Abstract
Background:
Several studies have evaluated the independent prognostic value of impairments in single geriatric-assessment (GA) components in elderly cancer patients. None identified homogeneous subgroups. Our aims were to identify such subgroups based on combinations of GA components and to assess their associations with treatment decisions, admission, and death.
Methods:
We prospectively included 1,021 patients aged ≥70 years who had solid or hematologic malignancies and who underwent a GA in one of two French teaching hospitals. Two geriatricians independently selected candidate GA parameters for latent class analysis, which was then performed on the 821 cases without missing data. Age, gender, tumor site, metastatic status, and inpatient versus outpatient status were used as active covariates and predictors of class membership. Outcomes were cancer treatment decisions, overall 1-year mortality, and 6-month unscheduled admissions. Sensitivity analyses were performed on the overall population of 1,021 patients and on 375 newly enrolled patients.
Results:
We identified four classes: relatively healthy (LC1, 28%), malnourished (LC2, 36%), cognitive and mood impaired (LC3, 15%), and globally impaired (LC4, 21%). Tumor site, metastatic status, age, and in/outpatient status independently predicted class membership (p < .001). In adjusted pairwise comparisons, compared to LC1, the three other LCs were associated with higher risks of palliative treatment, death, and unscheduled admission (p ≤ .05). LC4 was associated with 1-year mortality and palliative treatment compared to LC2 and LC3 (p ≤ .05).
Conclusion:
We identified four health profiles that may help physicians select cancer treatments and geriatric interventions. Researchers may find these profiles useful for stratifying patients in clinical trials.
Keywords: Frailty, Geriatric assessment, Outcomes, Cancer, Epidemiology
In Europe and the United States, approximately 60% of cancers are diagnosed in patients aged ≥65 years, and 70% of cancer deaths occur in this group (1,2). Older patients with cancer raise therapeutic challenges, as they constitute a heterogeneous population with various combinations of comorbidities, disabilities, and geriatric syndromes. The International Society of Geriatric Oncology and US National Comprehensive Cancer Network recommend a geriatric assessment (GA) to detect multidomain health problems potentially associated with poorer outcomes and to introduce interventions effective on those problems that may be reversible (3,4). Several studies have assessed associations between GA domains and adverse outcomes (5–8). However, most of them evaluated the prognostic value of single GA components in isolation from one another. Balducci and coworkers reported three profiles of older patients with cancer, based on theoretical considerations: fit, vulnerable, and frail. No studies have used a statistical approach to identify homogeneous health profiles (9). Applying statistical techniques to combinations of small numbers of GA components might identify health profiles associated with adverse outcomes, thereby providing treatment guidance and allowing the development of targeted interventions appropriate for the needs associated with each profile.
Latent class analysis (LCA) is a patient-centered approach specifically designed to reliably identify subgroups of patients when they exist (10). LCA has been used successfully to investigate, characterize, and validate disease subtypes, as well as to stratify patients into risk groups and to predict treatment responses (11–15).
We therefore used LCA in a population of older inpatients and outpatients with various cancer types to identify health profiles based on combinations of GA findings. We then assessed whether these profiles were associated with treatment decisions and/or with adverse outcomes such as 6-month unscheduled admissions and 1-year overall mortality.
Methods
Population and Data Source
We used data from the ELCAPA survey (ELderly CAncer PAtients), a prospective cohort study of consecutive patients aged ≥70 years who had newly diagnosed, histologically documented, solid, or hematologic malignancies; and who were referred by oncologists, radiotherapists, surgeons, or other specialists to one of two teaching hospitals in the Paris urban area, France, for a pretherapeutic GA (16); such referral occurred for about 60% of all older patients seen for cancer (8). For the present analysis (ELCAPA_09), we selected patients recruited between 2007 and 2012 (n = 1,021). Informed consent was obtained from all study patients prior to inclusion. The protocol was approved by the appropriate ethics committee (CPP Ile-de-France I, Paris, France).
At baseline, all patients underwent a GA before treatment decisions were made, as described previously (16). A trained geriatrician assessed the following GA domains: function and mobility, nutritional status, cognition, mood, social environment, comorbidities, and polypharmacy. Age, gender, cancer characteristics, and the planned cancer treatment were recorded. Vital status was determined from the medical records or public records office. Unscheduled admissions during the 6 months after study inclusion were identified by medical record review.
Indicators Used to Determine GA-Based Health Profiles
LCA is a multivariate regression technique designed to describe relationships between a set of observed dependent variables (ie, latent class indicators) and an unobserved categorical latent variable (17). The objective is to find the smallest number of groups such that patients in one group are similar to one another but distinct from patients in other groups (18,19). Appropriate selection of the latent class indicators is of paramount importance, as it constrains the ability to identify latent classes. We used a two-step approach for selection of the health indicators. First, we compiled a list of 46 potentially relevant health indicators (Online Supplement 1). Due to statistical considerations, and because many items were highly correlated (eg, Activities of Daily Living score [ADL] and ECOG-PS), two senior geriatricians specialized in geriatric oncology (P.C. and E.L.) independently selected 10 indicators, covering all GA domains, which they considered most relevant. The two geriatricians then discussed those indicators about which their opinions differed. They reached a consensus about six indicators, which were then used for LCA. These indicators were functional impairment (ADL ≤ 5/6) (20); cognitive impairment (Mini-Mental State Examination score < 24/30) (21); malnutrition defined as one or more of the following criteria as recommended by the French National Authority for Health: at least 10% weight loss in 6 months or 5% in 1 month and/or body mass index less than 21kg/m2 and/or Mini-Nutritional Assessment score less than 17/30 and/or serum albumin level less than 35g/L (22); inadequate social environment defined as absence of a primary caregiver or of adequate support at home or of a strong circle of family and friends able to meet the needs of the patient at the time of the evaluation (16); depression diagnosed by a semistructured interview to identify criteria for a major depressive episode from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (23); and number of severe (grade 3–4) comorbidities as assessed by the Cumulative Illness Rating Scale for Geriatrics (CIRS-G; 0,1, ≥2) (24). Second, we performed multiple correspondence analysis to ensure that each indicator selected by the experts contributed significantly to the first principal component of the corresponding GA domain (25). To characterize the classes and predict class membership, we considered the following active covariates: tumor site, metastatic status, age, gender, and in/outpatient status at the time of the GA. Age was dichotomized based on the median (≤80 versus >80 years).
Outcomes
The following outcomes were chosen to assess the prognostic value of the health profiles identified by LCA: overall 1-year mortality, unscheduled admissions within 6 months following the GA, and whether the final planned treatment strategy was palliative or curative. Curative treatment was defined as any necessary therapeutic strategy expected to achieve the cure of cancer. Palliative treatment was defined as any interventions or therapeutic strategy intended to relieve symptoms of the cancer (that may include palliative chemotherapy or radiation therapy in order to decrease the burden of malignant cells if responsible of these symptoms), and to ensure optimal patient comfort.
Statistical Analysis
Latent class analysis
We first used an iterative model-building process to perform LCA on the 821 patients without missing data, based on the six selected indicators. We obtained models containing one to eight classes. For each model, we used a one-step approach involving simultaneous estimation of the latent-class model of interest and of a logistic regression model whose latent classes were related to the set of the five active covariates. This method minimizes classification error (26–28). We used a variety of criteria to identify the best fitting LCA model, since no single approach is universally accepted (29–33). We considered the parameters listed in Table 1, and we selected the solution that had the greatest number of parameters in agreement: (a) nonsignificant bootstrap p value for the model fit using the likelihood-ratio chi-square statistic (L2); (b) lowest values for the Bayesian information criteria (BIC), sample-size-adjusted BIC (SABIC), and Akaike information criterion with 3 as the penalized factor (AIC3); and (c) nonsignificant improvement in fit between models having k classes versus k-1 classes, as assessed using the bootstrapped likelihood-ratio test (BLRT). Local independence was tested using bivariate residuals and discrimination was assessed using entropy, with values of 0.6 or higher indicating good class separation (34). LCA was performed using Latent Gold software 5.0 (Statistical Innovations, Belmont, MA).
Table 1.
Goodness-of-Fit Indices of Latent Class Models Comprising One to Eight Classes (n = 821 patients): The ELCAPA_09 Study
| No. of Classes | Bootstrap L2 | BIC (LL) | SABIC (LL) | AIC3 (LL) | BLRT | Entropy |
|---|---|---|---|---|---|---|
| p-Value | P-Value | |||||
| 1 | <.001 | 6750.76 | 6728.53 | 6724.78 | - | 1 |
| 2 | .008 | 6071.60 | 5992.21 | 5978.97 | <.001 | .76 |
| 3 | .048 | 6077.52 | 5940.97 | 5917.97 | <.001 | .70 |
| 4* | .140 | 6098.26 | 5904.55 | 5871.92 | <.001 | .71 |
| 5 | .102 | 6178.29 | 5927.42 | 5885.16 | .158 | .70 |
| 6 | .066 | 6259.65 | 5951.61 | 5899.73 | .154 | .71 |
| 7 | .072 | 6338.50 | 5973.30 | 5911.79 | .152 | .74 |
| 8 | .028 | 6421.39 | 5999.03 | 5927.89 | .302 | .76 |
Notes: AIC3 = adjusted Akaike information criterion; BIC = Bayesian information criterion; BLRT = bootstrapped likelihood ratio test; L2 = likelihood ratio; LL = log likelihood; SABIC = sample-size-adjusted Bayesian criterion.
*Model selected as providing the best fit, as demonstrated by a nonsignificant bootstrap L2 p value for goodness of fit and the lowest SABIC and AIC3 values; BLRT showed no improvement in fit of the five-class solution compared to the four-class solution. The null hypothesis for the BLRT test is that the k-1 model fits. Entropy values closer to 1 indicate better fit.
Outcomes associated with the identified latent classes
To assess the prognostic value of the latent classes, patients were assigned to classes based on their posterior class membership probabilities.
The log-rank test was used for global and pairwise comparisons of mortality across LCs. Then, stratified Cox models were built to deal with the time-dependent variable in/outpatient status (8,35). The models were adjusted for age; year of inclusion; final planned treatment strategy (curative/palliative/not reported), used as a confounder; and the composite variable including tumor site and metastatic status, to account for a previously reported interaction (8). Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated. The prevalences of planned palliative treatment and unscheduled admissions were compared globally across LCs using the Wald test obtained from logistic models adjusted for age, year of inclusion, in/outpatient status, tumor site and metastatic status, and final planned treatment strategy (this latter factor was included only in the model with admissions, as a confounder). Odds ratios (ORs) and their 95% CIs were estimated. Model discrimination was assessed using Harrell’s C-index with bootstrapped 95% CIs or ROC-AUC (95% CI), as appropriate. Calibration was assessed using the GrØnnesby and BØrgan or Hosmer-Lemeshow test (good fit if p > 0.20), as appropriate.
For all outcomes, analyses were performed using STATA software version 12.0 (StataCorp, College Station, TX), based on two-sided tests at the p ≤ 0.05 level. p Values for pairwise comparisons were corrected using the false discovery rate (FDR) method for multiple comparisons (36).
Sensitivity analyses
To test our results for robustness, three sensitivity analyses were performed. First, we used the missing data procedure implemented in Latent GOLD software under the missing-at-random hypothesis (n = 1,021) (33). Second, we applied the LCA according to metastatic status, using the missing data procedure and then validating the result on outcomes. Third, to validate our typology in a different patient sample and to test the stability over time of 1-year mortality prediction, we applied the posterior membership probabilities from our main analysis to a new sample of 375 patients enrolled prospectively in the ELCAPA cohort between 2012 and 2014.
Results
Identified Latent Classes
Of the 1,021 included elderly patients with cancer, 821 (80.4%) had complete data for all six selected indicators. Their median age was 80 [76–84] years, 52.0% were male, 20.3% had colorectal cancer, and 43.1% had metastatic disease. A four-class solution showed the best fit, with a nonsignificant bootstrap L2 p value and the lowest SABIC and AIC3 values; BLRT showed no improvement in fit of the five-class solution compared to the four-class solution (Table 1).
Table 2 reports the characteristics of the 821 patients with the conditional probabilities of the indicators and covariates for each of the four LCs, interpreted as the probability of each indicator being present in class members. Of the 821 patients, 232 (28.3%) belonged to LC1, 294 (35.8%) to LC2, 124 (15.1%) to LC3, and 171 (20.8%) to LC4. LC1 patients had low probabilities of GA indicator impairments; we therefore labeled this class “relatively healthy”. LC2 was characterized chiefly by a high probability of malnutrition and was labeled “malnourished”. LC3 patients had higher probabilities of cognitive and functional impairments, depressive mood, inadequate social environment, and one or more severe comorbidities compared with LC1 and LC2; in contrast, the probabilities of malnutrition, functional impairment, and having two or more severe comorbidities were lower than in LC4; we labeled this class “cognitive and/or mood impaired.” LC4 patients had high probabilities of functional and cognitive impairments, depressive mood, malnutrition, and severe comorbidities; compared with both LC1 and LC2, LC4 was also associated with a higher probability of inadequate social environment; this class was labeled “globally impaired.”
Table 2.
Parameter Estimates for the Four-Class Solution (n = 821 patients): The ELCAPA_09 Study
| Prevalence of Indicators Among the 821 Patients, N (%) | LC1 | LC2 | LC3 | LC4 | Global p-Value† | |
|---|---|---|---|---|---|---|
| Relatively Healthy, N = 232 | Malnourished, N = 294 | Cognitive and/or Mood Impaired, N = 124 | Globally Impaired, N = 171 | |||
| Probability of membership in each class expressed as % | 28.3 | 35.8 | 15.1 | 20.8 | ||
| Conditional probabilities* of (%) | ||||||
| Indicators | ||||||
| Functional impairment (ADL ≤ 5/6) | 267 (32.5) | 3.3 | 13.6 | 58.1 | 86.4 | |
| Cognitive impairment (MMSE < 24/30) | 222 (27.0) | 10.9 | 11.2 | 59.2 | 53.1 | |
| Malnutrition‡ | 421 (51.3) | 1.0 | 66.2 | 49.8 | 95.4 | |
| Inadequate social environment¶ | 163 (19.9) | 8.0 | 16.1 | 36.2 | 30.6 | |
| Depression (DSM IV criteria) | 236 (28.8) | 9.5 | 22.0 | 48.7 | 52.1 | |
| No. of severe comorbidities (grade 3–4 CIRS-G) | ||||||
| 0 | 353 (43.0) | 78.5 | 48.1 | 16.2 | 5.4 | |
| 1 | 219 (26.7) | 18.2 | 33.5 | 32.7 | 22.2 | |
| ≥2 | 249 (30.3) | 3.3 | 18.4 | 51.3 | 72.4 | |
| Active covariates | ||||||
| Tumor site | ||||||
| Colorectal | 167 (20.3) | 10.6 | 30.2 | 16.5 | 19.3 | <.001 |
| Breast | 147 (17.9) | 33.9 | 7.8 | 33.9 | 4.0 | |
| Prostate | 105 (12.8) | 27.5 | 4.1 | 1.5 | 15.9 | |
| Upper gastrointestinal tract/liver | 128 (15.6) | 3.5 | 25.5 | 0.1 | 26.1 | |
| Urinary system | 119 (14.5) | 16.1 | 17.3 | 10.9 | 10.2 | |
| Hematologic malignancies | 67 (8.2) | 3.1 | 8.1 | 14.0 | 10.9 | |
| Other tumors§ | 88 (10.7) | 5.1 | 7.1 | 26.0 | 13.6 | |
| Metastatic status | ||||||
| M0 | 345 (42.0) | 65.1 | 37.9 | 51.9 | 10.6 | <.001 |
| M1/Mx | 354 (43.1) | 24.7 | 50.7 | 19.3 | 72.4 | |
| Not reported or NA | 122 (14.9) | 10.2 | 11.4 | 28.9 | 17.0 | |
| Male gender | 427 (52.0) | 50.3 | 55.5 | 25.8 | 67.3 | .083 |
| Age >80 y | 376 (45.8) | 37.9 | 31.9 | 75.9 | 58.4 | <.001 |
| Outpatient at time of GA | 523 (63.7) | 98.8 | 66.6 | 66.0 | 9.4 | <.001 |
Notes: ADL = Activities of Daily Living; CIRS-G = Cumulative Illness Rating Scale for Geriatrics; DSM IV = Diagnostic and Statistical Manual of Mental Disorders; GA = geriatric assessment; LC = latent class; M0 = absence of distant metastases; M1 = presence of distant metastases; MMSE = mini mental state examination; Mx = metastatic status not assessable; NA = not applicable.
*Probability of each indicator being present in patients belonging to this class.
† p Value obtained using the Wald test.
‡Presence of one or more of the following criteria: at least 10% weight loss in 6 months or 5% in 1 month and/or body mass index less than 21kg/m2 and/or Mini Nutritional Assessment score less than 17/30 and/or serum albumin less than 35g/L.
§Upper gastrointestinal tract included stomach and esophagus; urinary system included bladder, upper urinary tract, and kidneys; and other tumors included tumors of the ovary, uterus, lung, head and neck, skin, thyroid, and unknown primary sites.
¶Inadequate social environment was defined as absence of a primary caregiver or of adequate support at home or of a strong circle of family and friends able to meet the needs of the patient at the time of the evaluation.
Factors independently associated with class membership were tumor site (p < 0.001), metastatic status (p < 0.001), age (p < 0.001), and in/outpatient status (p < 0.001; Table 2). LC1 was characterized by higher probabilities of nonmetastatic cancer, breast or prostate cancer, age ≤ 80 years, and outpatient status at the time of the GA; LC2 by higher probabilities of digestive cancer, metastatic disease, age ≤ 80 years, and outpatient status at the time of the GA; LC3 by higher probabilities of breast cancer or tumors in the “other” category (ovary, uterus, lung, head and neck, skin, thyroid, and unknown primary location), nonmetastatic disease, age > 80 years, and outpatient status at the time of the GA; and LC4 by higher probabilities of upper gastrointestinal tract/liver cancer, metastatic disease, age >80 years, and inpatient status at the time of the GA.
Outcomes
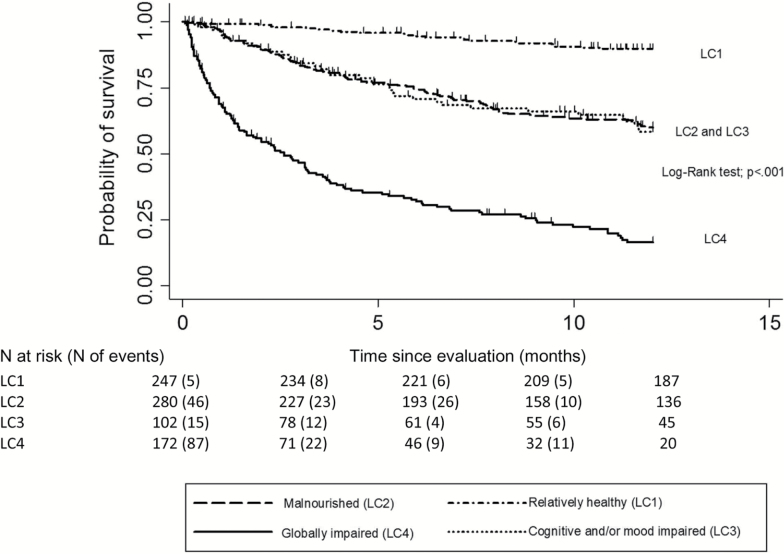
Vital status was unknown for 20/821 (2.4%) patients. The Kaplan–Meier 1-year mortality curves for each LC showed significant differences across classes (Figure 1). By univariate analysis, 1-year mortality was lower in LC1 (10.3%; 95% CI [7.0–14.9]) than in LC2 (40.0% [34.3–46.2]), LC3 (41.7% [32.1–52.8]), and LC4 (83.5% [76.8–89.1]; all p values <.001 in pairwise comparisons). No difference was found between LC2 and LC3 (p = .84). LC4 patients had the highest 1-year mortality rate (all p values <.001 in pairwise comparisons).
Figure 1.
Kaplan-Meier plots of overall 1-year survival after the comprehensive geriatric evaluation, for each of the four latent classes (n = 801 patients): the ELCAPA_09 study.
The multivariate analysis showed significant associations between LCs and 1-year overall mortality, 6-month unscheduled admissions, and planned treatment strategy (Table 3).
Table 3.
Comparisons of Outcomes in the Four Latent Classes (LCs; n = 821 patients): The ELCAPA_09 Study
| Outcomes | Overall Population | LC1 | LC2 | LC3 | LC4 | p Values for Pairwise Comparisons* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relatively Healthy | Malnourished | Cognitive and/or mood impaired | Globally Impaired | LC1 Versus | LC2 Versus | LC3 Versus | |||||||
| N = 821 | N = 250 | N = 282 | N = 110 | N = 179 | Adjusted p Value† | LC2 | LC3 | LC4 | LC3 | LC4 | LC4 | ||
| One-year mortality | N (%) | 295/801 (36.8) | 24/247 (9.7) | 105/280 (37.5) | 37/102 (36.3) | 129/172 (75.0) | .021 | .030 | .120 | .001 | .980 | .555 | .624 |
| HR (95% CI) | 1.00 (Ref) | 2.33 (1.41–3.85) | 3.15 (1.75–5.66) | 5.47 (3.09–9.68) | <.001 | .002 | .002 | .002 | .180 | .002 | .020 | ||
| Unscheduled 6-month admission | N (%) | 291/743 (39.2) | 51/235 (21.7) | 117/259 (45.2) | 38/91 (41.8) | 85/158 (53.8) | . | . | |||||
| OR (95% CI) | 1.00 (Ref) | 1.87 (1.10–3.20) | 2.32 (1.15–4.67) | 2.33 (1.12–4.87) | .047 | .048 | .048 | .048 | .643 | .643 | .985 | ||
| Planned palliative treatment | N (%) | 380/732 (51.9) | 49/223 (22.0) | 131/256 (51.2) | 53/90 (58.9) | 147/163 (90.2) | |||||||
| OR (95% CI) | 1.00 (Ref) | 3.11 (1.63–5.91) | 5.21 (2.37–11.41) | 15.83 (4.16–40.68) | <.001 | .002 | .002 | .002 | .177 | .002 | .023 | ||
Notes: CI = confidence interval; HR = hazard ratio; OR = odds ratio. Qualitative data are number (%). Bold values are significant.
*Corrected probabilities using the false discovery rate correction for multiple comparisons.
†Global p values by logistic regression adjusted for in/outpatient status, age, year of inclusion, tumor site and metastatic status (planned palliative treatment), and planned treatment approach (6-month unscheduled admissions), and by Cox models stratified by in/outpatient status and adjusted for age, year of inclusion, planned treatment approach, and a composite variable based on tumor site and metastatic status (1-year mortality).
Adjusted pairwise comparisons showed that LC1 was associated with a lower risk of 1-year overall mortality compared with the other LCs. Patients in LC4 had a higher risk of 1-year mortality compared with those in LC2 or LC3 (all p values ≤.05). Adjusted pairwise comparisons showed that both a palliative treatment decision and unscheduled admission were significantly less common among LC1 patients than among patients in the other LCs (Table 3). Compared to LC2 and LC3, LC4 was significantly associated with a palliative treatment decision (all p values ≤.05); unscheduled admissions did not differ significantly across L2, L3, and L4. All models demonstrated good discrimination (c-index mortality model, 0.76 [95% CI: 0.73–0.78]; admissions model, 0.75 [0.72–0.79]; and palliative treatment model, 0.90 [0.88–0.92]) and good calibration (p > .20 for all models).
Sensitivity Analyses
The sensitivity analysis performed on the overall population of 1,021 patients showed that the four-class solution was best. When applied separately to the patients with (n = 439) and without (n = 424) metastases, LCA showed persistence of the four-profile typology, with only minimal changes in prevalences and patterns that were chiefly ascribable to differences in the distribution of indicators and covariates (Online Supplement 4). The adjusted analysis of outcomes produced findings very similar to those in the overall population (Online Supplement 5). Of the 375 newly enrolled patients, 91 (24.3%) belonged to LC1, 131 (34.9%) to LC2, 87 (23.2%) to LC3, and 66 (17.3%) to LC4. Adjusted 1-year mortality showed similar findings as in the main analysis, and model discrimination was good (c-index, 0.76 [95% CI: 0.72–0.81] and calibration (p > 0.20; Online Supplement 5).
Discussion
We identified four profiles of older patients with cancer, namely, relatively healthy (LC1, 28%), malnourished (LC2, 36%), cognitive and/or mood impaired (LC3, 15%), and globally impaired (LC4, 21%). Factors independently associated with class membership were age, tumor site, metastatic status, and in/outpatient status. Compared with LC1 patients, LC2, LC3, and LC4 patients more often had a palliative treatment decision, unscheduled admissions within the next 6 months, and a fatal outcome within 1 year. Finally, patients belonging to LC4 more often had a higher 1-year mortality rate and planned palliative treatment compared with those in LC2 and LC3; for unplanned admissions, no differences were demonstrated across these three LCs. The same profiles were found in nonmetastatic and metastatic patients.
Comparison With the Literature
In previous studies of older patients with cancer, GA components such as mental health, comorbidities, polypharmacy, malnutrition, and mobility or functional impairment were associated with adverse outcomes including mortality (5–8,37). However, most studies assessed the prognostic value of single GA components. In contrast, we used a patient-centered approach to identify clusters of GA components that defined classes of patients. Few classifications of older patients with cancer have been published (9,38). The first was developed by Balducci and coworkers based on theoretical considerations and clinical expertise instead of on a statistical analysis designed to identify health profiles (9). Three groups were defined: fit patients, who were functionally independent and free of serious comorbidities; vulnerable patients with dependency for one or more IADLs and/or with one or two comorbid conditions; and frail patients, aged over 85 years and/or with dependency for one or more ADLs, three or more comorbid conditions, and one or more geriatric syndromes. A somewhat different classification is used in the International Society of Geriatric Oncology guidelines designed to assist in selecting cancer treatments and geriatric interventions in older men with prostate cancer (37): the factors taken into account in the previous classification, together with malnutrition, are used to distinguish four groups, namely, fit, vulnerable, frail, and too sick. Fit patients have no comorbidities (CIRS-G), IADL dependency, or malnutrition; vulnerable patients are dependent in one or more IADLs (but in no ADLs) or have one uncontrolled comorbidity (CIRS-G Grade 3) or are at risk for malnutrition; frail patients are dependent for one or more ADLs or have two or more uncontrolled comorbidities or severe malnutrition; and too sick patients have a very poor health status due to a combination of impairments.
Interestingly, our LCs partially match the classification described by Balducci and coworkers (9) the fit group resembles our relatively healthy class (LC1) and the frail group our globally impaired class (LC4) (9), whereas the malnourished class (LC2) and cognitive and/or mood impaired class (LC3) share features with the vulnerable group (38). The vulnerable group in previous classifications may encompass two distinct profiles (our LC2 and LC3) with different health care needs. Although these two LCs were associated with similar 1-year overall mortality, they might differ regarding toxicities and/or disabilities during follow-up.
In previous studies, the Balducci classification predicted adverse outcomes such as mortality (39,40). The 1-year mortality gradient from LC1 to LC2/LC3 and LC4 in our study supports the validity of our results. Malnutrition was not specifically considered by Balducci and coworkers (9) despite being a well-documented prognostic factor in older patients with cancer (8,41). In keeping with this adverse effect of malnutrition, the risks of unscheduled 6-month admissions and of 1-year mortality were similar to those in LC2, defined based on malnutrition, and in LC3, defined based on cognitive and/or mood impairment.
An original finding from our study is that malnutrition was the predominant characteristic of the LC2 profile, whereas LC3 and LC4 were defined chiefly by an accumulation of impairments and/or diseases. This could be related to the consequences of the cancer disease course, as suggested by the higher proportion in LC2 of patients with digestive cancer often associated with early malnutrition. Consequently, direct implication may be an extensive assessment and management of those patients for malnutrition (42,43). The extremely short life expectancy of patients in LC4, defined by global impairment, suggests that this profile may identify patients in whom early palliative care is likely to be the most beneficial management strategy. These associations linking cancer, demographic characteristics, and outcomes to specific latent classes support the validity of our typology.
Strengths and Limitations
To our knowledge, this is the first study investigating health profiles among elderly inpatients and outpatients with various cancers, based on GA findings and using a patient-centered statistical approach. The diversity of our population regarding cancer sites and stages reflects everyday practice and supports the external validity of our findings, together with the existence of all tumor sites (i.e., probabilities > 0) in each health profile (Table 2 and Online Supplement 2). Moreover, we chose to study a diverse population in order to determine whether shared factors predicted frailty and poor outcomes in older patients with cancer.
The assessment of GA domains using validated scales supports the applicability of our results to other health care institutions. We adjusted the Cox models for predictors of 1-year overall mortality previously identified by our group (8), excluding the indicators used to define the LCs. Neither an imputation of missing health-indicator data nor a reassessment of the prognostic value in a new ELCAPA cohort sample substantially changed our results, supporting the robustness of our findings. Moreover, the persistence of the same profiles in patients with and without metastases supports the external validity of our findings. In this subgroup analysis, the minimal changes in prevalences and patterns were chiefly ascribable to differences in the distribution of indicators and covariates between the two subgroups.
Our study has several limitations. First, identified LCs depend in part on the number and nature of the indicators selected for introduction into the model. However, we used a two-step approach to select the indicators: first, the experts chose indicators that accurately reflected the GA domains, and the relevance of these indicators was then checked using multiple correspondence analysis. Data on toxicities were not available. To validate the outcomes data, we used posterior class membership probabilities, which may have underestimated associations (27). Finally, although the inclusion of patients with various tumor sites and stages increases the general applicability of our results, the numbers of patients with each tumor site were too small for subgroup analyses.
Implications
The aim of our study was to understand the heterogeneity of populations of older patients with cancer, to describe combinations of impairments, and to identify naturally occurring uniform subgroups. Our findings have several clinical and research implications. First, the identification of four health profiles among elderly patients with cancer may help physicians to differentiate patients based on levels of risk. This improved risk stratification might translate into greater accuracy in determining the optimal cancer-treatment plan and allow the provision of geriatric interventions tailored to the needs of each profile. Second, the four profiles might prove useful to researchers for stratifying older patients in clinical trials of cancer treatments and/or geriatric interventions.
Future developments of our research project will seek to confirm the four-class typology and to assess its accuracy in predicting mortality, admissions, and toxicities in an independent external sample. Analyses of subgroups based on tumor site would be of interest. Our typology differs from the prognostic scores developed to help physicians distinguish levels of risk of severe toxicity in older patients with cancer (44,45), because latent class analysis is not developed from outcomes, being instead validated by outcomes. After external validation of our classification in an independent cohort, we plan to develop an online calculator based on the scoring equations and designed to classify patients, thereby providing treatment guidance and assisting in the development of interventions specifically targeted at the needs associated with each of the four profiles.
Conclusion
The identification of four health profiles among elderly inpatients and outpatients with various cancer types, based on findings from a GA, may help physicians to select interventions for both the cancer and any age-related impairments in these patients with complex health care needs. Researchers may find these profiles useful for stratifying patients in clinical trials.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by a grant from the French National Cancer Institute (Institut National du Cancer, INCA) and Ile-de-France Canceropole (Canceropôle Ile-de-France). The INCA and Canceropôle Ile-de-France had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary Material
Acknowledgment
We thank A. Wolfe, MD, for editing the manuscript. The ELCAPA Study Group was composed of five geriatricians (P. Caillet, M. Laurent, E. Liuu, E. Paillaud, and H. Vincent), two oncologists (S. Culine and Ch. Tournigand), one radiation oncologist (J-L. Lagrange), three epidemiologists (F. Canouï-Poitrine, S. Bastuji-Garin, and E. Audureau), one pharmacist (M. Carvahlo-Verlinde), one biostatistician (E. Guery), one clinical-research medical doctor (N. Reinald), and two clinical-research assistants (C. Poumba and J. Francese).
References
- 1. Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11:437–441. [DOI] [PubMed] [Google Scholar]
- 2. Micheli A, Mugno E, Krogh V, et al. Cancer prevalence in European registry areas. Ann Oncol. 2002;13:840–886. [DOI] [PubMed] [Google Scholar]
- 3. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Senior Adult Oncology Version 1 2013. https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf Accessed May 18, 2015.
- 5. Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104:1133–1163. doi:10.1093/jnci/djs285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. [DOI] [PubMed] [Google Scholar]
- 7. Caillet P, Laurent M, Bastuji-Garin S, et al. Optimal management of elderly cancer patients: usefulness of the Comprehensive Geriatric Assessment. Clin Interv Aging. 2014;9:1645–1660. doi:10.2147/CIA.S57849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrat E, Paillaud E, Laurent M, et al. Predictors of 1-year mortality in a prospective cohort of elderly patients with cancer. J Gerontol A Biol Sci Med Sci. 2015; 70:1148–1155. doi:10.1093/gerona/glv025 [DOI] [PubMed] [Google Scholar]
- 9. Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224–237. [DOI] [PubMed] [Google Scholar]
- 10. Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- 11. Crow SJ, Swanson SA, Peterson CB, et al. Latent class analysis of eating disorders: relationship to mortality. J Abnorm Psychol. 2012;121:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Starkstein S, Dragovic M, Jorge R, et al. Diagnostic criteria for depression in Parkinson’s disease: a study of symptom patterns using latent class analysis. Mov Disord. 2011;26:2239–2245. doi:10.1002/mds.23836 [DOI] [PubMed] [Google Scholar]
- 13. Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506–1511. doi:10.1038/jid.2012.472 [DOI] [PubMed] [Google Scholar]
- 14. Wolf EJ, Miller MW, Reardon AF, et al. A latent class analysis of dissociation and posttraumatic stress disorder. evidence for a dissociative subtype. Arch Gen Psychiatry. 2012;69:698–705. doi:10.1001/archgenpsychiatry.2011.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kao DP, Wagner BD, Robertson AD, et al. A personalized BEST. characterization of latent clinical classes of nonischemic heart failure that predict outcomes and response to bucindolol. PLoS One. 2012;7:e48184 doi:10.1371/journal.pone.0048184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–3642. doi:10.1200/JCO.2010.31.0664 [DOI] [PubMed] [Google Scholar]
- 17. McCutcheon AL. Latent Class Analysis. Thousand Oaks, CA: Sage Publications; 1987. [Google Scholar]
- 18. Vermunt JK, Magidson J. Can J Marketing Res. 2002;20:37–44. [Google Scholar]
- 19.Reinecke J. (2010) Latent class analysis. In Weiner IB, Craighead WE, eds. The Corsini Encyclopedia of Psychology. Vol. 4. John Wiley & Sons, Hoboken, NJ, pp. 912–914. doi:10.1002/9780470479216.corpsy0497 [Google Scholar]
- 20. Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL - A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 22. Haute Autorité de Santé. Nutritional support strategy for protein-energy malnutrition in the elderly 2007. http.//www.has-sante.fr/portail/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_630900 Accessed May 18, 2015.
- 23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24. Miller MD, Towers A. A Manual of Guidelines for Scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). Pittsburgh, PA: University of Pittsburgh; 1991. [Google Scholar]
- 25. Escofier B, Pagès J. Analyses factorielles simples et multiples. Paris: Dunod; 2008. [Google Scholar]
- 26. Huang GH, Bandeen-Roche K. Building an identifiable latent class model with covariate effects on underlying and measured variables. Psychometrika. 2004;69:5–32. [Google Scholar]
- 27. Vermunt JK. Latent class modeling with covariates. Two improved three-step approaches. Political Analysis. 2010;18450–469. [Google Scholar]
- 28. Vermunt Jk, Magidson J. Latent GOLD 4.0 User’s Guide. Belmont, MA: Statistical Innovations Inc; 2005. [Google Scholar]
- 29. Nylund KL, Asparouhov T, Muthe′n BO. Deciding on the number of classes in latent class analysis and growth mixture modeling. a Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 30. Croudace TJ, Jarvelin MR, Wadsworth ME, Jones PB. Developmental typology of trajectories to nighttime bladder control: epidemiologic application of longitudinal latent class analysis. Am J Epidemiol. 2003;157:834–842. [DOI] [PubMed] [Google Scholar]
- 31. Silverwood RJ, Nitsch D, Pierce M, et al. Characterizing longitudinal patterns of physical activity in mid-adulthood using latent class analysis. Results from a prospective cohort study. Am J Epidemiol. 2011;174:1406–1415. doi:10.1093/aje/kwr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dias J. Model selection for the binary latent class model. A Monte Carlo simulation. In: Batagelj V, Bock HH, Ferligoj A, iberna A, eds. Data Science and Classification. Berlin Heidelberg: Springer, 2006: 91–99. [Google Scholar]
- 33. Vermunt JK, Magidson J. Technical Guide for Latent GOLD 5.0. Basic, Advanced, and Syntax. Belmont, MA: Statistical Innovations Inc; 2013. [Google Scholar]
- 34.Asparouhov T, Muthén, B. Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Struct Equ Modeling. 2014;21:329–341. [Google Scholar]
- 35. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate. a practical and powerful approach to multiple testing. J R Statist Soc B (Methodological). 1995;57:289–300. [Google Scholar]
- 37. Cesari M, Cerullo F, Zamboni V, et al. Functional status and mortality in older women with gynecological cancer. J Gerontol A Biol Sci Med Sci. 2013;68:1129–1133. doi:10.1093/gerona/glt073 [DOI] [PubMed] [Google Scholar]
- 38. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106:462–469. doi:10.1111/j.1464-410X.2010.09334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basso U, Tonti S, Bassi C, et al. Management of Frail and Not-Frail elderly cancer patients in a hospital-based geriatric oncology program. Crit Rev Oncol Hematol. 2008;66:163–170. doi:10.1016/j.critrevonc.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 40. Tucci A, Ferrari S, Bottelli C, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115:4547–4553. doi:10.1002/cncr.24490 [DOI] [PubMed] [Google Scholar]
- 41. Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi:10.1200/JCO.2011.35.7442 [DOI] [PubMed] [Google Scholar]
- 42. Paillaud E, Liuu E, Laurent M, et al. ; ELCAPA Study Group Geriatric syndromes increased the nutritional risk in elderly cancer patients independently from tumour site and metastatic status. The ELCAPA-05 cohort study. Clin Nutr. 2014;33:330–335. doi:10.1016/j.clnu.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 43. Di Fiore F, Lecleire S, Rigal O, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi:10.1002/cncr.26646 [DOI] [PubMed] [Google Scholar]
- 45. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi:10.1200/JCO.2011.34.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.