participant. Data are expressed as coefficient ± 1 standard error, unless noted otherwise, and all tests were performed at the 5% significance level (2-tailed).
RESULTS
Participants, Samples, and Clinical Laboratory Tests
All 108 study participants were HIV-infected men who have sex with men (MSM) with recent HIV infection, who were followed for up to 135 months (median, 29.1 [IQR, 10.4–54.8] months). The median time from EDI to the initiation of ART was 4 months (IQR, 1–5 months), and half of our participants achieved viral suppression within 4.0 months (IQR, 0–6.8 months). Most were white, non-Hispanic (64.8%), and 27% were infected <70 days before study enrollment. At baseline (ie, first available timepoint at or shortly following ART initiation), participants were a median of 35 years old and had a median CD4+ and CD8+ count of 636 and 749 cells/µL, respectively; the median CD4/CD8 ratio was 0.8. They had a median of 3.2 log HIV RNA copies/mL and median CMV and EBV DNA counts of 0 (IQR, 0–3.6) and 26 (IQR, 5.4–79.6) copies per million cells, respectively. Clinical characteristics are summarized in Table 1.
Table 1.
Characteristic at First Sampled Timepoint (Post–Antiretroviral Therapy)
| Characteristic | Total (N = 108) |
|---|---|
| MSM, No. (%) | 108 (100) |
| Race/ethnicity, No. (%), (n = 107) | |
| White, non-Hispanic | 70 (64.8) |
| Hispanic | 22 (20.4) |
| Other | 15 (13.9) |
| Missing | 1 (0.9) |
| Stage of HIV infection, No. (%) | |
| Acute infection (≤70 d) | 32 (26.9) |
| Early infection (>70 d) | 76 (70.4) |
| CD4+ cell count, cells/µL, median (IQR) | 636.00 (487.50–821.00) |
| CD8+ cell count, cells/µL, median (IQR) | 748.84 (608.91–1055.84) |
| CD4:CD8 ratio, median (IQR) | 0.80 (0.55–1.05) |
| Age, y, median (IQR) | 35.00 (28.00–42.00) |
| Peak HIV RNA, log copies/mL, median (IQR) | 13.03 (11.70–14.69) |
| Months from EDI to ART start, median (IQR) | 3.00 (2.00–7.75) |
| CMV DNA, copies/µL, median (IQR) | 0.00 (0.00–3.57) |
| EBV DNA, copies/µL, median (IQR) | 26.00 (5.41–79.58) |
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; EBV, Epstein-Barr virus; EDI, estimated duration of infection; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men.
Characteristics of CD4+, CD8+, and CD4/CD8 Ratio Temporal Trajectories After the Initiation of ART
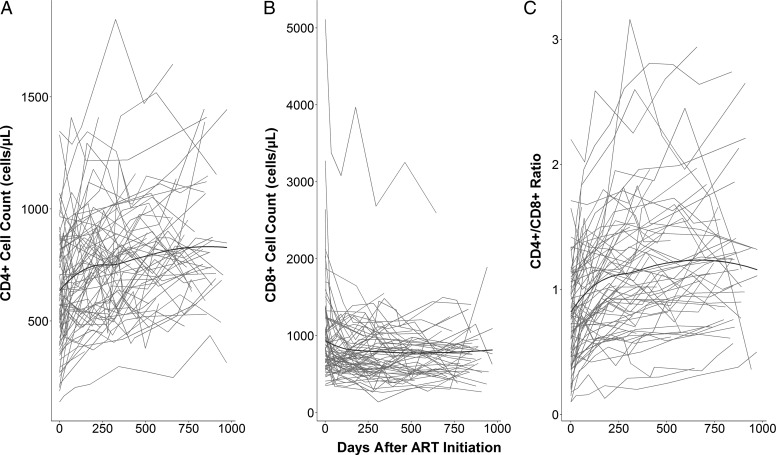
The CD4+ counts on average were 640 cells/μL at ART initiation, then gradually increased at a slowing rate of increase (Figure 1A). Mean CD8+ cell counts were 750 cells/μL at baseline and decreased over time and reached the asymptotic value within 250 days after ART initiation (Figure 1B). The CD4/CD8 ratio rapidly increased after the initiation of ART, then the rate of change slowed down before 250 days (Figure 1C). To model these patterns, we applied asymptotic regression models with random intercepts (Table 2). For all models, including random intercepts for Asym and Y0 and their covariation significantly improved model fit (P < .001) but not for LRC (P > .05). The model fit to CD4+ indicated that participants on average had a baseline value of 642 ± 23 and reached an asymptote of 834 ± 30 at a rate of 99.4% per day (99.4 = exp(−exp(−5.2))*100). These values indicate that participants would reach CD4+ cell counts of 800 in 284 days post-ART initiation. Standard deviations for Asym and Y0 random effects were 223 and 204, with a correlation of 0.92, suggesting that participants with higher baseline tended to a higher asymptote. Participants had a higher baseline CD8+ value of 933 cells/μL than their asymptotic value of 746 cells/μL, and their CD8+ cell counts changed at a rate of 94.6% per day. That is, it took only 23 days to reach cell counts of 800 cells/μL. Standard deviations for Asym and Y0 random effects were 329 cells/μL and 576 cells/μL, with a correlation of 0.81. Finally, for the ratio of CD4/CD8, participants were estimated to have baseline and asymptotic values of 0.83 and 1.25, and the ratio changed at a rate of 99.0% a day. On average, participants reached a ratio of 1.0 within 57 days, while their SDs for random Asym and Y0 effects were 0.49 and 0.35, respectively, with a correlation of 0.82.
Figure 1.
Individual trajectories of CD4+ cell counts (A), CD8+ cell counts (B), and the ratio of CD4+ to CD8+ (C) over time. The black line represents the local regression curve fitted to each outcome. Abbreviation: ART, antiretroviral therapy.
Table 2.
Predictors of Changes in CD4+, CD8+, and CD4+/CD8+ Ratio
| CD4+ |
CD8+ |
CD4+/CD8+ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Asymptote | Y0 | LRC | Asymptote | Y0 | LRC | Asymptote | Y0 | LRC | |
| Global Intercept | 834.45 ± 29.93*** | 641.6 ± 22.87*** | −5.24 ± 0.27*** | 746.29 ± 36.35*** | 933.47 ± 59.07*** | −2.90 ± 0.23*** | 1.25 ± 0.06*** | 0.83 ± 0.04*** | −4.57 ± 0.18*** |
| Random effects (σ) | 223*** | 204*** | NS | 329*** | 576*** | NS | 0.49*** | 0.35*** | NS |
| Predictors | |||||||||
| CMV Intercept | −24.3 ± 30.76 | −40.42 ± 28.36 | 0.37 ± 0.34** | 91.73 ± 40.45* | 72.43 ± 73.58 | −0.12 ± 0.23 | −0.14 ± 0.06* | −0.11 ± 0.05* | 0.48 ± 0.23* |
| EBV Intercept | NC | 66.44 ± 37.17+ | 133.54 ± 62.34* | −0.65 ± 0.24+ | −0.07 ± 0.06 | −0.09 ± 0.04* | 0.12 ± 0.16 | ||
| Age (year) | 17.68 ± 30.23 | 4.36 ± 23.81 | 0.42 ± 0.27 | −42.06 ± 37.56 | −61.28 ± 61.24 | 0.22 ± 0.53 | 0.13 ± 0.06* | 0.06 ± 0.04 | 0.18 ± 0.2 |
| Peak viral load (log RNA count) | −7.53 ± 32.49 | 7.49 ± 23.61 | 0.44 ± 0.27 | 53.72 ± 38.79 | 168.74 ± 59.15** | 1.12 ± 0.34 | 0.00 ± 0.06 | −0.08 ± 0.04+ | 0.22 ± 0.21 |
| Timing of ART (log month) | −46.99 ± 24.92 | −52.79 ± 24.54+ | 0.01 ± 0.26 | −44.38 ± 52.97 | −118.32 ± 54.72* | −1.91 ± 0.44** | −0.02 ± 0.06 | 0.01 ± 0.04 | −0.22 ± 0.23 |
N = 108, timepoint = 440. Data represent coefficients ±1 standard error for fixed effects and ±standard deviation for random effects. Predictors were standardized.
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; EBV, Epstein-Barr virus; LRC, natural logarithm of the rate constant; NC, model not converged; NS, not significant; Y0, baseline.
Not significant: P > .1 + P < .1; *P < .05; **P < .01; ***P < .001.
Model Results of CMV and EBV DNA Counts
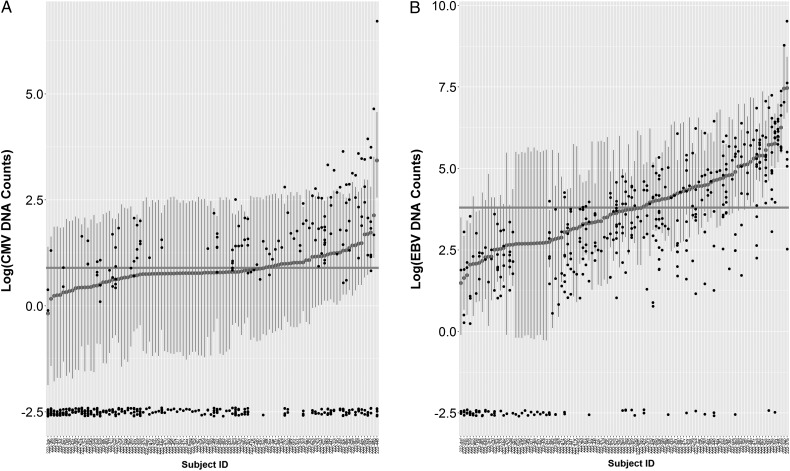
CMV and EBV DNA were detectable in 33% and 81%, respectively, of the PBMC samples; 63.0% and 88.9% of the study participants had at least 1 time point with detectable CMV and EBV, respectively, during follow-up. Both variables were analyzed with Bayesian hierarchical regression models with the negative binomial family with random intercepts. The models fit the data well: all R-hat values = 1.0, a lower LOOIC value than an alternative model with the Poisson family. For CMV, the intercepts ranged from −0.2 to 3.4 around the global intercept of 0.9 (Figure 2A), whereas for EBV the range of the random intercepts was 1.6–6.2 and the global intercept was estimated to be 3.8 (Figure 2B).
Figure 2.
Posterior medians and 95% credible intervals (CIs) for cytomegalovirus (CMV; A) and Epstein-Barr virus (EBV; B) DNA counts, derived from Bayesian hierarchical regression models. Small black dots indicate observed data. Large gray dots, vertical gray lines, and the horizontal gray line indicate estimated random intercepts for participants, 95% CIs, and the global intercept, respectively. Observed values are jittered to avoid overlap.
Associations of Predictors and Covariates With Changes in CD4+, CD8+, and CD4/CD8 T-Cell Dynamics
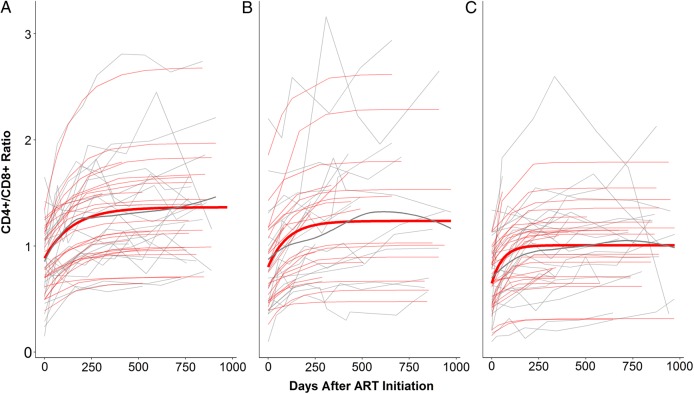
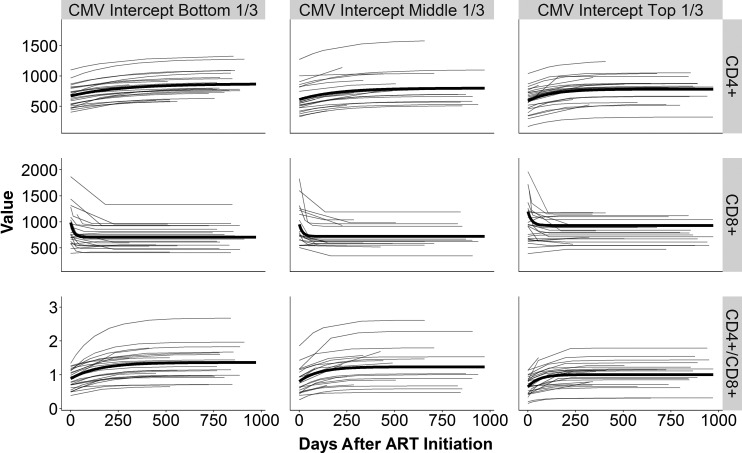
We included 2 predictors (CMV and EBV random intercepts) and 3 covariates (age, peak viral load, and timing of ART initiation) in the mixed-effects asymptotic regression model (Table 2). For CD4+ counts, each increase in the CMV intercept by 1 SD was associated with a faster rate of increase (P < .01; eg, 100 − exp(−exp(−5.24 + 0.37))/exp(−exp(−5.24))*100 = 0.2%), while the baseline and asymptotic values were unaffected (P > .1). For CD8+, CMV intercept levels were positively associated with asymptotic values (P < .05) but not with baseline values or rates of change (P > .1). Increased CMV levels were associated with lower baseline and asymptote values and with a faster rates of change in the CD4/CD8 ratio (P < .05). To visualize these negative effects of CMV on the ratio, we categorized participants into 3 groups by their CMV intercept levels. The bottom third group reached a higher level of the CD4/CD8 ratio over time than the other 2 groups or the global intercept (Figure 3C), while the top third group had a lower asymptote than the global intercept (Figure 3A). The asymptote of the middle third group roughly matched the intercept (Figure 3B). Figure 4 shows the CD4+ and CD8+ T-cell trajectory individually (top and middle row) related to the CD4/CD8 ratio (lower row). When the EBV intercept was included in a model for CD4+, the model did not reach convergence and was unable to produce results. Yet, increased EBV intercept levels were associated with higher baseline CD8+ cells (P < .05), while their associations with asymptote and the rate of change were not significant (P > .05). Reflecting the positive effect of the EBV intercept on CD8+ baseline, EBV intercept levels were negatively associated with CD4/CD8 baseline (P < .05), but not with asymptote of the rate of change (P > .1). Age was associated with the asymptote of CD4/CD8 (P < .05). Specifically, participants older by an SD from the mean were predicted to have a higher CD4/CD8 ratio at asymptote by 0.13. Age was not associated with any other parameters (P > .1). One SD increase in log of peak viral load from the mean was associated with higher baseline CD8+ cell counts by 169 (P < .01). Peak viral load was not associated with any other parameters (P > .05). The log of the timing of ART initiation was associated with CD8+ baseline and the rate of change (P < .05). A delay in ART initiation by 1 SD led to a lower CD8+ baseline value by 118 cells/μL and a slower rate of change. The timing of ART was not associated with any other variable (P > .05).
Figure 3.
Model-predicted trajectories of the CD4+/CD8+ ratio by cytomegalovirus (CMV) intercept level: bottom one-third (A), middle one-third (B), and top one-third (C). Light gray lines indicate individual participants. Thin and thick red lines indicate predicted trajectories for individuals and CMV groups, respectively. Thick gray line represents the local regression curve fitted to each group. Abbreviation: ART, antiretroviral therapy.
Figure 4.
Model-predicted trajectories for CD4+ (top row), CD8+ (middle row), and CD4/CD8 ratio (bottom row) by cytomegalovirus (CMV) intercept. Thin light gray lines indicate projected trajectories for each individual participant. Thick black lines show average estimates for each combination of the CMV groups and the outcomes. Abbreviation: ART, antiretroviral therapy.
Because both CMV intercepts and age affected the CD4/CD8 ratio, we included both variables in a multivariate model. The result remained the same; CMV still significantly affected all aspects of change (P < .05), while age's effect on asymptote remained significant (P < .05). These independent effects of CMV and age may reflect the fact that these variables were uncorrelated (Pearson r = −0.01).
We evaluated how robust the effects mentioned above were by fitting alternative models. We define robust effects as having P < .05 in 60% and P < .1 in 80% of the converged models (Table 3). The CMV effects on CD8+ asymptote and CD4+/CD8+ asymptote and baseline were robust, while the EBV effects on CD8+ and CD4+/CD8+ baseline emerged robust as well. Among the 3 covariates, the age effect on CD4+CD8+ asymptote, the peak viral load effect on CD8+ baseline, and the timing of the ART initiation on CD8+ baseline and the rate of change emerged as robust.
Table 3.
Summary of Sensitivity Analyses
| Predictor | Outcome | No. of Models |
No. (%) of Converged Models P < .05 (P < .1) |
|||
|---|---|---|---|---|---|---|
| Tested | Converged | Asymptote | Y0 | LRC | ||
| CMV intercept | CD4 | 6 | 4 | 0 | 0 | 50 (75) |
| CD8 | 6 | 5 | 60 (80) | 0 | 0 | |
| CD4/CD8 | 6 | 6 | 67 (83) | 67 (100) | 33 (50) | |
| EBV intercept | CD4 | 6 | 0 | NA | NA | NA |
| CD8 | 6 | 5 | 0 (80) | 80 (80) | 40 (80) | |
| CD4/CD8 | 6 | 6 | 0 | 67 (83) | 0 | |
| Age | CD4 | 4 | 2 | 0 | 0 | 0 |
| CD8 | 4 | 4 | 0 | 0 | 0 | |
| CD4/CD8 | 4 | 4 | 100 (100) | 25 (50) | 0 (25) | |
| Peak viral load (log) | CD4 | 4 | 3 | 0 | 0 | 0 |
| CD8 | 4 | 3 | 0 | 100 (100) | 33 (33) | |
| CD4/CD8 | 4 | 4 | 0 | 25 (75) | 0 | |
| Timing of ART in month (log + 1) | CD4 | 4 | 1 | 0 | 0 (100) | 0 |
| CD8 | 4 | 3 | 0 | 67 (100) | 67 (100) | |
| CD4/CD8 | 4 | 4 | 0 | 0 | 0 | |
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; EBV, Epstein-Barr virus; LRC, natural logarithm of the rate constant; NA, not available; Y0, baseline.
DISCUSSION
To better understand the role of asymptomatic CMV and EBV replication in blood cells on CD4/CD8 recovery, we investigated a well-characterized cohort of HIV/CMV/EBV-coinfected MSM who started ART during the early phases of HIV infection and sustained stable HIV RNA suppression. Levels of CMV measured in peripheral blood cells were negatively associated with lower CD4/CD8 ratio at baseline and with lower predicted maximum CD4/CD8 ratio during ART. When looking at CD4+ and CD8+ T cells individually, levels of CMV DNA were not associated with CD4+ T-cell counts at baseline nor with rate of change over time. On the other hand, higher levels of CMV DNA were positively associated with higher CD8+ T-cell levels over time. This suggests that the negative effect of CMV on the CD4/CD8 ratio is mainly driven by an expansion of CD8+ T cells rather than slower recovery of CD4+ T cells. As a comparison, levels of EBV DNA were associated with higher CD8+ T cells and lower CD4/CD8 at baseline, but did not affect the rate of CD4/CD8 recovery over time.
The only other factor associated with better CD4/CD8 recovery was older age. Older participants were predicted to have a higher CD4/CD8 ratio during ART. This is inconsistent with previous reports, which suggested that older HIV-infected people have a worse immune recovery [24, 25]. This analysis was biased by the narrow age range in our cohort (only 9 people aged >50 years), which might have affected our ability to determine the effect of older age on CD4/CD8 ratio.
Because both CMV and age affected the CD4/CD8 ratio, we included both variables in a multivariate model, and both factors remained significantly associated with CD4/CD8 during ART (P < .05).
Higher peak viral load and earlier initiation of ART after infection were associated with higher CD8+ T cells at baseline but not with CD4/CD8 ratio during ART. Previous studies reported that earlier ART initiation was associated with normalization of CD8+ counts and better CD4/CD8 recovery [26, 27]. Almost all of our participants started ART within the first year of HIV infection, and we did not include any chronically infected individuals. Our power to detect an effect of early ART was limited. One possible explanation for the inverse association between shorter time of ART initiation and higher CD8+ T cells might be that people with higher CD8+ T cells present more symptomatic primary HIV infection and, consequently, earlier diagnosis and treatment.
In summary, persistently low levels of CMV replication could contribute to incomplete recovery of CD4/CD8 ratio in people who started ART during the earliest phases of HIV infection. This is important as incomplete recovery of CD4/CD8 ratio is associated with increased mortality and morbidity during HIV infection [8].
This study had a number of limitations. Because this was an observational study, we cannot establish a causal relationship between CMV and T-cell dynamics, and the extent of immune activation during HIV infection could be a determinant of CMV shedding and CD8+ T-cell proliferation. Our data are consistent with previous studies showing that CMV drives CD8+ T-cell inflation [14]. As many as half of all CD8+ T cells can be CMV reactive [28–30], and the percentage of CD8+ T cells specific for CMV antigens is further increased in HIV-infected persons [31–34]. Although the precise mechanism connecting CMV and CD8+ T-cell expansion is unclear, administration of the anti-CMV drug valganciclovir to HIV-infected patients with incomplete CD4+ T-cell recovery reduced CD8+ T-cell activation [35], suggesting that reactivation of CMV could be a contributor of T-cell activation.
This study provides insights regarding connections between asymptomatic CMV replication and CD4/CD8 dynamics during the earliest phase of HIV infection. Even among individuals who started ART early, asymptomatic CMV reactivation is associated with slower CD4/CD8 recovery. This effect seemed unique to CMV as levels of EBV were not associated with any difference in the CD4/CD8 ratio. Carefully designed clinical trials targeting CMV will be crucial to understand the complex relationships between CMV and HIV pathogenesis and immune response and to direct the design of clinical strategies that will have a positive effect on HIV disease progression and aging-related complications.
Notes
Acknowledgments. We are grateful to all the participants in the San Diego Primary Infection cohort, the Center for AIDS Research Genomic, Translational Virology and Flow Cytometry Cores. Primer and probe for quantification of herpesviruses as well as the plasmids and quantification standards were kindly provided by Fred Lakeman and Rich Whitley.
Author contributions. D. M. S. participated in the study design and data analysis, and wrote the primary version of the manuscript; M. N. performed the primary data analysis and wrote the primary version of the manuscript; M. L. F. participated in the study design and revised the manuscript; C. M. A. contributed to the study design and data analysis and revised the manuscript; M. F. O. performed laboratory experiments and revised the manuscript; S. J. L. performed laboratory experiments, participated in the study design, enrolled all participants, and revised the manuscript. S. G. participated in the study design, participated in the data analyses, and wrote the primary version of the manuscript. All authors read and approved the final manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported primarily by a grant from the National Institutes of Health (NIH), University of California, San Francisco–Gladstone Institute of Virology and Immunology Center for AIDS Research, P30-AI027763 (Creative and Novel Ideas in HIV Research [CNIHR]), California HIV Research Program IDEA award to S. G., by the department of Veterans Affairs, the James B. Pendleton Charitable Trust, and additional grants from the NIH (grant numbers AI100665, MH100974, MH097520, DA034978, AI007384, AI027763, AI106039, AI43638, AI074621, AI036214, MH101012, UL1TR000100, CARE U19 AI096113, MH062512, MH107345, and AI068636-09). M. F. O. was supported by the CNPq-Brazil postdoctoral fellowship.
Potential conflicts of interest. D. M. S. has received grant support from ViiV Pharmaceuticals and consultant fees from GenProbe and Testing Talent Services. S. J. L. has received grant support from Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–6. [DOI] [PubMed] [Google Scholar]
- 4.Taylor JM, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquir Immune Defic Syndr 1989; 2:114–24. [PubMed] [Google Scholar]
- 5.Serrano-Villar S, Gutierrez C, Vallejo A et al. . The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect 2013; 66:57–66. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Villar S, Sainz T, Lee SA et al. . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caby F, Guihot A, Lambert-Niclot S et al. . Determinants of a low CD4/CD8 ratio in HIV-1-Infected individuals despite long-term viral suppression. Clin Infect Dis 2016; 62:1297–303. [DOI] [PubMed] [Google Scholar]
- 8.Sainz T, Serrano-Villar S, Diaz L et al. . The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013; 27:1513–6. [DOI] [PubMed] [Google Scholar]
- 9.Bastard JP, Soulie C, Fellahi S et al. . Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther 2012; 17:915–9. [DOI] [PubMed] [Google Scholar]
- 10.Lee SA, Sinclair E, Jain V et al. . Low proportions of CD28- CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis 2014; 210:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianella S, Massanella M, Wertheim JO, Smith DM. The sordid affair between human herpesvirus and HIV. J Infect Dis 2015; 212:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman ML, Lederman MM, Gianella S. Partners in crime: the role of CMV in immune dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep 2016; 13:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman ML, Mudd JC, Shive CL et al. . CD8 T cell expansion and inflammation linked to CMV co-infection in ART-treated HIV infection. Clin Infect Dis 2015; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16:367–77. [DOI] [PubMed] [Google Scholar]
- 15.Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS 2014; 28:2045–9. [DOI] [PubMed] [Google Scholar]
- 16.Dan J, Massanella M, Spina C et al. . Effect of CMV and HIV replication on T cell exhaustion and senescence during ART. J Acquir Immune Defic Syndr 2016; 72:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianella S, Massanella M, Richman DD et al. . Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little SJ, Frost SD, Wong JK et al. . Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J Virol 2008; 82:5510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le T, Wright EJ, Smith DM et al. . Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strain MC, Lada SM, Luong T et al. . Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 2013; 8:e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianella S, Anderson CM, Var SR et al. . Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol 2016; 90:3944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massanella M, Gianella S, Lada SM, Richman DD, Strain MC. Quantification of total and 2-LTR (long terminal repeat) HIV DNA, HIV RNA and herpesvirus DNA in PBMCs. 2015. Bio Protoc Available at: http://www.bio-protocol.org/e1492. [DOI] [PMC free article] [PubMed]
- 23.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. 3rd ed London: Chapman and Hall/CRC, 2013. [Google Scholar]
- 24.Douek DC, McFarland RD, Keiser PH et al. . Changes in thymic function with age and during the treatment of HIV infection. Nature 1998; 396:690–5. [DOI] [PubMed] [Google Scholar]
- 25.Lederman MM, McKinnis R, Kelleher D et al. . Cellular restoration in HIV infected persons treated with abacavir and a protease inhibitor: age inversely predicts naive CD4 cell count increase. AIDS 2000; 14:2635–42. [DOI] [PubMed] [Google Scholar]
- 26.Cao W, Mehraj V, Trottier B et al. . Early initiation rather than prolonged duration of antiretroviral therapy in HIV infection contributes to the normalization of CD8 T-cell counts. Clin Infect Dis 2016; 62:250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornhill J, Inshaw J, Oomeer S et al. . Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc 2014; 17:19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan N, Shariff N, Cobbold M et al. . Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 2002; 169:1984–92. [DOI] [PubMed] [Google Scholar]
- 29.Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Human Immunol 2004; 65:456–64. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang Q, Wagner WM, Wikby A et al. . Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol 2003; 23:247–57. [DOI] [PubMed] [Google Scholar]
- 31.Naeger DM, Martin JN, Sinclair E et al. . Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsue PY, Hunt PW, Sinclair E et al. . Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006; 20:2275–83. [DOI] [PubMed] [Google Scholar]
- 33.Stone SF, Price P, Khan N, Moss PA, French MA. HIV patients on antiretroviral therapy have high frequencies of CD8 T cells specific for immediate early protein-1 of cytomegalovirus. AIDS 2005; 19:555–62. [DOI] [PubMed] [Google Scholar]
- 34.Stone SF, Price P, French MA. Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J Antimicrob Chemother 2006; 57:585–8. [DOI] [PubMed] [Google Scholar]
- 35.Hunt PW, Martin JN, Sinclair E et al. . Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]