Abstract
Background:
Grip strength is a noninvasive method of risk stratification; however, the association between changes in strength and mortality is unknown. The purposes of this study were to examine the association between grip strength and mortality among older Mexican Americans and to determine the ability of changes in strength to predict mortality.
Methods:
Longitudinal data were included from 3,050 participants in the Hispanic Established Population for the Epidemiological Study of the Elderly. Strength was assessed using a hand-held dynamometer and normalized to body mass. Conditional inference tree analyses were used to identify sex- and age-specific weakness thresholds, and the Kaplan–Meier estimator was used to determine survival estimates across various strata. We also evaluated survival with traditional Cox proportional hazard regression for baseline strength, as well as with joint modeling of survival and longitudinal strength change trajectories.
Results:
Survival estimates were lower among women who were weak at baseline for only 65- to 74-year-olds (11.93 vs 16.69 years). Survival estimates were also lower among men who were weak at baseline for only ≥75-year-olds (5.80 vs 7.39 years). Lower strength at baseline (per 0.1 decrement) was significantly associated with mortality (hazard ratio [HR]: 1.10; 95% confidence interval [CI]: 1.01–1.19) for women only. There was a strong independent, longitudinal association between strength decline and early mortality, such that each 0.10 decrease in strength, within participants over time, resulted in a HR of 1.12 (95% CI: 1.00–1.25) for women and a HR of 1.15 (95% CI: 1.04–1.28) for men.
Conclusions:
Longitudinal declines in strength are significantly associated with all-cause mortality in older Mexican Americans.
Keywords: Mexican American, Mortality, Strength, Handgrip, Diabetes
A growing body of evidence supports the role of muscular strength capacity as a protective factor for function, cardiometabolic health, and longer survival across populations. Recent research supports the role that muscle atrophy and weakness may play on the progression of secondary complications with aging or disease, and national efforts to identify thresholds for low strength (1,2) among adults may help clinicians screen patients for prognostic risk. However, despite the known links between weakness, cardiometabolic disease (3,4), incident disability (5), and early mortality (6–13), the extent to which age-related longitudinal declines in muscle strength contribute to an increased risk phenotype is largely unknown. Joint modeling of survival and longitudinal nonsurvival data may compliment traditional Cox regression by providing a more accurate representation of the quantitative influence of time-varying factors, such as strength capacity, on the survival estimates (14,15).
The lifetime risks of obesity, diabetes, and disability are elevated among older Hispanic and Mexican Americans (16–18), a population projected to increase by nearly 115% between 2014 and 2060 in the United States (19). Therefore, the overall goal of this study was to better understand the interrelationships between grip strength and mortality in a large cohort of older Mexican Americans and to explore the longitudinal link between strength change and survival endpoints. Our hypothesis was that baseline muscle weakness would be strongly associated with earlier mortality, even after adjusting for known predictors such as diabetes and disability. We further hypothesized that longitudinal rate of strength decline would be a greater independent risk factor for all-cause mortality than baseline weakness.
Methods
Design and Sample Population
This study used data from the Hispanic Established Population for the Epidemiological Study of the Elderly (H-EPESE), a longitudinal study of Mexican Americans aged 65 and older, residing in Texas, New Mexico, Colorado, Arizona, and California. The H-EPESE was modeled after the previous EPESE studies in East Boston, New Haven, rural Iowa, and North Carolina (20). As previously described (21), the H-EPESE used an area probability sample design developed by listing counties in the Southwestern states to ensure representativeness of the older Mexican American population of those regions. Starting in 1993–1994 (Wave 1), in-home interviews and limited medical assessments were conducted with a response rate of 83%, and the 3,050 baseline Mexican American participants were followed up in waves: (2) 1995–1996 (n = 2,437), (3) 1998–1999 (n = 1,980), (4) 2000–2001 (n = 1,678), (5) 2004–2005 (n = 1,166), (6) 2006–2007 (921), and (7) 2010–2011 (659).
Exposure Variable
Strength was assessed using a hydraulic handgrip dynamometer (Jamar Hydraulic Dynamometer, model 5030J1; JA Preston, Clifton, NJ), as previously described in detail (22,23). Briefly, a trained examiner explained and demonstrated the protocol to the participant, then adjusted the grip size of the dynamometer to the participant’s hand size, and asked the participant to squeeze the dynamometer for a practice trial. Thereafter, the participant was assigned to start the test with his/her dominant hand and was asked to squeeze the dynamometer as hard as possible, exhaling while squeezing. Two trials were performed. The grip test was performed in the standing position unless the participant was physically limited. Because there is substantial covariance between strength capacity and body mass, grip strength was normalized as strength per body mass (ie, Grip strength [kg] / Body mass [kg]) and expressed as normalized grip strength (NGS). For each participant, grip strength was adjusted to the respective body mass ascertained during the same wave of testing.
Covariates
Baseline sociodemographic variables potentially related to mortality included age (in years), sex, marital status (not married, married), and number of formal years of education (1 = less than high school graduate, 2 = high school graduate/general educational development or equivalent, and 3 = college graduate or above). The presence of diabetes was determined with a series of questions asking participants whether a doctor had ever told them that they had diabetes. All who responded yes indicated that they had been diagnosed with type 2 diabetes. Presence of a functional disability was determined by individual questions pertaining to difficulty performing specific, everyday tasks. The questions chosen as most relevant for this research involved reporting an inability (ie, answering “no”) to perform specific tasks most relevant to mobility and lower extremity functional capacity [“Can you do light housework without help (dishwashing and bed making, etc.)?”, “Can you walk up and down stairs to the second floor without help?”, “Can you walk half a mile without help?”], or answering “need help” or “unable to do” to the following question: “Do you need help from another person or special equipment or a device for walking across a small room?” Presence of a functional disability was recorded for any individual who had one or more limitations as determined by these questions.
All-cause Mortality
Deaths through December 31, 2011 were ascertained through reports by living relatives and confirmed by a search of the National Death Index and the Social Security Administration’s Death Master File. Of the 3,050 participants, 2,178 (71.4%) died over the 18-year follow-up period. Participants who survived were censored at their last interview date. Of the initial cohort, there were 2,026 participants with grip strength data at baseline. Of those 2,026 participants, 1,289 (63.6%) died over the 18-year follow-up, and 257 were lost to follow-up (12.7%). Baseline characteristics and mortality estimates from participants not included in the analyses, due to missing data on grip strength, are provided in Supplementary File 1.
Statistical Analyses
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive characteristics are provided as means, standard errors, and percentages. Differences in these baseline characteristics across age categories were tested using two-sample t tests and chi-squared tests for continuous and categorical variables, respectively. A similar strategy was used to test differences for outcomes between men and women across equivalent age categories (ie, 65–74 years and ≥75 years).
Threshold analysis
Conditional inference tree analyses were used to determine risk thresholds of normalized strength in differentiating the overall survival from the initial enrollment time (ie, thresholds which provide the largest separations among Kaplan–Meier curves for select strata). Unlike a recent study in older adults that used classification and regression tree analysis to identify cut points for weakness (2), we chose not to incorporate this method because it tends to overfit. The conditional inference tree method recursively partitions participants into mutually exclusive groups defined by predictor cut points, grouping together participants with similar outcome probabilities (24). However, in contrast with classification and regression tree analysis, it utilizes a formal statistical framework to evaluate the recursive partitioning, taking into account both the distributional properties of the measures and multiple comparison between groups. This technique is also free of modeling assumptions, which allows for optimal concurrent validity, by identifying those cut points with the strongest association with the outcome. Moreover, it does not require an a priori specified number of cut points, and thus it can provide more than a single threshold to predict the outcome. The analysis was conducted using R software with the party package (24). To preserve consistency of sample across models, we included only those individuals with complete data for all variables and covariates.
Survival and longitudinal joint modeling
The Kaplan–Meier estimator was used to determine survival estimates and curves across various strata including sex, age category (65–74 years and ≥ 75 years), and weakness status at enrollment. We also evaluated survival with traditional Cox proportional hazard regression for baseline strength as a continuous variable (ie, with units reflecting 0.1 decrements of NGS between participants), adjusted for age, sex, diabetes, disability status, and various sociodemographic variables. As a high NGS is indicative of decreased risk, the inverse of NGS was used in the model. To utilize the longitudinal strength data obtained throughout the study, we used joint modeling of survival and longitudinal nonsurvival data, modeling the trajectory of strength changes through a linear mixed-effect model, and the effects of the time-dependent exposure (strength) on overall survival through an extension of the traditional Cox model as previously described (15,25).
The participant hazard function was modeled as:
where M(t) denotes NGS at time t (measured from the enrollment time); X represents baseline covariates including gender, age, diabetes, disability status, and various sociodemographic variables; h 0 denotes the baseline risk function modeled as basis splines with 9degrees; and parameter γ quantifies the effect of the underlying longitudinal change in the exposure (ie, change in NGS) on the hazard for death. Because M(t) data were observed only at the designed follow-up times, the longitudinal trajectory of M(t) for the ith patient was modeled as basis splines with 5degrees with participant-specific random effects.
Results
Baseline descriptive data are presented as means, standard errors, and percentages across age categories in Table 1. In both men and women, prevalence of physical disabilities was significantly higher among those aged 75 and older, and women had higher prevalences than men. Both obesity and diabetes were more prevalent among 65- to 74-year-olds as compared with those among ≥75-year-olds; however, obesity was significantly more prevalent among women across all ages. There were no significant differences in diabetes between men and women at either age category. Across both age categories, men were stronger than women, in terms of both absolute and NGS capacity. For both men and women, grip strength showed a significant stepwise decline among individuals aged 75 and older.
Table 1.
Demographic and Cardiometabolic Characteristics of the Study Population by Sex and Age Category
| Men | Women | |||
|---|---|---|---|---|
| Age 65–74 y | Age ≥ 75 y | Age 65–74 y | Age ≥ 75 y | |
| n = 885 | n = 406 | n = 1,168 | n = 591 | |
| Age, y | 69.78 (2.99) | 81.82 (5.00)† | 69.52 (3.00) | 81.75 (4.84)† |
| BMI, kg/m2 | 27.57 (4.53)† | 25.81 (4.04) | 29.07 (5.85)* | 26.97 (5.23)* |
| Obesity (BMI > 30kg/m2), % | 25.4† | 17.3 | 39.2*,† | 25.5* |
| Level of education | ||||
| Less than HS, % | 87.9 | 90.3 | 90.2 | 92.7 |
| HS graduate, % | 7.7 | 7.6 | 6.7 | 5.4 |
| Some college, % | 2.3 | 1.3 | 1.5 | 0.7 |
| College graduate, % | 2.1 | 0.8 | 1.6 | 1.2 |
| Married, % | 79.4 | 66.0† | 50.3* | 22.8*,† |
| Grip strength, kg | 32.39 (7.92) | 25.56 (7.61)† | 20.35 (5.88)* | 16.05 (5.63)*,† |
| NGS (relative to body mass) | 0.43 (0.11) | 0.37 (0.11)† | 0.30 (0.09)* | 0.26 (0.10)*,† |
| Functional disability, % | 19.3 | 43.1† | 27.1* | 57.0*,† |
| Diabetes, % | 25.8† | 20.0 | 25.7 | 21.7 |
| Systolic blood pressure, mmHg | 136.41 (15.86) | 135.14 (17.51) | 135.57 (16.19) | 136.58 (17.48) |
| Diastolic blood pressure, mmHg | 81.13 (10.25)*,† | 77.62 (12.35) | 79.82 (11.14) | 78.9 (9.94) |
Notes: BMI = body mass index; HS = high school; NGS = normalized grip strength.
*Significant difference between men and women in equivalent age category (p < .01).
†Significant difference between ages 65–74 y and 75 and older (p < .01).
Threshold Analysis
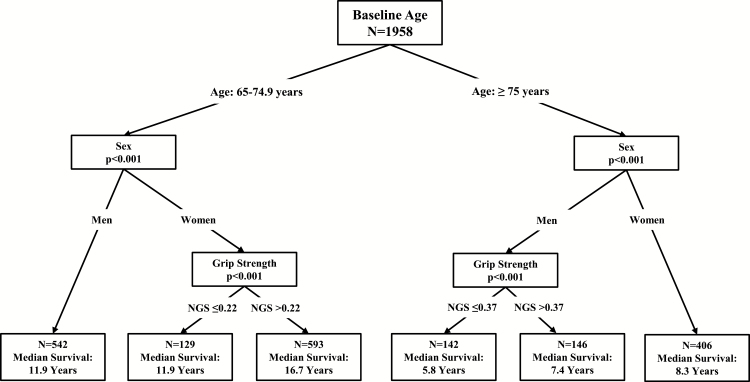
Conditional inference trees predicting mortality confirmed different low strength thresholds for men and women. Figure 1 provides results for the primary definitions. No NGS cutoffs were identified in women aged 75 and older or in men aged 65–74 years. The cutoff identified in men aged 75 and older was based on having NGS less than or equal to 0.37 (ie, grip strength in kg ≤ [0. 37 × body mass in kg]) versus greater than 0.37. Among women aged 65–74 years, the identified NGS cutoff was based on having NGS less than or equal to 0.22 versus greater than 0.22.
Figure 1.
Conditional inference trees for age, sex, and baseline strength as predictors of overall mortality in men and women.
Survival Analyses
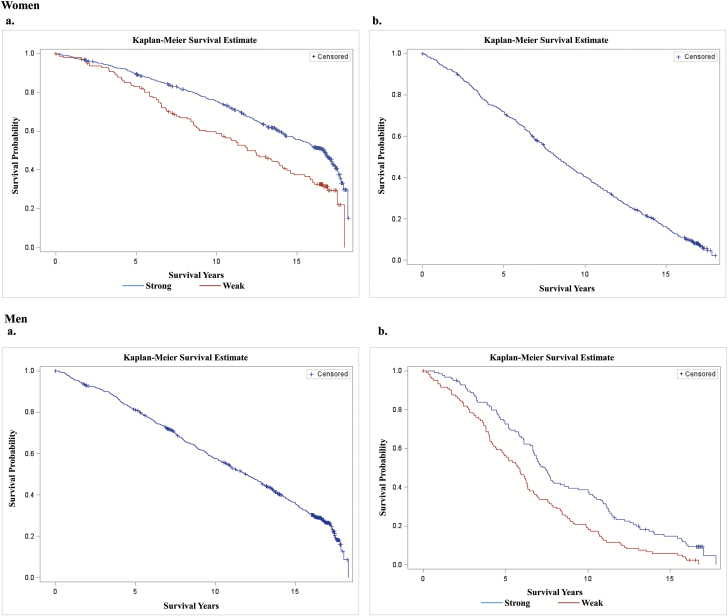
Of the 2,026 individuals who had a baseline measure of NGS, there were 1,289 deaths (63.6%), 257 were lost to follow-up (12.7%), and a final sample of 2,002 had complete data on the exposure variable (NGS) and all covariates. Figure 2 provides Kaplan–Meier survival curves for men and women across categorical age and NGS strata. The median survival rates for women aged 65–74 years (14.98 years, 95% confidence interval [CI]: 14.19–15.87) and ≥ 75 years (8.25 years, 95%CI 7.55–8.91) were greater than those for men aged 65–74 years (11.87 years; 95% CI: 10.99–12.70) and ≥75 years (6.34 years; 95% CI: 5.93–6.77). Survival estimates were lower among women who were weak at baseline for 65- to 74-year-olds (median survival: 11.93 vs 16.69 years; 10-year survival: 63.3% vs 75.4%; p < .001). Survival estimates were also lower among men who were weak at baseline for ≥75-year-olds (median: 5.80 vs 7.39 years; 10-year survival: 18.2% vs 37.9%; p = .001).
Figure 2.
Kaplan–Meier curves for men and women aged (A) 65–74 years and (B) 75 and older.
Traditional Cox proportional hazard regression demonstrated that, for every 0.1 lower NGS between participants, the crude hazard of mortality was 1.20 (95% CI: 1.12–1.29) and 1.29 (95% CI: 1.19–1.39) for men and women, respectively. After adjusting for sex, age, baseline diabetes status, baseline disability status, marital status, and education, baseline diabetes status (hazard ratio [HR]: 1.70; 95% CI: 1.51–1.92) and disability status (HR: 1.43; 95% CI: 1.26–1.62) were also strong independent predictors of early mortality. Sex-stratified analyses revealed that lower strength at baseline (per 0.1 decrement) was significantly associated with mortality (HR: 1.10; 95% CI: 1.01–1.19) only for women (Table 2).
Table 2.
Traditional Cox Proportional Hazard Regression for Baseline Variables and Mortality for Men and Women
| Model Predictor(s) | Men | Women | ||||
|---|---|---|---|---|---|---|
| HR | 95% CIs | p Value | HR | 95% CIs | p Value | |
| Age (y) | 1.08 | 1.07–1.10 | <.001 | 1.08 | 1.06–1.09 | <.001 |
| Married | 0.74 | 0.62–0.89 | .001 | 0.88 | 0.75–1.02 | .09 |
| Baseline disability status (reference: no disabilities) | 1.40 | 1.15–1.70 | <.001 | 1.4 | 1.19–1.64 | <.001 |
| Baseline diabetes status (reference: no diabetes) | 1.74 | 1.46–2.09 | <.001 | 1.73 | 1.47–2.03 | <.001 |
| Education | 1.09 | 0.93–1.26 | .29 | 1.04 | 0.89–1.23 | .61 |
| NGS | 1.04* | 0.96–1.12** | .35 | 1.10* | 1.01–1.19** | .03 |
Notes: CI = confidence interval; HR = hazard ratio; NGS = normalized grip strength.
*HR for every 0.10 (10%) decrement in NGS, between participants.
**95% CIs for every 0.10 (10%) decrement in NGS, between participants.
Joint Modeling of Longitudinal and Survival Data
Longitudinal parameterization of NGS had a significant, time-dependent effect on mortality (p < .001). There was a strong longitudinal association between strength decline and early mortality, such that every 0.1 decrease in NGS, within participants over time, resulted in an increased HR of 1.12 (95% CI: 1.00–1.25) for women and a HR of 1.15 (95% CI: 1.04–1.28) for men, after adjusting for baseline age, baseline diabetes status, baseline disability status, marital status, and education. Table 3 shows the fully adjusted, stratified models with all covariates.
Table 3.
Stratified Joint Modeling of Survival and Longitudinal Analysis for Men and Women. The Effects of Baseline Covariates (ie, age, marital status, disability, etc.) and Time-dependent Covariate (NGS) Were Modeled for Overall Survival
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Model Predictor(s) | HR | 95% CIs | p Value | HR | 95% CIs | p Value |
| Age (y) | 1.09 | 1.08–1.10 | <.001 | 1.09 | 1.08–1.11 | <.001 |
| Married | 0.92 | 0.78–1.08 | .30 | 0.97 | 0.85–1.12 | .71 |
| Baseline disability status (reference: no disabilities) | 1.29 | 1.09–1.53 | .003 | 1.39 | 1.21–1.60 | <.001 |
| Baseline diabetes status (reference: no diabetes) | 1.74 | 1.48–2.05 | <.001 | 1.75 | 1.53–2.02 | <.001 |
| Education | 1.07 | 0.99–1.23 | .35 | 1.03 | 0.89–1.11 | .70 |
| NGS | 1.15* | 1.04–1.28** | .008 | 1.12* | 1.00–1.25** | .05 |
Notes: CI = confidence interval; HR = hazard ratio; NGS = normalized grip strength.
*HR for every 0.10 incremental decrease in NGS.
**95% CIs for every 0.10 incremental decrease in NGS.
Discussion
Our principal finding was that grip strength was significantly associated with all-cause mortality in older Mexican Americans. Although men had earlier mortality than women, Kaplan–Meir estimates for having “weakness” at baseline demonstrated substantially lower survival rates among both younger and older women and a decreased survival only among men who were aged 75 and older. Moreover, Cox regression demonstrated that lower strength at baseline was significantly associated with mortality among women only. More importantly, we have demonstrated for the first time that strength change coefficients from the longitudinal analysis were larger than those from the baseline only analysis. Specifically, for every 0.10 decrease in strength relative to body mass, over time, the risk of death increased 12% and 15% for women and men, respectively, even after adjustment for all baseline covariates. This HR is considerably more prominent than that of baseline weakness, because it represents a more efficient and precise use of longitudinal data from joint modeling (15). These findings lend strong support to the growing body of literature revealing a link between muscle strength and risk of mortality.
We found that the predictive power of baseline NGS on survival was stronger for Mexican American women than for men. This result may be explained by how we operationalized strength as a normalized measure (ie, accounting for body mass), as opposed to using an absolute measure. The Foundation of the National Institute of Health (FNIH) Sarcopenia Project (12,26) compared relative versus absolute measures of grip strength and found that, although weakness was associated with slower walking speed, the use of a normalized measure of grip strength was more predictive of outcomes among women. In the FNIH study, obese women were more likely to be classified as weak using a normalized measure of grip strength and also had higher odds of slow walking speed compared with the absolute measure, which was more predictive for men. In our study, obesity was more prevalent in women than in men across all age groups. Thus, by accounting for body mass, NGS may have explained more variability in all-cause mortality for women than for men. However, it is also plausible that weakness or declines in muscular strength, relative to body mass, poses greater risk of mortality for women than men. Regardless, because there is substantial covariance between strength capacity and body mass, the normalization of individual-level strength to body mass is critical in improving sensitivity of cutoff values and screening efforts.
Interestingly, when stratified by sex, the longitudinal analyses revealed that declines in strength were strongly associated with mortality in both men and women. The results of this study have important implications for the long-term health of Mexican Americans. Strength loss has been linked to a host of negative health outcomes, including impaired physical functioning and incident disability (5). However, muscular atrophy and weakness are also closely linked with various cardiometabolic conditions, such as chronic hyperglycemia (27), diabetes (28), and the metabolic syndrome (3), particularly in those with advancing age. In a recent study by Norman and colleagues (29), grip strength was independently associated with chronic inflammatory markers, even after adjusting for sociodemographic covariates. Because the elevated lifetime risk of cardiometabolic disease is highest among Hispanic men and women, at just more than 50% (18), early screening and health promotion efforts are vital in reducing the burden of disease, incidence of comorbidities, and health care utilization in this population. The Centers for Medicare and Medicaid Services (CMS) Clinical Quality Measures, which specifies the recommended core measures for adult recipients of both Medicare and Medicaid, has not recommended the assessment of muscle strength as part of their priority health care improvement goals. This has important implications given that 30% and 8% of Medicaid and Medicare recipients are Hispanic, respectively (30,31).
Our findings underscore the potential value of grip strength measures for clinical screening. In recent findings from the PURE study, Leong and colleagues demonstrated that, among nearly 140,000 adults across 17 socioculturally and economically diverse countries, weak grip strength was an even stronger predictor of all-cause and cardiovascular mortality than systolic blood pressure (6). Yet, despite growing consensus that muscle strength and functional capacity are essential components of health and longevity (7,32), functional capacity is rarely assessed in the clinical setting. The use of multiple measurements of strength over time may offer a unique opportunity for screening to inform the timing of specific interventions, such as physical activity, strength training participation, and healthy body weight maintenance, prior to the onset of adverse health outcomes. Among older individuals, the decline in strength undergoes a steeper, more precipitous decline than muscle mass (33), so regular screening may be important to provide patients with feedback regarding risk.
We acknowledge that our study has several limitations. First, although these results highlight an important relationship between longitudinal grip strength trajectories and survival among Mexican Americans, our findings are not necessarily generalizable to other populations. Second, we cannot rule out time-varying confounding because baseline measurements of all covariates, with the exception of grip strength, were included in our final model. Whether declines in NGS “cause” an elevated risk for early mortality, or if incident disease processes (eg, diabetes) themselves, are a cause of diminished muscle function (ie, competing risks, as implied from the study by Kalyani and colleagues (27)), is an interesting and complex topic. Indeed, we were unable to determine whether other competing risks or unmeasured confounding (ie, other risk factors [eg, smoking] or existing diseases [eg, cardiovascular disease]) may have influenced the observed survival trajectories. Third, as with any long-term, prospective study, loss to follow-up may have resulted in an underestimation of the true association if sicker individuals were more likely to drop out. Despite these limitations, this study has various strengths. Most notably, whereas previous studies looked at strength as a baseline risk exposure in traditional survival analyses, we used 18 years of longitudinal data to examine the association between grip strength trajectories and survival. Moreover, joint modeling procedures were incorporated for repeated measurement and time-to-event data, a novel analytic technique largely underutilized in clinical and epidemiologic research. Finally, we examined this research question within a Mexican American sample, the largest subgroup of Hispanics in the United States. By 2060, the Hispanic population is estimated to make up nearly 30% of the U.S. population (19). Therefore, given the expected rise of this demographic group combined with the rapidly aging population, our results have important public health relevance for understanding future survival trajectories and for informing efforts to meaningfully intervene in preventing health disparities in this population.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study was supported by the National Institutes of Health (R01AG10939, K.S.M., PI; R01AG17638, R24HD065702, K.J.O., PI). M.D.P. is funded by the National Institutes of Health (1KO1 HD074706). The funders had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance of Sarah Toombs Smith, PhD, ELS, in manuscript preparation. Dr. Sarah Toombs Smith received no special remuneration for this assistance.
References
- 1. Spruit MA, Sillen MJ, Groenen MT, Wouters EF, Franssen FM. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc. 2013;14:775.e5–775.11. doi:10.1016/j.jamda.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 2. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi:10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Senechal M, McGavock JM, Church TS, et al. Cut points of muscle strength associated with metabolic syndrome in men. Med Sci Sports Exerc. 2014;46:1475–1481. doi:10.1249/MSS.0000000000000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peterson MD, Zhang P, Choksi P, Markides KS, Al Snih S. Muscle weakness thresholds for prediction of diabetes in adults. Sports Med. In Press. doi:10.1007/s40279-015-0463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirani V, Blyth F, Naganathan V, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc. 2015;16:607–613. doi:10.1016/j.jamda.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 6. Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi:10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 7. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 8. Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. doi:10.1046/j.1532-5415.2002.50312.x [DOI] [PubMed] [Google Scholar]
- 9. Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi:10.1136/bmj.a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper R, Strand BH, Hardy R, Patel KV, Kuh D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ. 2014;348:g2219. doi:10.1136/bmj.g2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lopez-Jaramillo P, Cohen DD, Gomez-Arbelaez D, et al. Association of handgrip strength to cardiovascular mortality in pre-diabetic and diabetic patients: a subanalysis of the ORIGIN trial. Int J Cardiol. 2014;174:458–461. doi:10.1016/j.ijcard.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 12. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi:10.1093/gerona/glu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stenholm S, Mehta NK, Elo IT, Heliovaara M, Koskinen S, Aromaa A. Obesity and muscle strength as long-term determinants of all-cause mortality—a 33-year follow-up of the Mini-Finland Health Examination Survey. Int J Obesity. 2014;38:1126–1132. doi:10.1038/ijo.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asar Ö, Ritchie J, Kalra PA, Diggle PJ. Joint modelling of repeated measurement and time-to-event data: an introductory tutorial. Int J Epidemiol. 2015;44:334–344. doi:10.1093/ije/dyu262 [DOI] [PubMed] [Google Scholar]
- 15. Lawrence Gould A, Boye ME, Crowther MJ, et al. Joint modeling of survival and longitudinal non-survival data: current methods and issues. Report of the DIA Bayesian joint modeling working group. Stat Med. 2015;34:2181–2195. doi:10.1002/sim.6141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. J Am Med Assoc. 2012;307:491–497. doi:10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- 17. Markides KS, Stroup-Benham CA, Black SA, Satish S, Perkowski LC, Ostir GV. The health of Mexican American elderly: selected findings from the Hispanic EPESE. In: Wykle M, Ford A, eds. Serving Minority Elders in the 21st Century. New York, NY: Springer; 1999. [Google Scholar]
- 18. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2:867–874. doi:10.1016/S2213-8587(14)70161-5 [DOI] [PubMed] [Google Scholar]
- 19. Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. Current Population Reports. U.S. Department of Commerce; 2015:13. [Google Scholar]
- 20. Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging. 1993;5:27–37. [DOI] [PubMed] [Google Scholar]
- 21. Markides KS, Samper-Ternent R, Al Snih S. Aging and health in Mexican Americans: selected findings from the Hispanic EPESE. In: Bangs RL, Davis LE, eds. Race and Social Problems. New York, NY: Springer; 2015:171–186. [Google Scholar]
- 22. Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res. 2004;16:481–486. [DOI] [PubMed] [Google Scholar]
- 23.Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 61(8):859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15:651–674. [Google Scholar]
- 25. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28:2796–2801. doi:10.1200/JCO.2009.25.0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38:82–90. doi:10.2337/dc14-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585–592. doi:10.1016/j.jamda.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 29. Norman K, Stobäus N, Kulka K, Schulzke J. Effect of inflammation on handgrip strength in the non-critically ill is independent from age, gender and body composition. Eur J Clin Nutr. 2014;68:155–158. doi:10.1038/ejcn.2013.261 [DOI] [PubMed] [Google Scholar]
- 30. Kaiser Family Foundation. Distribution of the Nonelderly With Medicaid by Race/Ethnicity. Menlo Park, CA: Kaiser Family Foundation; 2014. [Google Scholar]
- 31. Kaiser Family Foundation. Distribution of Medicare Beneficiaries by Race/Ethnicity. Menlo Park, CA: Kaiser Family Foundation; 2014. [Google Scholar]
- 32. Fragala MS, Kenny AM, Kuchel GA. Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports Med. 2015;45:641–658. doi:10.1007/s40279-015-0305-z [DOI] [PubMed] [Google Scholar]
- 33. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.