Abstract
Southern Zambia is the focus of strategies to create malaria-free zones. Interventions being rolled out include test and treat strategies and distribution of insecticide-treated bed nets that target vectors that host-seek indoors and late at night. In Macha, Choma District, collections of mosquitoes were made outdoors using barrier screens within homesteads or UV bulb light traps set next to goats, cattle, or chickens during the rainy season of 2015. Anopheline mosquitoes were identified to species using molecular methods and Plasmodium falciparum infectivity was determined by ELISA and real-time qPCR methods. More than 40% of specimens caught were identified as Anopheles squamosus Theobald, 1901 of which six were found harboring malaria parasites. A single sample, morphologically identified as Anopheles coustani Laveran, 1900, was also found to be infectious. All seven specimens were caught outdoors next to goat pens. Parasite-positive specimens as well as a subset of An. squamosus specimens from either the same study or archive collections from the same area underwent sequencing of the mitochondrial cytochrome oxidase subunit I gene. Maximum parsimony trees constructed from the aligned sequences indicated presence of at least two clades of An. squamosus with infectious specimens falling in each clade. The single infectious specimen identified morphologically as An. coustani could not be matched to reference sequences. This is the first report from Zambia of infections in An. squamosus, a species which is described in literature to display exophagic traits. The bionomic characteristics of this species needs to be studied further to fully evaluate the implications for indoor-targeted vector control.
Keywords: malaria, vector competence, mosquito-borne disease, vector ecology
In Macha, Southern Province, Zambia, malaria cases have dropped by over 90% in the past decade (Moss et al. 2011, 2012; Mharakurwa et al. 2012). Current community surveys indicate the prevalence by rapid diagnostic test to be <1% (W.J. Moss unpublished data) and elimination strategies are now being rolled out. These methods are primarily based on reactive case detection; cases reporting at health facilities are followed up and household members and neighbors are screened and treated with antimalarials if infected. Vector control relies on the routine distribution of long-lasting insecticidal nets (LLINs) to pregnant women and children under 5 yr of age at health facilities and mass distribution of LLINs every 2–3 yr (NMCC 2011). LLINs are currently the most effective tool in preventing exposure to indoor foraging malaria vectors that predominantly feed when people are asleep (Lengeler 2004). However, this assumption of late night endophagy (Gillies and De Meillon 1968, Pates and Curtis 2005, Killeen et al. 2006) is being challenged in some areas; studies in other parts of Sub-Saharan Africa have shown replacement of vector populations with species that can evade indoor control (Gillies and Smith 1960, Lindblade et al. 2006, Bayoh et al. 2010), or have demonstrated changes in the foraging behavior of existing primary vectors, resulting in biting time shifts, and outdoor feeding and resting (Reddy et al. 2011, Russell et al. 2011, Moiroux et al. 2012, Yohannes and Boelee 2012, Sougoufara et al. 2014, Cooke et al. 2015). Recent studies have focused on determining the extent of exposure to vectors that may not be controlled by indoor targeted methods (Geissbuhler et al. 2007, Govella et al. 2010, Seyoum et al. 2012, Killeen 2014, Cooke et al. 2015). Currently the only vector control deployed in southern Zambia is use of LLINs, which do not combat exposure to malaria mosquito vectors either outdoors or indoors at times prior or after bed net use.
The majority of programmatic entomological surveillance relies on morphological identification of samples using standard, albeit dated, keys (Gillies and Coetzee 1987). In sub-Saharan Africa, discrimination of specimens focuses on separating and quantifying collections of the main malaria vector complexes, Anopheles gambiae Giles, 1902 and Anopheles funestus Giles, 1900. Due to limitations of infrastructure in country, samples are rarely identified to sibling species within these complexes by standard PCR-based tools (Scott et al. 1993, Koekemoer et al. 2002), and little attention is paid to other species which are often discarded. However, studies have demonstrated presence of Plasmodium falciparum in secondary and unrecognized vectors (Gillies 1964, Nigatu et al. 1994, Wilkes et al. 1996, Antonio-Nkondjio et al. 2006, Stevenson et al. 2012, Degefa et al. 2015, Nepomichene et al. 2015, St. Laurent et al. 2016). Early studies relied on dissection of salivary glands to detect sporozoites, but these labor-intensive methods have generally been superseded by the use of circumsporozoite (CSP) ELISAs of homogenates of mosquito head and thoraces (Burkot et al. 1984). The use of CSP ELISA for zoophagic species has been reported to result in false positives (Durnez et al. 2011) and so some studies have confirmed infectivity by detection of parasite DNA by PCR in the mosquito. In a recent study in central Madagascar, infections of both P. vivax and P. falciparum detected by CSP ELISA were confirmed by PCR in An. coustani Laveran, 1900 caught both indoors and outdoors (Nepomichene et al. 2015). Worryingly, infectivity rates and entomological inoculation rates (the number of infectious bites received per person per annum, EIRs) were comparable with that of An. funestus, the recognized vector in the area. Indoor application of insecticides, the mainstay of vector control in Madagascar, as in most other African countries, is unlikely to prevent exposure to An. coustani that displays both endophagic and exophagic (indoor and outdoor feeding) behaviors (Gillies and De Meillon 1968, Fornadel et al. 2011, Mwangangi et al. 2013, Degefa et al. 2015, Nepomichene et al. 2015). PCR methods also confirmed presence of P. falciparum-positive mosquitoes in the highlands of western Kenya that did not belong to the An. gambiae or An. funestus species complexes (Stevenson et al. 2012, St. Laurent et al. 2016). Genetic sequencing of these samples to identify the infectious vector species resulted in no match to mosquito species that have been previously sequenced. Many of the infectious specimens were trapped outdoors where they may avoid current control measures. These findings highlight the importance of expansion of entomological surveillance to include potential secondary vectors, especially in low transmission areas where recognized primary vector populations may be marginalized, and programs start to focus on elimination strategies.
As part of the International Centers of Excellence in Malaria Research (ICEMR) in Southern Africa (Conn et al. 2015), outdoor collection methods for anopheline malaria vectors were evaluated in Macha, Choma district, Southern Zambia. Using two 4x4 Latin Square designs, miniature CDC UV light traps, updraft UV light traps (John W Hock Co., Gainesville, FL) and barrier screens (Burkot et al. 2013) were rotated through eight consenting households for a period of 48 nights in the rainy season between February and April 2015. UV light traps were set next to cow, chicken, or goat enclosures, whilst barrier screens were erected within the homestead between houses and breeding site. At the laboratories in Macha, mosquitoes were identified to species level using morphological keys (Gillies and De Meillon 1968, Gillies and Coetzee 1987) and identities confirmed using standard diagnostic PCRs for An. gambiae and An. funestus species complexes, the latter which targets a polymorphic region of the ribosomal intergenic spacer 2 (ITS2) region of DNA (Scott et al. 1993, Koekemoer et al. 2002, Kent et al. 2006). Addition of primers to this PCR designed to amplify the ITS2 region of other African anophelines, allowed for detection of species not of the An. funestus and An. gambiae complexes (Das et al. 2016). All samples underwent CSP ELISA and positive samples were determined by OD readings 2-fold greater than the negative controls (Burkot et al. 1984). Real-time qPCR was performed on DNA extracts from the head and thorax to detect P. falciparum.
All parasite-positive samples were sent to Johns Hopkins Bloomberg School of Public Health, Baltimore, for mosquito species confirmation by amplification and alignment of a 698 bp fragment from the mitochondrial cytochrome oxidase subunit I gene (COI) (Norris and Norris 2015). Consensus sequences were aligned and Maximum Parsimony (MP) trees constructed (1,000 bootstraps) with An. coustani as an outgroup, using MEGA 6.0 (Tamura et al. 2013) as previously described by Norris and Norris (Norris and Norris 2015). The blood feeding host preference of all samples identified as Anopheles squamosus Theobald, 1901 were analyzed by PCR (Kent and Norris 2005).
A total of 834 female anophelines were caught during the study from the outdoor light traps and barrier screens. Morphological and molecular identifications were successfully conducted on 812 specimens and revealed domination of catches by An. squamosus (40.3%) and An. arabiensis (24.8%). Other species identified were An. coustani (6.0%), An. rufipes (5.5%), An. quadriannulatus (4.8%), An. parensis (3.7%), An. leesoni (1.2%), An. longipalpis (1.0%), An. pretoriensis (1.0%), An. rivulorum (0.1%), and An. rivulorum-like (0.1%). Of the 812 samples, 51 were blood-fed with 27 of these identified as An. squamosus. These 27 bloodmeals were identified as nonhuman, with 78% of bloodmeals taken from goats. Following standard CSP ELISA, seven of the 812 samples had OD values 2-fold greater than the negative controls. These samples were morphologically identified as An. squamosus (n = 6) and An. coustani (n = 1). All samples were analyzed by qPCR of which four gave positive signals for P. falciparum (Table 1).
Table 1.
Details of anopheline specimens sequenced and referenced, GPS locations where specimens were trapped, and results of CSP ELISA and qPCR for P. falciparum
| Specimen and infection status | GenBank | Longitude | Latitude |
|---|---|---|---|
| Anopheles sp. 475 +* | KU524734 | 26.9004 | −16.2537 |
| Anopheles squamosus 526 | KU524735 | 27.0154 | −16.3737 |
| Anopheles squamosus 540 | KU524736 | 27.0154 | −16.3737 |
| Anopheles squamosus 564+* | KU524737 | 27.0154 | −16.3737 |
| Anopheles squamosus 609+ | KU524738 | 26.9168 | −16.2500 |
| Anopheles squamosus 610 | KU524739 | 26.9168 | −16.2500 |
| Anopheles squamosus 671+ | KU524740 | 26.9168 | −16.2500 |
| Anopheles squamosus 539 | KU524741 | 27.0154 | −16.3737 |
| Anopheles squamosus 541 | KU524742 | 27.0154 | −16.3737 |
| Anopheles squamosus 613 | KU524743 | 26.9168 | −16.2500 |
| Anopheles squamosus 614 | KU524744 | 26.9168 | −16.2500 |
| Anopheles squamosus 440 | KU524745 | 26.8784 | −16.2937 |
| Anopheles squamosus 508 | KU524746 | 26.9168 | −16.2500 |
| Anopheles squamosus 510 | KU524747 | 26.9168 | −16.2500 |
| Anopheles squamosus 611 | KU524748 | 26.9168 | −16.2500 |
| Anopheles squamosus 620+* | KU524749 | 26.9168 | −16.2500 |
| Anopheles squamosus 649+ | KU524750 | 26.9168 | −16.2500 |
| Anopheles squamosus 706B+* | KU524751 | 26.9168 | −16.2500 |
| Anopheles coluzzi | KU524752 | Keele Strain | |
| Anopheles gambiae | KU524753 | 28.8072 | −9.2569 |
| Anopheles squamosus SQ5 | KU524754 | 26.7906 | −16.3929 |
| Anopheles squamosus SQ3 | KU524755 | 26.7906 | −16.3929 |
| Anopheles squamosus SQ15 | KU524756 | 26.7906 | −16.3929 |
| Anopheles squamosus SQ16 | KU524757 | 26.7906 | −16.3929 |
| Anopheles squamosus 392 | KU524758 | 26.9532 | −16.3786 |
| Anopheles squamosus 433 | KU524759 | 26.8412 | −16.4425 |
| Anopheles squamosus 327 | KU524760 | 26.9004 | −16.2537 |
| Anopheles squamosus | JN994170.1 | Norris and Norris 2015 | |
| Anopheles pharoensis | JN994163.1 | ||
| Anopheles coustani | JN994154.1 | ||
| Anopheles rufipes | JN994169.1 | ||
| Anopheles pretoriensis | JN994164.1 | ||
| Anopheles funestus | JN994155.1 | ||
| Anopheles parensis | JN994162.1 | ||
| Anopheles vaneedeni | JN994172.1 | ||
| Anopheles leesoni | JN994158.1 | ||
| Anopheles rivulorum | JN994168.1 | ||
| Anopheles theileri | JN994171.1 | ||
| Anopheles arabiensis | JN994153.1 | ||
| Anopheles quadriannulatus | JN994165.1 | ||
+—CSP-ELISA positive, *—P. falciparum qPCR positive.
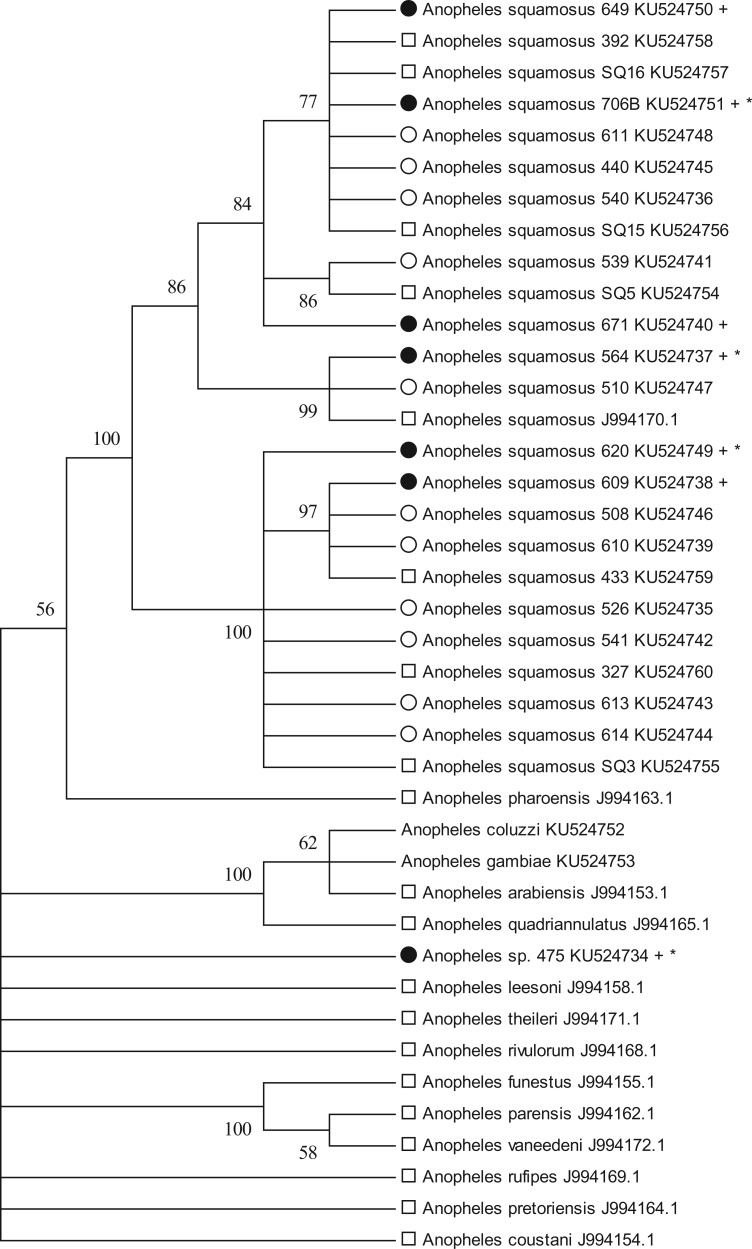
A 698 bp fragment from the mosquito mitochondrial COI was amplified and sequenced from all seven ELISA/qPCR-positive specimens (Norris and Norris 2015). Included with these samples were 11 randomly selected Plasmodium-negative specimens caught during the same week, from light traps and identified morphologically as An. squamosus, as this morphological taxon dominated the collection. Also included in the phylogenetic analysis were seven mosquitoes morphologically identified as An. squamosus from other studies in the Macha area, and specimens of An. gambiae s.s. from Nchelenge district, Luapula Province, northern Zambia and An. coluzzii specimens from colonies kept at Johns Hopkins Bloomberg School of Public Health (Table 1). These samples were included to address consistency of morphological identifications over time and across studies. The MP analysis and resulting trees revealed that all specimens morphologically identified as An. squamosus cluster together with 100% support (Fig. 1). The existence of at least two molecular COI clades is also apparent and strongly supported within An. squamosus and warrant further investigation. The molecular identity of specimen 475 remains ambiguous, despite morphological identification as An. coustani. This sequence does not cluster with any significance to any other available anopheline COI sequence, most notably any recognized vector species. This lone P. falciparum-positive specimen may suggest the existence of yet another potentially important unreported malaria vector species.
Fig. 1.
Cytochrome oxidase subunit I (COI) Maximum Parsimony tree, one of the two most parsimonious trees that did not differ in any arrangements after collapse of all branches with <50% bootstrap support (1,000 replicates). Circle—UV trap outdoors, Square—CDC standard light trap indoors, Filled Circle—CSP-ELISA positive, An. gambiae from barrier collection in northern Zambia, An. coluzzi from insectary at Johns Hopkins Bloomberg School of Public Health, +—CSP-ELISA positive, *—P. falciparum qPCR positive.
Studies are increasingly reporting the potential importance of secondary vectors (Awono-Ambene et al. 2004, Okorie et al. 2011, Stevenson et al. 2012, Animut et al. 2013, Mwangangi et al. 2013, Nepomichene et al. 2015, St. Laurent et al. 2016). Whilst there is the possibility of false positives resulting from ELISAs conducted on zoophagic species (Durnez et al. 2011, Charlwood et al. 2015), our current study in Zambia used both antigen and DNA-based detection methods to determine infection rates and confirm the presence of P. falciparum sporozoites and DNA in An. squamosus mosquitoes. These anophelines have not been associated with malaria transmission in this area, although historic reports have implicated An. squamosus in malaria transmission by sporozoite visualization in nearby Tanzania and Zimbabwe (Gillies 1964, Gillies and De Meillon 1968). The fact that there are no animal reservoirs of P. falciparum apart from humans, supports the potential role of An. squamosus in malaria transmission in Africa. The COI data generated in this study demonstrated presence of two strongly supported molecular clades in mosquitoes identified as An. squamosus, and three specimens which were positive by both ELISA and qPCR fell into each of the two clades. These mosquitoes were caught outdoors near goat pens and although the extent of their outdoor foraging behavior requires further investigation, such behaviors could undermine current elimination efforts that rely on vector control targeting indoor human sleeping structures. This study did not reveal presence of human blood in any of the An. squamosus specimens caught, but previous studies from the area have demonstrated significant anthropophily of An. squamosus (Fornadel et al. 2011).
Residual transmission may explain the continued presence of cases in the Macha area. Our findings highlight the utility of molecular tools for both determination of infectivity and accurate identification of anopheline species, and stress the importance of rigorous entomological studies that are not limited to known malaria vectors but also incorporate sympatric anophelines active both indoors and outdoors. Such studies are essential to fully evaluate the epidemiological importance of secondary vectors and to develop appropriate vector control tools. To our knowledge, this is the first molecular confirmation of An. squamosus harboring P. falciparum sporozoites. Evidence of the existence of Plasmodium-infectious exophagic An. squamosus and other unidentified taxa indicates that species other than well-recognized malaria vectors could play a role in malaria transmission in Southern Africa, which may jeopardize current malaria elimination efforts, where vector control is solely indoor based. Larval source management which targets both indoor and outdoor resting mosquitoes, is the only recommended programmatic intervention by the World Health Organization (Tusting et al. 2013, WHO 2013), but its use is generally limited to areas where breeding sites are identifiable and limited. It requires a large investment in terms of capacity for sustained program management and entomological monitoring and surveillance. If exophagic and zoophilic species such as An. squamosus are found to play an important role in transmission, there are a number of interventions that can reduce exposure to mosquitoes outdoors, such as the use of topical and spatial repellents(Achee et al. 2012, Debboun and Strickman 2013, Wilson et al. 2014), application of topical and systemic insecticides to animals (Hewitt and Rowland 1999, Rowland et al. 2001, Habtewold et al. 2004, Chaccour et al. 2013, Franco et al. 2014, Poche et al. 2015), and deployment of odor-baited traps (Okumu et al. 2010). However, most of these interventions have not been demonstrated to have a marked impact on malaria incidence across multiple sites and so have not received endorsement by the WHO at present. There is urgent need for these technologies to be fully evaluated.
Acknowledgments
We gratefully acknowledge the Southern Africa ICEMR field teams in Macha for their logistical support and participation in field collections and laboratory analysis. We are also very grateful to the communities in Zambia in whose households collections were made. This study was carried out following ethical approval from the Tropical Disease Research Centre IRB, Ndola (TDRC/ERC/2010/4/11) and Johns Hopkins Bloomberg School of Public Health IRB (00003467). This work was supported in part, through funding from the Southern Africa ICEMR (U19AI089680-01) to D.E.N. C.M.J. was supported by a NIH T32 Grant (2T32AI007417-16) and a Johns Hopkins Malaria Research Institute Fellowship. J.C.P. was supported in part by the Johns Hopkins Malaria Research Institute.
References
- Achee N., Bangs M., Farlow R., Killeen G., Lindsay S., Logan J., Moore S., Rowland M., Sweeney K., Torr S., et al. 2012. Spatial repellents: From discovery and development to evidence-based validation. Malar. J. 11: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animut A., Balkew M., Gebre-Michael T., Lindtjorn B. 2013. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malar. J. 12: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio-Nkondjio C., Kerah C. H., Simard F., Awono-Ambene P., Chouaibou M., Tchuinkam T., Fontenille D. 2006. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J. Med. Entomol. 43: 1215–1221. [DOI] [PubMed] [Google Scholar]
- Awono-Ambene H., Kengne P., Simard F., Antonio-Nkondjio C., Fontenille D. 2004. Description and bionomics of Anopheles (Cellia) ovengensis (Diptera: Culicidae), a new malaria vector species of the Anopheles nili group from south Cameroon. J. Med. Entomol. 41: 561–568. [DOI] [PubMed] [Google Scholar]
- Bayoh M. N., Mathias D. K., Odiere M. R., Mutuku F. M., Kamau L., Gimnig J. E., Vulule J. M., Hawley W. A., Hamel M. J., Walker E. D. 2010. Anopheles gambiae: Historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkot T. R., Williams J. L., Schneider I. 1984. Identification of Plasmodium falciparum-infected mosquitoes by a double antibody enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 33: 783–788. [DOI] [PubMed] [Google Scholar]
- Burkot T., Russell T., Reimer L., Bugoro H., Beebe N., Cooper R., Sukawati S., Collins F., Lobo N. 2013. Barrier screens: A method to sample blood-fed and host-seeking exophilic mosquitoes. Malar. J. 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaccour C., Kobylinski K., Bassat Q., Bousema T., Drakeley C., Alonso P., Foy B. 2013. Ivermectin to reduce malaria transmission: A research agenda for a promising new tool for elimination. Malar. J. 12: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood J. D., TomÁS E.V.E., Cuamba N., Pinto J. 2015. Analysis of the sporozoite ELISA for estimating infection rates in Mozambican anophelines. Med. Vet. Entomol. 29: 10–16. [DOI] [PubMed] [Google Scholar]
- Conn J. E., Norris D. E., Donnelly M. J., Beebe N. W., Burkot T. R., Coulibaly M. B., Chery L., Eapen A., Keven J. B., Kilama M., et al. 2015. Entomological monitoring and evaluation: diverse transmission settings of ICEMR projects will require local and regional malaria elimination strategies. Am. J. Trop. Med. Hyg. 93: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M., Kahindi S., Oriango R., Owaga C., Ayoma E., Mabuka D., Nyangau D., Abel L., Atieno E., Awuor S., et al. 2015. ‘A bite before bed': Exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar. J. 14: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Muleba M., Stevenson J. C., Norris D. E. 2016. Habitat partitioning of malarial vectors in Nchelenge District, Zambia. Am. J. Trop. Med. Hyg. pii: 150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debboun M., Strickman D. 2013. Insect repellents and associated personal protection for a reduction in human disease. Med. Vet. Entomol. 27: 1–9. [DOI] [PubMed] [Google Scholar]
- Degefa T., Zeynudin A., Godesso A., Michael Y., Eba K., Zemene E., Emana D., Birlie B., Tushune K., Yewhalaw D. 2015. Malaria incidence and assessment of entomological indices among resettled communities in Ethiopia: A longitudinal study. Malar. J. 14: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnez L., Van Bortel W., Denis L., Roelants P., Veracx A., Trung H. 2011. False positive circumsporozoite protein ELISA: A challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 10: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornadel C., Norris L., Franco V., Norris D. 2011. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis. 11: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A. O., Gomes M. G., Rowland M., Coleman P. G., Davies C. R. 2014. Controlling malaria using livestock-based interventions: A one health approach. PLoS ONE 9: e101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissbuhler Y., Chaki P., Emidi B., Govella N., Shirima R., Mayagaya V., Mtasiwa D., Mshinda H., Fillinger U., Lindsay S., et al. 2007. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar. J. 6: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M. T. 1964. The role of secondary vectors of malaria in North-East Tanganyika. Trans. R. Soc. Trop. Med. Hyg. 58: 154–158. [DOI] [PubMed] [Google Scholar]
- Gillies M., Smith A. 1960. Effect of a residual house-spraying campaign on species balance in the Anopheles funestus group: The replacement of Anopheles gambiae Giles with Anopheles rivulorum leesoni. Bull. Entomol. Res. 51: 248–252. [Google Scholar]
- Gillies M. T., De Meillon B. 1968. The Anophelinae of Africa South of the Sahara. South African Institute for Medical Research, Johannesburg, South Africa. [Google Scholar]
- Gillies T., Coetzee M. 1987. A supplement to the anophelinae of Africa South of the Sahara: Afrotropical Region, South African Institute for Medical Research, Johannesburg, South Africa. [Google Scholar]
- Govella N., Okumu F., Killeen G. 2010. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am. J. Trop. Med. Hyg. 82: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtewold T., Prior A., Torr S., Gibson G. 2004. Could insecticide-treated cattle reduce Afrotropical malaria transmission? Effects of deltamethrin-treated Zebu on Anopheles arabiensis behaviour and survival in Ethiopia. Med. Vet. Entomol. 18: 408–417. [DOI] [PubMed] [Google Scholar]
- Hewitt S., Rowland M. 1999. Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Trop. Med. Intl. Health 4: 481–486. [DOI] [PubMed] [Google Scholar]
- Kent R., Norris D. 2005. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am. J. Trop. Med. Hyg. 73: 336–342. [PMC free article] [PubMed] [Google Scholar]
- Kent R. J., Coetzee M., Mharakurwa S., Norris D. E. 2006. Feeding and indoor resting behaviour of the mosquito Anopheles longipalpis in an area of hyperendemic malaria transmission in southern Zambia. Med. Vet. Entomol. 20: 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen G. 2014. Characterizing, controlling and eliminating residual malaria transmission. Malar. J. 13: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen G., Kihonda J., Lyimo E., Okech F., Kotas M., Mathenge E., Schellenberg J., Lengeler C., Smith T., Drakeley C. 2006. Quantifying behavioural interactions between humans and mosquitoes: Evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect. Dis. 6: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekemoer L., Kamau L., Hunt R., Coetzee M. 2002. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 66: 804–811. [DOI] [PubMed] [Google Scholar]
- Lengeler C. 2004. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. CD000363. [DOI] [PubMed] [Google Scholar]
- Lindblade K., Gimnig J., Kamau L., Hawley W., Odhiambo F., Olang G., Ter Kuile F., Vulule J., Slutsker L. 2006. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. J. Med. Entomol. 43: 428–432. [DOI] [PubMed] [Google Scholar]
- Mharakurwa S., Thuma P. E., Norris D. E., Mulenga M., Chalwe V., Chipeta J., Munyati S., Mutambu S., Mason P. R. 2012. Malaria epidemiology and control in Southern Africa. Acta Trop. 121: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroux N., Gomez M., Pennetier C., Elanga E., Djenontin A., Chandre F., Djegbe I., Guis H., Corbel V. 2012. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect Dis. 206: 1622–1629. [DOI] [PubMed] [Google Scholar]
- Moss W. J., Hamapumbu H., Kobayashi T., Shields T., Kamanga A., Clennon J., Mharakurwa S., Thuma P. E., Glass G. 2011. Use of remote sensing to identify spatial risk factors for malaria in a region of declining transmission: A cross-sectional and longitudinal community survey. Malar. J. 10: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss W. J., Norris D. E., Mharakurwa S., Scott A., Mulenga M., Mason P. R., Chipeta J., Thuma P. E. 2012. Challenges and prospects for malaria elimination in the Southern Africa region. Acta Trop. 121: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangangi J., Muturi E., Muriu S., Nzovu J., Midega J., Mbogo C. 2013. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomichene T., Tata E., Boyer S. 2015. Malaria case in Madagascar, probable implication of a new vector, Anopheles coustani. Malar. J. 14: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigatu W., Petros B., Lulu M., Adugna N., Wirtz R. 1994. Species composition, feeding and resting behaviour of the common anthropophilic anopheline mosquitoes in relation to malaria transmission in Gambella, south west Ethiopia. Intl. J. Trop. Insect Sci. 15: 371–377. [Google Scholar]
- NMCC. 2011. National Malaria Strategic Plan 2011–2015. Government of Zambia, Ministry of Health, National Malaria Control Programme, Lusaka, Zambia. [Google Scholar]
- Norris L. C., Norris D. E. 2015. Phylogeny of anopheline (Diptera: Culicidae) species in southern Africa, based on nuclear and mitochondrial genes. J. Vector Ecol. 40: 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorie P. N., McKenzie F. E., Ademowo O. G., Bockarie M., Kelly-Hope L. 2011. Nigeria Anopheles vector database: an overview of 100 years' research. PLoS ONE 6: e28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu F. O., Moore S. J., Govella N. J., Chitnis N., Killeen G. F. 2010. Potential benefits, limitations and target product-profiles of odor-baited mosquito traps as a means of malaria control. PLoS ONE 5: e11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pates H., Curtis C. 2005. Mosquito behavior and vector control. Annu. Rev. Entomol. 50: 53–70. [DOI] [PubMed] [Google Scholar]
- Poche R. M., Burruss D., Polyakova L., Poche D. M., Garlapati R. B. 2015. Treatment of livestock with systemic insecticides for control of Anopheles arabiensis in western Kenya. Malar. J. 14: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. R., Overgaard H. J., Abaga S., Reddy V. P., Caccone A., Kiszewski A. E., Slotman M. A. 2011. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 10: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M., Durrani N., Kenward M., Mohammed N., Urahman H., Hewitt S. 2001. Control of malaria in Pakistan by applying deltamethrin insecticide to cattle: A community-randomised trial. Lancet 357: 1837–1841. [DOI] [PubMed] [Google Scholar]
- Russell T., Govella N., Azizi S., Drakeley C., Kachur K., Killeen G. 2011. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Brogdon W., Collins F. 1993. Identification of single specimens of the Anopheles gambiae complex by the Polymerase Chain Reaction. Am. J. Trop. Med. Hyg. 49: 520–529. [DOI] [PubMed] [Google Scholar]
- Seyoum A., Sikaala C., Chanda J., Chinula D., Ntamatungiro A., Hawela M., Miller J., Russell T., Briet O., Killeen G. 2012. Most exposure to Anopheles funestus and Anopheles quadriannulatus in Luangwa valley, South-East Zambia occurs indoors, even for users of insecticidal nets. Parasit Vectors 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougoufara S., Diedhiou S., Doucoure S., Diagne N., Sembene P., Harry M. 2014. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: A new challenge to malaria elimination. Malar. J. 13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Laurent B., Cooke M., Krishnankutty S. M., Asih P., Mueller J. D., Kahindi S., Ayoma E., Oriango R. M., Thumloup J., Drakeley C., et al. 2016. Molecular characterization reveals diverse and unknown malaria vectors in the western Kenyan highlands. Am. J. Trop. Med. Hyg. 94: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J., St Laurent B., Lobo N. F., Cooke M. K., Kahindi S. C., Oriango R. M., Harbach R. E., Cox J., Drakeley C. 2012. Novel vectors of malaria parasites in the western highlands of Kenya. Emerg. Infect. Dis. 18: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusting L., Thwing J., Sinclair D., Fillinger U., Gimnig J., Bonner K., Bottomley C., Lindsay S. 2013. J. G., Bonner KE, Bottomley C, Lindsay SW: Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev 8: CD008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2013. Larval source management – a supplementary measure for malaria vector control. An operational manual. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Wilkes T., Matola Y., Charlwood J. 1996. Anopheles rivulorum, a vector of human malaria in Africa. Med. Vet. Entomol. 10: 108–110. [DOI] [PubMed] [Google Scholar]
- Wilson A. L., Chen-Hussey V., Logan J. G., Lindsay S. W. 2014. Are topical insect repellents effective against malaria in endemic populations? A systematic review and meta-analysis. Malar. J. 13: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes M., Boelee E. 2012. Early biting rhythm in the Afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 26: 103–105. [DOI] [PubMed] [Google Scholar]