Abstract
Vascularization of bone defects is considered a crucial component to the successful regeneration of large bone defects. Although vascular endothelial growth factor (VEGF) has been delivered to critical-size bone defect models to augment blood vessel infiltration into the defect area, its potential to increase bone repair remains ambiguous. In this study, we investigated whether integrin-specific biomaterials modulate the effects of VEGF on bone regeneration. We engineered protease-degradable, VEGF-loaded polyethylene glycol (PEG) hydrogels functionalized with either a triple-helical, α2β1 integrin-specific peptide (GFOGER) or an αvβ3 integrin-targeting peptide (RGD). Covalent incorporation of VEGF into the PEG hydrogel allowed for protease degradation-dependent release of the protein while maintaining VEGF bioactivity. When applied to critical-size segmental defects in the murine radius, GFOGER-functionalized VEGF-free hydrogels exhibited significantly increased vascular volume and density and resulted in a larger number of thicker blood vessels compared to RGD-functionalized VEGF-free hydrogels. VEGF-loaded RGD hydrogels increased vascularization compared to VEGF-free RGD hydrogels, but the levels of vascularization for these VEGF-containing RGD hydrogels were similar to those of VEGF-free GFOGER hydrogels. VEGF transiently increased bone regeneration in RGD hydrogels but had no effect at later time points. In GFOGER hydrogels, VEGF did not show an effect on bone regeneration. However, VEGF-free GFOGER hydrogels resulted in increased bone regeneration compared to VEGF-free RGD hydrogels. These findings demonstrate the importance of integrin-specificity in engineering constructs for vascularization and associated bone regeneration.
Keywords: vascularization, cell adhesion, mesenchymal stem cells
1. Introduction
Vascularization is a crucial factor in bone development as well as the repair of bone defects 1-3. In the developing skeleton, long bones are formed through endochondral ossification which involves the invasion and sprouting of blood vessels into the intermediate cartilage tissue followed by osteoprogenitor cell migration and mineralization of the cartilaginous anlage 4. Blocking infiltration of blood vessels into cartilage causes enlarged hypertrophic zones associated with incomplete and delayed onset of ossification and suboptimal bone formation 5. In terms of bone repair, large bone defects arising from trauma or cancer resection suffer from poor vascularization and impaired healing 6. Co-induction of a tibial fracture and vascular injury in the form of hind limb ischemia in a mouse model increases the chances of a non-union compared to the fracture alone 7. Anti-angiogenic treatment to inhibit the initial revascularization response following a critical-size segmental defect also results in lower levels of bone formation and a higher prevalence of non-union 8. Although current gold-standard of autografts and allografts are extensively used in the clinic, these constructs are significantly limited by donor-site morbidity, supply, bioactivity and risk of infection 9. Additionally, the revascularization of these grafts remains limited without micro-surgical procedures which often results in high degrees of local tissue morbidity 10,11. Therefore, incorporating cues to augment the vascularization response can greatly enhance the efficacy of these treatments while diminishing their limitations.
Vascular endothelial growth factor (VEGF) has routinely been delivered to increase vascularization in vivo. By interacting with two main tyrosine kinase receptors, VEGFR1 and VEGFR2, VEGF destabilizes existing blood vessel walls and allows endothelial cells to proliferate and migrate in the direction of highest VEGF concentration 12. VEGF is instrumental in bone development as blocking VEGF activity results in reduced angiogenesis, massive chondrocyte death and severely under-developed bone 13,14. However, in terms of bone repair, the efficacy of exogenous VEGF in increasing vascularization and associated bone regeneration remains ambiguous. One of the first studies utilizing VEGF for bone treatment demonstrated that continuous delivery of VEGF to a rabbit critical-size defect through an osmotic pump resulted in increased bone formation compared to no VEGF treatment 8. Subsequent efforts have focused on engineering scaffolds to deliver VEGF through more practical methodologies. VEGF incorporated into β-tricalcium phosphate and PLGA scaffolds increased blood vessel invasion and bone formation in critical-size defects 15,16. Other reports, however, have shown VEGF to induce minimal bone formation despite enhanced blood vessel formation 17-21.
Synergistic interactions between VEGF receptors and integrin adhesion receptors provide signals regulating vascularization. For example, blocking antibodies against the α2 subunit of the collagen-binding α2β1 integrin inhibits VEGF-dependent endothelial cell chemotaxis 22. Consistent with these results, deletion of the β1 integrin subunit using the Cre-lox system negatively impacts angiogenic sprouting 22. VEGF has also been shown to upregulate endothelial cell surface expression of α2β1 23. In addition to the α2β1 integrin the αvβ3 integrin plays a central role in angiogenesis and vascularization 24-26. While the mechanism of integrin-dependent angiogenesis remains unclear, antagonists to αvβ3 have been used to prevent abnormally active angiogenesis within tumors as activated endothelium within these environments exhibit greatly enhanced expression of αvβ3 27-31. Extensive cross-talk also exists between αvβ3 and VEGF as expression of VEGF is in part induced through αvβ3 ligation, clustering and association of the β3 subunit with phosphorylated p66 Shc 32. The β3 subunit is also involved in the activation of VEGFR2 in response to VEGF 33,34.
Because of the interplay between VEGF and integrins α2β1 and αvβ3, the objective of this study was to investigate whether presentation of integrin-specific peptides within a hydrogel in combination with exogenous VEGF modulates vascularization and bone formation in a murine segmental bone defect model. We synthesized protease-degradable poly(ethelyene glycol)-based hydrogels functionalized with VEGF and either the collagen I-mimetic α2β1-targeting GFOGER peptide or the fibronectin-derived αvβ3-targeting RGD peptide. We then implanted these constructs within critical-size murine radial bone defects and evaluated blood vessel formation and newly formed bone tissue. Based on previous studies showing that GFOGER-functionalized biomaterials promote osteoblastic differentiation in vitro 35 and enhance osseointegration of metal implants in rat tibiae 36, we hypothesized that VEGF-functionalized GFOGER hydrogels would increase vascularization and subsequent bone regeneration compared to VEGF-functionalized RGD hydrogels.
2. Materials and Methods
2.1 PEG hydrogel synthesis and VEGF release kinetics
Four-arm maleimide-end functionalized PEG macromer (PEG-MAL 20 kDa MW, Laysan Bio, >95% purity) was functionalized with recombinant human VEGF-A165 (Invitrogen) for 15 min at room temperature in 10 mM HEPES buffer pH=7.4 followed by functionalization with either GFOGER peptide, GGYGGGP(GPP)5GFOGER(GPP)5GPC (AAPPTec), or RGD peptide (GRGDSPC) (AAPPTec). Functionalized macromers were cross-linked using the bi-cysteine peptide VPM (GCRDVPMSMRGGDRCG) (AAPPTec). The PEG-MAL hydrogels were synthesized to obtain a final concentration of 1.0 mM adhesive peptide and 10 μg/mL VEGF unless otherwise noted. The concentration of cross-linker used for the synthesis of each hydrogel was calculated by matching the number of cysteine residues on the cross-linker to the number of residual maleimides on the PEG-MAL macromer following adhesive peptide and VEGF functionalization. Hydrogels were allowed to gel at 37°C for 15 min before swelling in PBS. For verification of VEGF tethering to the PEG-MAL macromer, the VEGF-PEG-MAL product was run on SDS-PAGE gel followed by protein visualization with Sypro Red (Life Technologies) staining.
To assess VEGF release kinetics, VEGF was labelled with NHS-AlexaFluor 488 (Life Technologies), purified, and incorporated into hydrogels as described. Hydrogels were incubated in either PBS or 50 μg/mL collagenase (Worthington Biomedical). At specified time points, supernatant was collected and analyzed for fluorescence.
2.2 Rheology
The storage and loss moduli of hydrogels were assessed by dynamic oscillatory strain and frequency sweeps performed on a MCR 302 stress-controlled rheometer (Anton Paar, Austria) with a 9 mm diameter, 2° cone and plate geometry. The hydrogels were synthesized as described and loaded between the cone and plate, after which the measuring system was lowered to a 39 μm gap. Initial strain amplitude sweeps were performed at an angular frequency of 10 rad s-1 to determine the linear viscoelastic range of the hydrogel. Oscillatory frequency sweeps were then used to examine the storage and loss moduli (ω= 0.5-100 rad s-1) at a strain of 1%.
2.3 Bioactivity of PEGylated VEGF
Human umbilical vein endothelial cells (HUVECs, Lonza) were grown in complete endothelial growth media EGM-2 (Lonza). Cells were synchronized in growth factor-free basal media (EBM-2, Lonza) with 1% fetal bovine serum (FBS) for 8 hr followed by addition of soluble VEGF, VEGF-conjugated PEG-MAL macromer, or control (VEGF-free) media for 48 hr. Cell metabolic activity was measured using CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega). To assess the activity of endothelial cells on VEGF-functionalized hydrogels, GFOGER-modified hydrogels were synthesized with or without incorporated VEGF. HUVECs were seeded at 10,000 cells/cm2 and incubated for 4, 8 or 15 hr. Samples were fixed in 3.7% paraformaldehyde, stained with AlexaFluor 488-conjugated phalloidin and DAPI, and imaged on a Nikon C2 confocal microscope. Endothelial cell network length and cell numbers were quantified using a custom macro in ImageJ.
2.4 3D endothelial cell network formation
To study 3D endothelial cell network assembly, a co-culture of GFP-expressing HUVECs (Angioproteomie) and mouse embryo 10T1/2 cells (ATCC) was used. GFP-expressing HUVECs and 10T1/2 cells were encapsulated in PEG-MAL hydrogels presenting either GFOGER or RGD at final cell densities of 4 ×106 cells/mL for 10T1/2 cells and 15 ×106 cells/mL for HUVECs. For gels containing VEGF, gels were incubated in EGM-2 media without supplemented VEGF. For gels synthesized without VEGF, gels were incubated in EGM-2 media with or without soluble VEGF. The cell-laden hydrogels were cultured for 48 hr, rinsed, and fixed in 3.7% paraformaldehyde. GFP-expressing HUVECs were imaged on a Nikon C2 confocal microscope and 3D network formation analyzed using a custom ImageJ macro.
2.5 Bone defect surgery
A critical-size bone defect model in the mouse radius was used to evaluate bone formation as previously described 37. All animal experiments were performed with the approval of the Georgia Tech Animal Care and Use Committee within the guidelines of the Guide for the Care and Use of Laboratory Animals. C57BL/6J wild-type male mice (8-10 weeks old, Jackson Laboratories) were anesthetized under isoflurane and fur was removed from the right forelimb. Prior to surgery, mice were administered a single dose of slow-release buprenorphine for pain relief. The right forelimb was then swabbed with chlorohexidine and alcohol, and a 1.5 cm incision was made in the skin. Muscle tissue surrounding the ulna and radius was dissected away, and a 2.5 mm complete excision in the radius was made using a custom-built bone cutter. 3.0 μL of hydrogel was cast within a 4-mm long polyimide sleeve with 4 rows of laser machined holes spaced 200 μm apart to improve cell invasion and nutrient transport. This sleeve was used to hold the hydrogel within the defect, and we previously showed that the sleeve does not interfere with the bone healing process 37. The hydrogel-containing sleeve was then carefully inserted over the ends of the defect, the soft tissue repositioned over the bone and the incision was closed using Vicryl sutures. Mice were monitored post-surgery for lethargy, weight loss, normal eating habits and signs of distress.
2.6 MicroCT angiography
Animals were euthanized by CO2 inhalation at 8 weeks post-surgery. Radiopaque contrast agent-enhanced microCT angiography was performed using a protocol modified from Phelps et al. 38-40. Briefly, an incision was made across the lower abdomen of the mouse followed by a continuing incision up the midline of the mouse exposing the entire abdominal cavity. The thoracic cavity was cut to carefully expose the heart. A butterfly needle was inserted into the left ventricle followed by cutting of the lower vena cava. Mice were then sequentially perfused with saline, 10% neutral buffered formalin, saline and lead chromate-based radiopaque contrast agent at a 30:60:10 v/v mixture of MV-122 Yellow:MV-diluent:MV curing agent (Microfill MV-122, Flow Tech). Samples were kept at 4°C overnight to allow the contrast agent to polymerize, and the forearms were then incubated for 72 hr in Krajian decalcification solution (Ricca Chemical), rinsed with PBS and scanned using a μCT50 scanning system (7 μm resolution, 55 kVp, 145 μA, Scanco Medical).
2.7 Immunohistochemistry
Following euthanasia by CO2 inhalation, the ulna and radius were excised and fixed in 10% neutral-buffered formalin, decalcified using Krajian decalcification solution, processed for paraffin embedding and embedded in paraffin wax. Sections (5 μm thick) were deparaffanized and incubated in antigen retrieval solution (10 mM sodium citrate buffer, pH=6.0) overnight at 60°C. Sections were then stained with either rabbit anti-endomucin or rat anti-CD31 antibodies overnight at 4°C followed by secondary staining with AlexaFluor 488- and AlexaFluor 555-conjugated goat antibodies for 1 hr at room temperature. Sections were imaged on a Nikon C2 confocal microscope.
2.8 VEGF, FGF-2 secretion
Human mesenchymal stem cells (hMSCs, Texas A&M University) were grown in α-MEM containing 16% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. hMSCs were encapsulated in VEGF-free integrin-specific hydrogels at a concentration of 5 ×106 cells/mL and cultured for 24 hr in growth media. After 24 hr, the media was exchanged for osteogenic media (growth media with 10 nM dexamethasone, 20 mM Na-β-glycerolphosphate, and 50 μM L-ascorbic acid 2-phosphate). After 7 days in culture, the conditioned media was collected and assayed for VEGF and FGF-2 levels using ELISA (Life Technologies, USA).
2.9 MicroCT imaging of bone formation
In vivo μCT imaging was performed on anesthetized mice using a VivaCT imaging system (Scanco Medical) at a voltage of 55 kVp and a current of 142 μA. Mice were centered such that the 2.5 mm radial defect was scanned within a 3.2 mm scan length window. Bone volume was evaluated as previously described 37. Briefly, 2D slices were contoured to solely include the radius followed by application of a Gaussian filter (sigma=1, support=1, threshold= 540 mg HA/ccm). While 3D reconstructions displayed the full 3.2 mm scanned length, only the middle 2.0 mm of the defect was analyzed for bone volume.
2.10 Statistics
Error bars on graphs represent SEM. Comparisons among multiple groups was performed by one-way analysis of variance (ANOVA) with post-hoc Tukey tests. Comparisons between two groups were done through a t-test in GraphPad Prism 6. A p value of <0.05 was considered significant.
3. Results
3.1 Synthesis and characterization of VEGF-releasing PEG-MAL hydrogels
We engineered hydrogels based on a maleimide-functionalized 4-arm PEG macromer which allows for peptide tethering onto the polymer precursor via a Michael-type addition reaction between the terminal-maleimide group and free thiols present on the biomolecules. Subsequent reaction with bi-cysteine cross-linking peptides (VPM) containing a protease cleavage site resulted in the formation of a cross-linked PEG hydrogel network sensitive to protease degradation (Fig. 1A). Prior to cross-linking, the PEG-MAL macromer was functionalized with VEGF by reacting the cysteine available for conjugation on VEGF165 to the maleimide moiety on the PEG macromer 41. The VEGF-functionalized macromer was further reacted with either the RGD or GFOGER cell adhesion peptides in order to investigate the coupled effects of biomaterials-based VEGF delivery and integrin-specificity on vascularization and bone regeneration. By design, covalent incorporation of VEGF onto the hydrogel backbone provides for cell-demanded release of VEGF as the construct degrades. Covalent tethering of VEGF to the PEG-MAL macromer was verified through the expected increase in molecular weight for VEGF reacted with PEG-MAL macromer (Fig. 1B).
Figure 1.
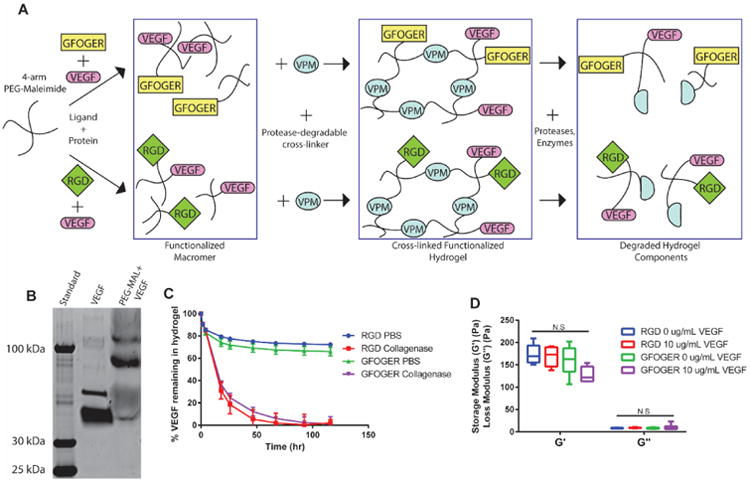
Protease-degradable integrin-specific PEG-MAL hydrogels for degradation-dependent release of covalently-tethered VEGF. (A) Schematic detailing PEG-MAL synthesis, stoichiometric ligand functionalization and growth factor incorporation. (B) Gel electrophoresis of VEGF and PEG-MAL+VEGF demonstrating increased MW of VEGF tethered onto PEG-MAL macromer. (C) VEGF release profile from integrin-specific hydrogels treated in PBS or collagenase as measured through fluorescence (Mean ± SEM, n=4). (D) Rheological properties of integrin-specific hydrogels measured through storage and loss moduli from dynamic oscillatory frequency tests (Box-whisker plot show min-max, n= 6) N.S., not significant.
To determine the kinetics of VEGF release from integrin-specific hydrogels in vitro, VEGF was fluorescently labelled and incorporated into RGD- or GFOGER-functionalized hydrogels. Hydrogels subjected to collagenase treatment degraded within the first 48 hr and released 100% of the incorporated VEGF (Fig. 1C). The high collagenase concentration used was selected to fully degrade the gel by 2-3 days; we expect slower in vivo protease-dependent degradation kinetics. In contrast, hydrogels incubated in PBS remained intact and, following an initial burst release due to hydrogel swelling, retained approximately 70% of the loaded VEGF over a period of 5 days. The loss of approximately 30% of loaded VEGF is attributed to incomplete covalent tethering of VEGF onto the PEG-MAL macromer as shown by the low intensity band on the SDS-PAGE gel at the MW of unconjugated VEGF in the PEG-MAL + VEGF lane (Fig. 1B). To investigate whether adhesive peptide or VEGF incorporation influences the hydrogel's mechanical properties, we performed dynamic oscillatory rheological testing to assess the storage (G′) and loss (G″) moduli of the different hydrogel conditions (Fig. 1D). Importantly, no significant differences in moduli were detected among the hydrogel conditions tested (average storage and loss moduli equal to 160 ± 6.3 and 8.1 ± 0.69 Pa, respectively).
3.2 Bioactivity of PEGylated and hydrogel-tethered VEGF
We next investigated the bioactivity of VEGF tethered to the PEG-MAL macromer. HUVECs cultured in media containing either PEG-MAL-conjugated or unmodified VEGF displayed similar dose-dependent responses in metabolic activity over the course of 48 hr (Fig. 2A). This result indicates that VEGF tethered to the PEG-MAL macromer retains bioactivity and that the PEG macromer does not interfere with VEGF-dependent signaling.
Figure 2.
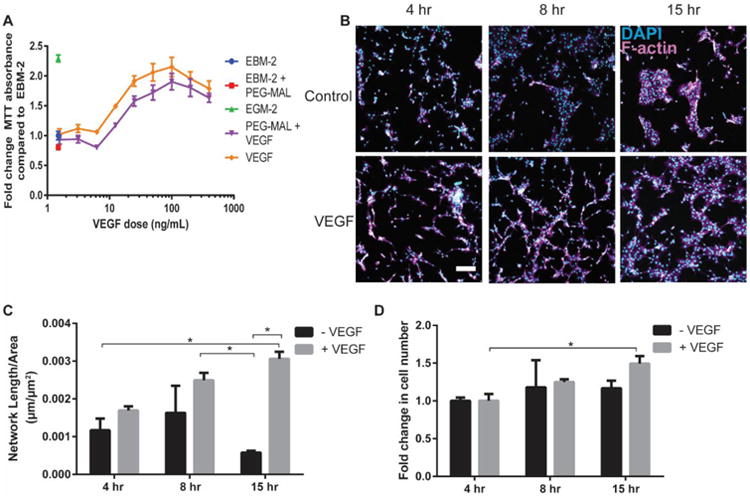
Bioactivity of PEGylated VEGF. (A) Endothelial cell metabolic assay for VEGF vs. PEG-MAL-VEGF (Mean ± SEM, n=6). (B) Images of endothelial cell networks on top of GFOGER-functionalized hydrogels either functionalized with (VEGF) or lacking VEGF (Control) over 15 hrs (Scale bar = 200μm, DAPI=cyan, F-actin=magenta). (C) Quantification of endothelial cell network length measured through custom ImageJ macro. (Mean ± SEM, n=4) (D) Quantification of endothelial nuclei count (Mean ± SEM, n=4) *p<0.05.
We further examined the bioactivity of VEGF tethered to hydrogels by assessing the proliferation and network formation of endothelial cells cultured on top of hydrogels with either soluble or tether VEGF. GFOGER-functionalized hydrogels supported cell adhesion regardless of VEGF incorporation (Fig. 2B). By 8 and 15 hr after seeding, endothelial cells adhering to VEGF-containing hydrogels exhibited elevated levels of network formation compared to hydrogels lacking VEGF (Fig. 2B,C). By quantifying the number of nuclei, we also found a significant increase in the number of endothelial cells on VEGF-containing hydrogels compared to VEGF-free gels (Fig. 2D). Taken together, these results show that tethering VEGF to the PEG-MAL macromer either in soluble form or after incorporation into a hydrogel network maintains the bioactivity of VEGF in regards to endothelial cell activity.
3.3 Integrin-dependent 3D endothelial cell tubulogenesis
After confirming the biological activity of PEGylated VEGF, we examined the effect of different integrin ligands in conjunction with VEGF presentation on the vasculogenic response to these materials. We used a model for 3D vascular tubulogenesis consisting of GFP-expressing HUVECs encapsulated with 10T1/2 cells, which act as a supporting pericyte-like cell, in hydrogels functionalized with either the GFOGER or RGD adhesive peptide. For both adhesive peptides, endothelial cells encapsulated within VEGF-free hydrogels and cultured in VEGF-free media exhibited minimal tubulogenesis as the cells remained largely rounded and failed to form protrusions to adjacent cells (Fig. 3A). In contrast, endothelial cells cultured in the presence of soluble VEGF in the media showed increased network formation in both RGD and GFOGER hydrogels (Fig. 3B). Importantly, VEGF-containing hydrogels cultured in VEGF-free media displayed enhanced endothelial cell-based network formation compared to hydrogels in VEGF-free conditions for both RGD- and GFOGER-functionalized hydrogels (Fig. 3B). For GFOGER-functionalized hydrogels, VEGF containing hydrogels exhibited similar levels of increased network formation compared to that of GFOGER-functionalized hydrogels cultured in VEGF-containing media. VEGF-containing RGD-functionalized hydrogels displayed elevated network formation compared to RGD hydrogels in VEGF-free conditions and RGD hydrogels cultured in media with VEGF (Fig. 3B). Taken together, the data demonstrates that hydrogels containing VEGF induce similar levels of endothelial cell tubulogenesis in vitro compared to soluble VEGF present in the media. Furthermore, this increase in tubulogenesis is independent of the adhesive peptide presented in the hydrogel.
Figure 3.
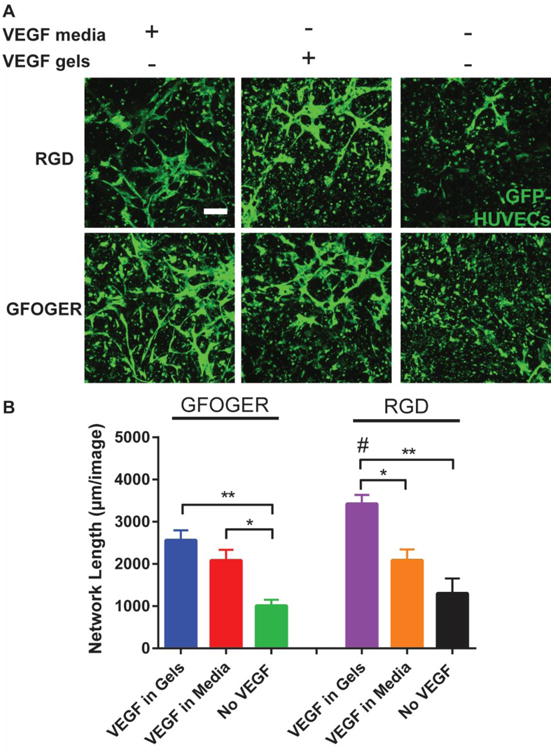
Integrin-specific hydrogels demonstrate VEGF-dependent increases in 3D endothelial cell network formation. (A) Projected Z-stack images of GFP-HUVECs cultured in either media containing VEGF, hydrogels containing VEGF or VEGF-free conditions for 3 days (scale bar = 200 μm). (B) Quantification of 3D network length in varying conditions (Mean ± SEM, n=5). *p < 0.05, **p < 0.01.
3.4 Effects of integrin specificity and VEGF incorporation on vascularization
We evaluated the potential of VEGF-functionalized hydrogels presenting integrin-specific peptides to enhance vascularization in a murine critical-size radial bone defect model. We tested two different VEGF doses (50 and 250 ng, referred to as low and high doses, respectively) as well as hydrogels lacking VEGF (0 ng) for both RGD and GFOGER-functionalized hydrogels. Hydrogels were implanted into 2.5 mm long unilateral murine radial defects and evaluated for vascular morphometric parameters within the bone defect area at 8 weeks via micro-computed tomographic (microCT) analysis of a perfused radiopaque polymer (Fig. 4A). Importantly, this technique measures functional vasculature connected to the host vasculature as the radiopaque polymer is perfused through the left ventricle and exits out the inferior vena cava to perfuse the vasculature. VEGF-free, RGD hydrogels exhibited very low levels of vascularization (Fig. 4A). With low and high doses of VEGF, RGD hydrogels exhibited significantly increased vessel number as well as significantly decreased vessel spacing denoting a higher density of vessels for both VEGF doses (Fig. 4C-D). Low doses of VEGF also significantly increased the total blood vessel volume and resulted in higher frequency of both small diameter (25-50 μm) and larger diameter vessels (80-100 μm) compared to VEGF-free RGD hydrogels (Fig. 4B,E).
Figure 4.
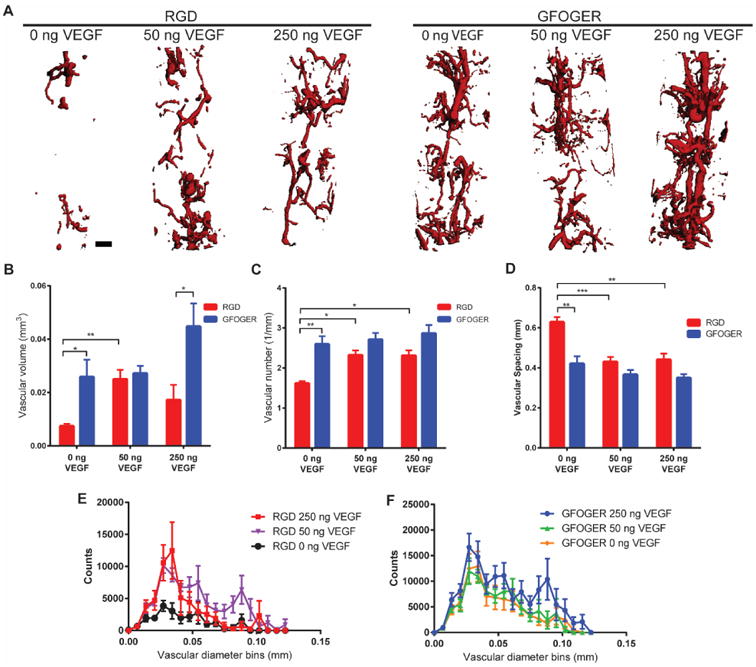
Vascularization of bone defects. (A) Representative 3D reconstructions of vascular structures within the bone defect for different adhesive peptides and VEGF doses (scale bar = 200 μm). (B,C,D) Vascular volume, number and spacing in bone defect respectively. (E,F) Vascular diameter histogram indicating blood vessel size distribution. (Mean ± SEM, n=5-8) *p < 0.05, **p < 0.01, ***p < 0.001.
Remarkably, VEGF-free GFOGER hydrogels displayed significantly increased vascularization across all quantified parameters (vessel volume, number and spacing) compared to VEGF-free RGD hydrogels (Fig. 4B-D). The levels of vascularization present within VEGF-free GFOGER hydrogels were equivalent to those of VEGF-containing RGD hydrogels. Furthermore, at high VEGF doses, while GFOGER and RGD hydrogels showed similar levels of vascular number and spacing, GFOGER hydrogels demonstrated elevated levels of total vascular volume compared to those of RGD hydrogels. The high levels of vascularization exhibited in GFOGER hydrogels were insensitive to delivery of either dose of exogenous VEGF as shown by the equivalent levels of vessel volume, spacing, and number (Fig. 4B-D). Whereas no differences were seen in these parameters, high doses of VEGF did increase the frequency of larger diameter vessels for GFOGER hydrogels compared to VEGF-free and low VEGF dose conditions (Fig. 4F). Taken together, the microCT vascular analysis shows that for growth-factor free conditions, RGD hydrogels result in poor vascularization; however, addition of VEGF to these hydrogels increases vascularization of these bone defects. In contrast, GFOGER hydrogels show elevated levels of vascularization regardless of delivery of exogenous VEGF. The observation that VEGF-free GFOGER hydrogels show significantly increased vascularization compared to VEGF-free RGD hydrogels and similar levels of vascularization compared to low dose VEGF-delivering RGD hydrogels emphasizes the importance of integrin-specificity in these biomaterials. By simply functionalizing scaffolds with differing integrin-specific ligands, robust increases in vascularization can be achieved.
We also performed immunostaining for CD31 and endomucin, which are specific markers for endothelial cells within capillary networks 42 (Fig. 5). RGD hydrogels having low and high doses of VEGF exhibited increased levels of CD31 and endomucin staining compared to VEGF-free RGD hydrogels. The staining also showed the presence of larger diameter vessels in the low dose group compared to the high dose group and VEGF-free gels, in agreement with the microCT vascular analyses. GFOGER hydrogels displayed robust staining for CD31 and endomucin independent of the VEGF dose. The histological analysis is fully consistent with the microCT results showing VEGF dose-dependent increases in endothelial markers for RGD hydrogels and no VEGF-dependence in GFOGER hydrogels, underscoring the importance of integrin-specificity in the vascularization response to these biomaterials.
Figure 5.
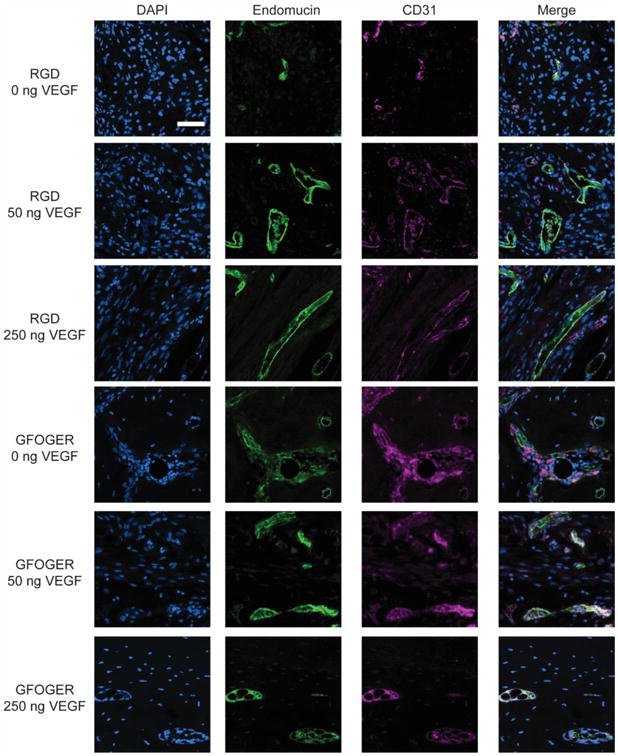
Representative images of endomucin and CD31 staining in bone defect samples (scale bar = 50 μm).
3.5 Effects of integrin-specificity on vasculogenic protein secretion by hMSCs
A major finding from the in vivo vascularization study is the enhancement in vascularization for defects treated with GFOGER-functionalized hydrogels compared to RGD-presenting gels. The network formation assay showed no differences in endothelial cell tubulogenesis between these two integrin-specific ligands (Fig. 3). Therefore, we investigated whether integrin binding specificity could influence the vasculogenic potential of non-endothelial cells such as mesenchymal stem cells (MSCs). During bone repair, MSCs secrete paracrine factors that increase the recruitment and proliferation of endothelial cells to form functional neovessels 43,44. Due to the influx of MSCs and osteoprogenitors immediately after bone injury 45, we speculated that differential integrin binding could result in differences in secreted factors by MSCs. We thus investigated the effect of integrin-specificity on the secretion of angiogenic proteins from MSCs encapsulated within GFOGER or RGD-functionalized hydrogels. Integrin-specific hydrogels containing human MSCs were cultured in osteogenic media for 7 days after which the conditioned media was analyzed for VEGF and FGF-2 levels. Conditioned media from GFOGER hydrogels showed a significantly elevated concentration of VEGF when compared to that of RGD hydrogels (Fig. 6A). The GFOGER-dependent upregulation in angiogenic factor secretion was specific to VEGF, as secretion of FGF-2 was below the limit of detection of the assay for both GFOGER and RGD hydrogels (Fig 6B). These results demonstrate that MSCs cultured within hydrogels presenting the α2β1-specific GFOGER secrete higher levels of VEGF compared to that of MSCs cultured in RGD hydrogels. This finding provides an explanation for the enhancements in vascularization observed in bone defects treated with GFOGER-functionalized hydrogels compared to defects treated with RGD-presenting hydrogels.
Figure 6.
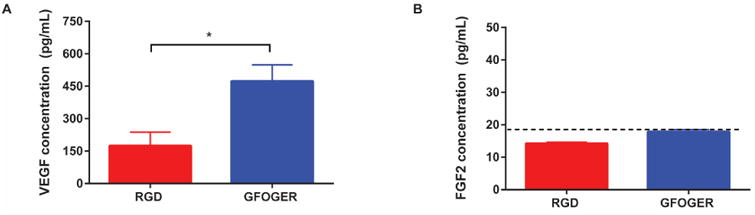
hMSCs encapsulated within GFOGER-functionalized hydrogels exhibit enhanced secretion of VEGF compared to hMSCs encapsulated within RGD-functionalized hydrogels. (A,B) Quantification of VEGF or FGF-2 in the supernatant of hMSCs encapsulated within integrin-specific hydrogels in osteogenic differentiation conditions (Mean ± SEM, n=4). Dotted line indicates reliable limit of detection. *p < 0.05.
3.6 Effect of VEGF-delivering hydrogels on bone repair
We next analyzed the effects of VEGF delivery within integrin-specific hydrogels on the repair of non-healing bone defects. Hydrogel conditions and surgical procedures were consistent with the vascularization study, and bone healing was evaluated by microCT at 4 and 8 weeks post-surgery. Bone defects treated with VEGF-free GFOGER-modified hydrogels exhibited 3-4 fold higher bone volume compared to those treated with VEGF-free RGD-modified hydrogels at 4 and 8 weeks post-surgery (Fig. 7B,D). For GFOGER hydrogels, VEGF had no significant effect on bone formation at 4 and 8 weeks post-surgery, consistent with the vascularization analyses. For RGD hydrogels, low doses of VEGF significantly increased bone volume at 4 weeks, whereas high doses of VEGF showed similar levels of bone volume compared to VEGF-free RGD hydrogels. However, by 8 weeks post-surgery, there were no differences among VEGF-containing and VEGF-free RGD hydrogels (Fig. 7B,D). These results demonstrate that in VEGF-free conditions, GFOGER hydrogels exhibit significantly increased bone formation compared to RGD hydrogels. Additionally, although increases in vascularization are noted, especially within RGD hydrogels, delivery of exogenous VEGF does not enhance bone repair in this murine critical-size defect for either integrin-specific hydrogel.
Figure 7.
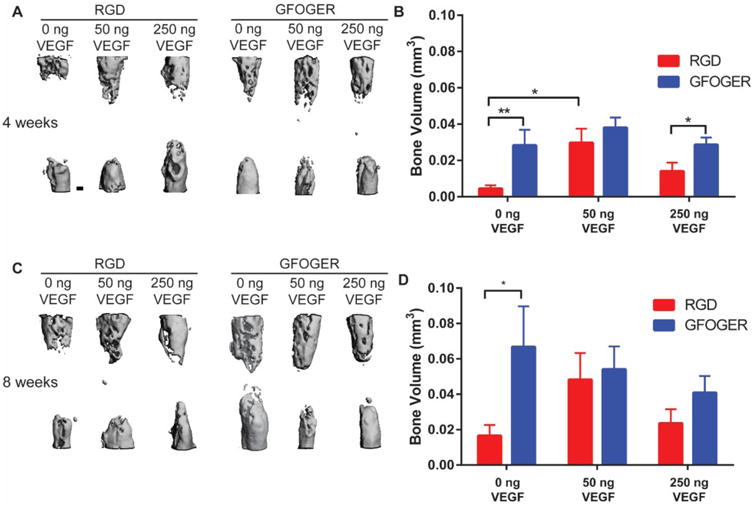
Bone volume for bone defects treated with VEGF-containing hydrogels. (A,C) Representative 3D reconstructions of radial defect at 4 and 8 weeks respectively with differing ligand and VEGF doses (scale bar = 200 μm). (B,D) Quantification of bone volume of defects at 4 and 8 weeks respectively. (Mean ± SEM, n=7-9) *p < 0.05, **p < 0.01.
4. Discussion
Non-healing bone defects and fractures represent a serious clinical problem with over 1 million surgical procedures necessitating bone grafts performed annually in the USA alone and costing over $5 billion 46. While the creation of a functional vascular network is considered a crucial factor in successful regeneration of bone defects, it is still unclear whether delivery of vasculogenic factors such as VEGF enhances bone repair 8,16,17,47. Additionally, while the role that integrins play in the progression of angiogenesis in tumors has been of particular interest recently 48, the ability of integrin binding to direct vascularization in the context of biomaterial-directed tissue repair has not been investigated. In this study, we examined whether incorporation of VEGF into synthetic hydrogels functionalized with either the α2β1 integrin-targeting GFOGER ligand or the RGD peptide that mainly binds to αvβ3 integrin modulates both the vascularization and bone regeneration of critical-size bone defects.
Controlled and sustained delivery of VEGF constitutes an important parameter in the design of the hydrogel. In the strategy described here, VEGF is covalently tethered to the hydrogel precursor thus allowing for high retention efficiency once cross-linked. The protease-sensitive nature of the cross-linked material provides for controlled release of VEGF based on cell-mediated invasion and degradation of the hydrogel. Another crucial parameter when designing a growth-factor based release system is retaining the biological activity of the protein. For delivery vehicles based on the encapsulation of proteins within solid matrices such as microparticles, the bioactivity of the protein is likely reduced 49. In the described system, VEGF remains in a hydrated state throughout gelation. Furthermore, the conjugation of the PEG macromer to free thiols within VEGF does not affect the protein's biological activity as endothelial cells exhibited heightened 3D network formation when encapsulated within VEGF-tethered hydrogels compared to those in VEGF-free conditions.
Whereas no differences were observed for in vitro endothelial network formation between integrin-specific hydrogels, significant differences were observed in the vascularization of bone defects in vivo for hydrogels presenting different adhesive peptides. For VEGF-free conditions, GFOGER-functionalized hydrogels exhibited significantly increased vascular volume and density compared to RGD-functionalized hydrogels. This difference may be attributed to multiple factors. Activation of the α2β1 integrin has been reported to increase osteogenic activity resulting in increased pro-angiogenic signals as osteoblasts and osteoprogenitors secrete large quantities of VEGF to support the survival and proliferation of surrounding endothelial cells 50-52. This observation potentially explains the findings that VEGF-free GFOGER-functionalized hydrogels produce higher bone regeneration in vivo compared to VEGF-free RGD-functionalized hydrogels. Additionally, while the αvβ3 integrin has often been implicated in angiogenesis, more recent studies have shown that the role of this integrin in vascularization is context-dependent 53. Depending on the environment, cell type, and which molecules the integrin interacts with, the αvβ3 integrin can play either a pro- or anti-angiogenic role 54-56.
For hydrogels incorporating VEGF, RGD hydrogels exhibited increases in blood vessel volume, number and density within the bone defect area compared to VEGF-free controls. This result is consistent with the well-established cross-talk between the β3 integrin subunit and VEGFR2. Mutation of the cytoplasmic tail of the β3 integrin results in impaired interactions with VEGFR2 which leads to inefficient phosphorylation of the dimerized VEGFR2 complex 33. Downstream activation of FAK and JNK signaling pathways from VEGFR2 activity is also dependent on the co-activation of the αvβ3 integrin 57. The ability for RGD hydrogels to activate the αvβ3 integrin could thus cause heightened sensitivity to the presence of VEGF within the defect microenvironment. In contrast, the level of vascularization for GFOGER-functionalized hydrogels was independent of VEGF dose, with VEGF-free as well as low and high doses of VEGF exhibiting high levels of vascularization. Notably, when MSCs were encapsulated within GFOGER hydrogels in vitro, the cells exhibited increased secretion of VEGF compared to those encapsulated within RGD hydrogels. This finding provides an explanation as to why in vivo vascularization in GFOGER hydrogels was elevated and insensitive to exogenous VEGF as the interaction between the hydrogel and invading MSCs may provide abundant levels of endogenous VEGF. Overall, the finding that VEGF-free, GFOGER hydrogels show elevated levels of in vivo vascularization compared to VEGF-free, RGD hydrogels and similar levels of vascularization compared to VEGF-containing RGD hydrogels is noteworthy. The ability for integrin-specificity alone to instruct and guide levels of vasculogenesis and regulate biological activity of vasculogenic proteins highlights the importance of exploiting integrin-specificity in regenerative medicine applications. With the expensive and often significant regulatory issues associated with growth factor therapies, the ability to engineer scaffolds to reduce or completely eliminate the need for growth factors through simple functionalization techniques can greatly enhance the clinical efficacy of future regenerative medicine constructs.
In addition to differences in vascularization for the integrin-specific hydrogels, integrin specificity also played a role in the regeneration of bone within the critical-size defects. VEGF-free, GFOGER-functionalized hydrogels exhibited higher bone repair at both 4 and 8 weeks in comparison to VEGF-free RGD-presenting hydrogels. Addition of VEGF, however, had no effect on bone formation at 8 weeks indicating that exogenously delivered VEGF is not sufficient to repair critical-size bone defects in this model. This conclusion does not rule out an important role for VEGF in bone repair. On the contrary, endogenous VEGF is necessary for bone repair as treatment with VEGF blocking antibodies inhibits the healing of bone defects 8. Furthermore, mice lacking the VEGF165 and VEGF188 isoforms show abnormal vascular patterning along with significant decreases in trabecular bone volume and bone growth 58. However, in terms of delivering exogenous VEGF to regenerate bone defects, studies demonstrate conflicting results with some reports showing enhanced bone formation while others showing no effect after delivery of VEGF 15-18,59,60. The inconsistencies between these reports could be related to the in vivo model, scaffold, or delivery kinetics. Exploration into strategies utilizing VEGF in conjunction with other stimuli such as other growth factors (e.g., BMP-2), further scaffold engineering, cell therapy or gene therapy could enhance its effects.
5. Conclusion
While endogenous VEGF is known to be essential to osteogenesis, the ability of exogenously delivered VEGF to significantly repair bone defects remains ambiguous. In this study, we investigated whether integrin-specific biomaterials could constitute a crucial, yet, up to now, unexplored role in vascularization and osteogenesis in bone defects. VEGF-free GFOGER-presenting hydrogels exhibited significantly increased vascularization and bone formation in a non-healing segmental bone defect compared to RGD-functionalized hydrogels. Furthermore, RGD-presenting hydrogels exhibited exogenous VEGF dose dependent increases in vascularization. Nevertheless, addition of VEGF to these hydrogels did not enhance bone repair in this bone defect model. This study demonstrates interplay between biomaterial integrin specificity and VEGF in tissue vascularization.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01-AR062368 and R01-AR062920. JRG was supported by the Cell and Tissue Engineering NIH Biotechnology Training Grant (T32-GM008433). The authors thank Dr. Nick Willet for his assistance with the Microfill perfusion technique.
References
- 1.Garcia JR, Garcia AJ. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv Transl Res. 2015 doi: 10.1007/s13346-015-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao RR, Stegemann JP. Cell-based approaches to the engineering of vascularized bone tissue. Cytotherapy. 2013;15(11):1309–22. doi: 10.1016/j.jcyt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, Kang Y, Yang Y, Khademhosseini A. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev. 2012;18(5):363–82. doi: 10.1089/ten.teb.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97(8):4052–7. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8(21):980–9. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 7.Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25(1):51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Frohlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;3(4):254–64. doi: 10.2174/157488808786733962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 13.Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113(Pt 1):59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 14.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131(9):2161–71. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 15.Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, Siebenrock KA, Hofstetter W, Klenke FM. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 16.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249–55. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15(9):2347–62. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez A, Reyes R, Sanchez E, Rodriguez-Evora M, Delgado A, Evora C. In vivo osteogenic response to different ratios of BMP-2 and VEGF released from a biodegradable porous system. J Biomed Mater Res A. 2012;100(9):2382–91. doi: 10.1002/jbm.a.34183. [DOI] [PubMed] [Google Scholar]
- 20.Geuze RE, Theyse LF, Kempen DH, Hazewinkel HA, Kraak HY, Oner FC, Dhert WJ, Alblas J. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng Part A. 2012;18(19-20):2052–62. doi: 10.1089/ten.tea.2011.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De la Riva B, Sanchez E, Hernandez A, Reyes R, Tamimi F, Lopez-Cabarcos E, Delgado A, Evora C. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J Control Release. 2010;143(1):45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160(1):195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94(25):13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 25.Silva R, D'Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28(10):1703–13. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Wang F, Chen X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev Res. 2008;69(6):329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buerkle MA, Pahernik SA, Sutter A, Jonczyk A, Messmer K, Dellian M. Inhibition of the alpha-nu integrins with a cyclic RGD peptide impairs angiogenesis, growth and metastasis of solid tumours in vivo. Br J Cancer. 2002;86(5):788–95. doi: 10.1038/sj.bjc.6600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One. 2009;4(2):e4449. doi: 10.1371/journal.pone.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA, Tucker GC, Van Obberghen-Schilling E. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood. 2006;108(9):3035–44. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- 30.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79(7):1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 31.Danhier F, Le Breton A, Preat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9(11):2961–73. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 32.De S, Razorenova O, McCabe NP, O'Toole T, Qin J, Byzova TV. VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci U S A. 2005;102(21):7589–94. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101(6):570–80. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–85. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes CD, Garcia AJ. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69(4):591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 36.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–35. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, Garcia AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35(21):5453–61. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107(8):3323–8. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287(1):H302–10. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 40.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22(2):286–97. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 41.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjic N. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273(32):20556–67. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 42.dela Paz NG, D'Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res. 2009;335(1):5–16. doi: 10.1007/s00441-008-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18(5):1026–34. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491–8. doi: 10.1007/s00264-013-2059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927–38. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 47.Krishnan L, Willett NJ, Guldberg RE. Vascularization strategies for bone regeneration. Ann Biomed Eng. 2014;42(2):432–44. doi: 10.1007/s10439-014-0969-9. [DOI] [PubMed] [Google Scholar]
- 48.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8(8):604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4(4):298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 50.Steiner D, Lampert F, Stark GB, Finkenzeller G. Effects of endothelial cells on proliferation and survival of human mesenchymal stem cells and primary osteoblasts. J Orthop Res. 2012;30(10):1682–9. doi: 10.1002/jor.22130. [DOI] [PubMed] [Google Scholar]
- 51.Grellier M, Granja PL, Fricain JC, Bidarra SJ, Renard M, Bareille R, Bourget C, Amedee J, Barbosa MA. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials. 2009;30(19):3271–8. doi: 10.1016/j.biomaterials.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 52.Grellier M, Ferreira-Tojais N, Bourget C, Bareille R, Guillemot F, Amedee J. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem. 2009;106(3):390–8. doi: 10.1002/jcb.22018. [DOI] [PubMed] [Google Scholar]
- 53.Robinson SD, Hodivala-Dilke KM. The role of beta3-integrins in tumor angiogenesis: context is everything. Curr Opin Cell Biol. 2011;23(5):630–7. doi: 10.1016/j.ceb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8(1):27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 55.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203(11):2495–507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur S, Kenny HA, Jagadeeswaran S, Zillhardt MR, Montag AG, Kistner E, Yamada SD, Mitra AK, Lengyel E. {beta}3-integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer. Am J Pathol. 2009;175(5):2184–96. doi: 10.2353/ajpath.2009.090028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masson-Gadais B, Houle F, Laferriere J, Huot J. Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress Chaperones. 2003;8(1):37–52. doi: 10.1379/1466-1268(2003)8<37:ivrfva>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111(1-2):61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 59.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21(5):735–44. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 60.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–25. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]


