Abstract
Background:
Few cohort studies have examined longitudinal associations between age-related changes in cognition and physical performance. Further, whether these associations differ for men versus women or can be attributed to differences in physical activity (PA) is unknown.
Methods:
Participants were 2,876 initially well-functioning community-dwelling older adults (aged 70–79 years at baseline; 52% female; 39% black) studied over a 9-year period. Usual gait speed, self-reported PA, and two cognitive measures—Digit Symbol Substitution Test (DSST) and Mini-Modified Mental State examination (3MS)—were assessed years 0 (ie, baseline), 4, and 9.
Results:
Early decline between years 0 and 4 in gait speed predicted later decline between years 4 and 9 in performance on the 3MS (β = 0.10, p = .004) and on the DSST (β = 0.16, p < .001). In contrast, the associations between early decline in cognition and later decline in gait speed were weaker and were non-significant after correcting for multiple comparisons (β = 0.08, p = .019 for 3MS and β = .06, p = .051 for DSST). All associations were similar for women and men and were unaltered when accounting for PA levels.
Conclusions:
The results indicate declining gait speed as a precursor to declining cognitive functioning, and suggest a weaker reciprocal process among older women and men.
Keywords: Cognition, Gait, Physical activity, Physical function.
Aging is frequently accompanied by decline in aspects of cognitive functioning (1) and physical performance (2). Although age-related decline in these domains is related (3), the degree to which decline in one domain predicts decline in the other remains unclear. Several cohort studies have shown that better baseline cognitive performance predicts slower rate of decline in physical functioning (4–10) and others have shown that better baseline physical performance predicts slower rate of decline in cognition (11,12). Still others have shown that decline in cognition and physical performance are correlated over time (9,13).
However, none of these previous studies directly examined whether decline in one domain predicts decline in the other. Poor baseline performance is an imperfect proxy for age-related decline, as poor performance might reflect long-standing suboptimal functioning that predates older age. Furthermore, correlated change over time does not inform the directionality of the relationship. Ideally, physical performance and cognition should be assessed over several time points and then early change in one domain can be used to predict later change in the other using cross-lagged longitudinal models, as concluded in a review of the literature (3). Although these types of studies are rare, one notable example (14) found bidirectional influences between physical performance and cognition over time in a relatively small cohort of older women. Whether these findings replicate in a larger cohort or whether these associations are similar for men and women is unknown. Another important consideration is the role of physical activity (PA) as a possible behavioral mechanism linking physical performance to cognition among older adults (9,13). Given that regular PA improves cognitive and brain health (15), decline in physical performance might lead to decreases in regular PA, and in turn, exacerbate cognitive decline.
This study aimed to address these outstanding issues using data from a large cohort of initially well-functioning older women and men followed over 9 years. Cognition (both general cognition and the specific domain of psychomotor/executive functioning), physical performance (specifically, gait speed), and PA (as indexed by self-reported time spent walking) were assessed at years 0 (ie, baseline), 4, and 9. We utilized cross-lagged longitudinal models, similar to those employed by Krall and colleagues (14), to test the hypothesis that changes in gait speed and cognition would be related to one another over time and to explore whether early change in gait speed was a stronger predictor of later change in cognition, or vice versa. By including two measures of cognitive performance, we investigated whether the nature of the cognition-physical performance association might differ across cognitive domain (14). We then examined whether longitudinal changes in PA might account for the association between gait speed and cognitive decline. Finally, in light of potential sex differences in aging (16), we determined whether these associations varied as a function of participant sex.
Methods
Study Design and Participants
Participants were from the Health, Aging, and Body Composition study, a prospective cohort study of adults aged 70–79 years at baseline. Participants were community dwelling, and living in Pittsburgh or Memphis. A random sampling of eligible white adults was recruited and every eligible black adult was recruited. The baseline assessment occurred between May 1997 and June 1998. Of the 3,075 total participants, 2,876 (94%) had complete baseline data on the main measures of interest (ie, gait speed, cognition, and self-reported time spent walking) and were included in this study. The study was approved by the institutional review boards at the University of Pittsburgh, the University of Tennessee Memphis, and the University of California San Francisco. All participants gave written informed consent.
Measures
Repeatedly Measured Variables
Executive functioning and information processing were measured using the Digit Symbol Substitution Test (DSST), which consists of a series of numbers (1–9) and corresponding symbols. Participants are requested to draw the correct symbols for given digits during a 90-s time period (17). General cognitive functioning was measured using the modified mini-mental status examination (3MS). The 3MS is a comprehensive test of orientation, attention, calculation, language, and short-term memory (18). Self-reported time spent walking (min/week) was based on the previous week using a standardized questionnaire (19). Gait speed (m/s) at usual pace is a valid and reliable marker of physical performance in older adults (20) and was assessed over a 6-m walkway (years 0 and 9) and over a 20-m walkway (year 4). Similar to previous research (21,22), 20-m gait speed was converted to 6-m gait speed using a conversion formula derived using data from 1,342 individuals who completed both gait speed assessments at year 9:
Covariates
Potential confounding variables were assessed at baseline and included demographics, body mass index (kg/m2), self-reported chronic disease conditions and health behavior, and study site. Demographics included age, sex, race, and educational attainment. Health behavior included smoking status (current, former, or never) and frequency of alcohol consumption. Self-reported chronic disease conditions included cerebrovascular disease, diabetes, and coronary heart disease. Diabetes was confirmed by medication use.
Statistical Analyses
Data distributions were visually screened, and 3MS and self-reported walking were log10 transformed to normalize their distributions. Next, the longitudinal relationships between cognition and gait speed were analyzed using structural equation modeling (SEM) in the statistical package Mplus 7.3 (23). All models employed maximum likelihood estimation with robust standard errors, thus including all individuals with baseline data (n = 2,876). Acceptable fit was obtained in the final models based on comparative fit index ≥.95 and root mean square estimate of approximation ≤.05 (24). The primary analyses involved cross-lagged latent change models to determine whether early change in gait speed (ie, between years 0 and 4) predicted later change in cognition (ie, between years 4 and 9), and/or vice versa. This modeling framework is useful in examining change over time in two concurrently measured variables because the inclusion of autoregressive effects (eg, the regression of later change in gait on early change in gait) allows the researcher to rule out the possibility that the cross-lagged effect (eg, the predictive association between early change in cognition and later change in gait speed) is due to a cross-sectional correlation (25). See the Supplementary Material for additional information on this statistical approach. Models were adjusted for the following: study site, education, race, baseline age, BMI, sex, baseline smoking and drinking status, and prevalent diabetes, cardiovascular disease, and cerebrovascular disease. Both the baseline and change scores were adjusted for the covariates. For the primary analyses, standardized estimates (β) are reported with significance based on a Bonferroni-corrected p < .0056 (α/n = .05/9 = .0056) to adjust for the nine cross-domain estimates within each model.
To determine whether any associations between gait speed and cognition could be explained by PA, we added years 0, 4, and 9 self-reported walking as an additional covariate to control for baseline PA and changes in PA over time. We also stratified the sample by sex and used the model test feature in Mplus to determine whether the size of the physical performance-cognition associations differed between men and women. Finally, five sets of follow-up sensitivity analyses were conducted. Because individuals who completed the year 9 assessment were younger, healthier, and had higher cognitive scores at baseline compared to those who did not complete the year 9 assessment (see Supplementary Table 1 in the Supplement for a detailed comparison), the first three sensitivity analyses handled follow-up missing data in different ways. First, multiple imputation using Bayesian analysis (26,27) was employed to impute missing follow-up data and to determine whether the pattern of findings using non-imputed data was consistent with the findings using imputed data. The imputation process included only those individuals known to be alive at year 9 (n = 2,084) and used all study variables and covariates to create 40 imputed data sets; parameter estimates and standard errors were pooled across the 40 data sets. Second and third, we limited the sample to individuals with gait speed data from at least two time points and from all three time points, respectively. Fourth and fifth, we determined whether the pattern of findings were similar when excluding individuals with baseline 3MS scores lower than 80 (n = 222) or when excluding individuals with baseline gait speed lower than 1.0 m/s (n = 586), which is associated with cognitive impairment (18) and physiological impairment (28,29), respectively.
Results
Baseline characteristics of the sample are summarized in Table 1. Descriptive statistics and the number of observations at each time point for the repeatedly measured variables are provided in the top portion of Table 2. The estimated change over time using maximum likelihood estimation is provided in the bottom portion of Table 2. There was significant group-level decline between years 0–4 and years 4–9 (p < .001) with the exception that 3MS performance did not change significantly between years 0 and 4 (p = .88). Statistical comparison using the model test feature in Mplus showed that the amount of group-level change was greater from years 4–9 than from years 0–4 for all variables (p < .05) except DSST (p = .83). Lastly, there was significant individual-level variance in change in each variable during each segment of time (p < .001), which indicates that some individuals showed much faster rates of decline whereas others showed slower rates of decline than the average.
Table 1.
Baseline Characteristics of the Study Sample.
| Mean (SD) or n (%) | |
|---|---|
| Demographics | |
| Age, years | 73.6 (2.9) |
| Sex, female | 1,499 (52%) |
| Race, black | 1,134 (39%) |
| Education, > high school | 1,247 (44%) |
| Body mass index (kg/m2) | 27.4 (4.8) |
| Chronic disease conditions | |
| Coronary heart disease | 482 (17%) |
| Cerebrovascular disease | 208 (7%) |
| Diabetes | 426 (15%) |
| Health behavior | |
| Current or former smoker | 1,619 (56%) |
| Alcohol consumption, ≥ once per week | 854 (30%) |
| Study site, Pittsburgh | 1,467 (51%) |
Table 2.
Information on the Time-Varying Variables of Cognition, Self-Reported Time Spent Walking, and Gait Speed.
| Observed Mean Scores and Standard Deviations at Each Time Point | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3MS* (# of Points Earned) | DSST (# of Correct Matches) | Self-Reported Walking* (min/week) | Usual Gait Speed over 6 m† (m/s) | |||||
| Year | # | Mean (SD) | # | Mean (SD) | # | Mean (SD) | # | Mean (SD) |
| 0 | 2,876 | 90.8 (7.2) | 2,876 | 36.6 (13.5) | 2,876 | 125.3 (239.9) | 2,876 | 1.18 (.23) |
| 4 | 2,227 | 90.7 (8.3) | 2,204 | 34.9 (14.1) | 2,457 | 83.9 (156.2) | 2,159 | 1.09 (.18) |
| 9 | 1,528 | 89.1 (10.1) | 1,497 | 34.0 (14.0) | 1,769 | 68.9 (135.9) | 1,423 | 1.01 (.25) |
| Estimated change over time using maximum likelihood estimation (n = 2,876)‡ | ||||||||
| Average early change (95% CI) | −0.001 (−0.01, 0.01) | −2.82*** (−3.21, −2.42) | −0.20*** (−0.25, −0.15) | −0.10*** (−0.11, −0.10) | ||||
| Variance in early change (95% CI) | 0.09*** (0.08, 0.09) | 88.70*** (79.04, 98.36) | 1.52*** (1.43, 1.61) | 0.04*** (0.03, 0.04) | ||||
| Average late change (95% CI) | −0.10*** (−0.11, −0.08) | −2.90*** (−3.39, −2.40) | −0.11*** (−0.16, −0.06) | −0.13*** (−0.14, −0.12) | ||||
| Variance in late change (95% CI) | 0.08*** (0.07, 0.09) | 83.57*** (72.87, 94.27) | 1.37*** (1.27, 1.46) | 0.04*** (0.03, 0.04) | ||||
Notes: # = number of observations at each time point; 3MS = Modified Mini-Mental State Examination; DSST = digit symbol substitution test; SD = standard deviation; SE = standard error.
*3MS and self-reported walking scores were log-transformed for statistical analyses. For the observed means and standard deviations we report the raw scores; however, we estimated change over time using the log-transformed values.
†Gait speed over 6 m at year 4 was estimated from gait speed over 20 m using the following equation: Ŷ6mgaitspeed = 0.171+20m gait speed * 0.834. This equation was derived from 1,342 individuals who completed both a 6-m walk and a 20-m walk at year 9.
‡Maximum likelihood estimation implicitly imputes missing data. As such the estimated change scores do not necessarily match the means and standard deviations derived from the observed scores in the top part of the table.
***p < .001.
Longitudinal Associations between Gait Speed and Cognition
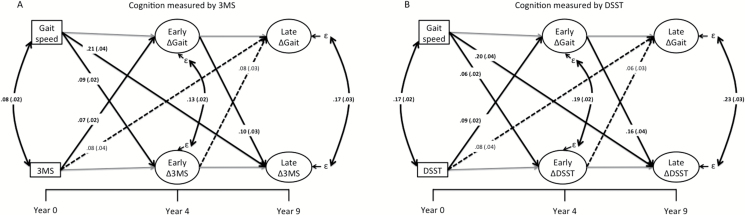
Figure 1A provides the standardized estimates for the cross-lagged longitudinal model using 3MS as the cognitive outcome. We observed that baseline gait speed was a significant predictor of early and late changes in 3MS (β = .09 and β = .21, respectively, p < .001). Baseline 3MS predicted early change in gait speed (β = .07, p < .001) but not late change in gait speed (β = .08, p = .03). Early change in gait speed predicted late change in 3MS (β = .10, p = .004), but early change in 3MS did not significantly predict late change in gait speed (β = .08, p = .019).
Figure 1.
Depiction of the main findings of the study when using modified mini-mental state examination (A) or digit symbol substitution test (B). Standardized estimates (and standard errors) are provided for cross-domain associations. Gray lines are within-domain associations and are not of interest in this study. To simplify the model, covariates are not shown but included clinical site, education, age, race, baseline BMI, gender, baseline smoking and drinking status, and prevalent diabetes, cardiovascular disease, and cerebrovascular disease. 3MS = Modified mini-mental state examination. DSST = Digit symbol substation test. Gait = gait speed. ε = Residual change in the latent variable not accounted for covariates or previous assessments. Solid lines with bolded estimates indicate associations that are significant based on Bonferroni correction (p < .0056).
Similar results were obtained when using DSST as the cognitive outcome (Figure 1B). Specifically, baseline gait speed was a significant predictor of early and late changes in DSST (β = .06 and β = .20, respectively, p < .005). Baseline DSST predicted early change in gait speed (β = .09, p < .001) but not late change in gait speed (β =.08, p = .04). Early change in gait speed was a significant predictor of late change in DSST (β = .16, p < .001), but early change in DSST did not significantly predict late change in gait speed (β = .06, p = .05). In both models (ie, 3MS and DSST), the residual correlations between change scores were highly significant (p < .001). This indicates that even after accounting for the predictive paths (as well as covariates), change in gait speed correlated with change in cognition during both the early and late phase of the study.
The above estimates were unaltered when further controlling for changes in PA with no standardized estimate changing by more than 0.01 units (Table 3). In sex-stratified models, we found no evidence that the associations between gait speed and cognition differed significantly between men and women (Table 4). The various ways of dealing with missing data replicated the findings described above (Supplementary Tables 2–4), as did excluding individuals with baseline 3MS < 80 (Supplementary Table 5) or with baseline gait speed < 1.0 m/s (Supplementary Table 6).
Table 3.
Effects of Physical Activity on the Associations Between Gait Speed and Cognition.
| Cognition Measured by 3MS | Cognition Measured by DSST | |||
|---|---|---|---|---|
| Adjusted for Covariates* | Adjusted for Covariates* and ΔPA | Adjusted for Covariates* | Adjusted for Covariates* and ΔPA | |
| Predictive associations | ||||
| BL Gait→ Early Cognition | .09 (.02) | .09 (.02) | .06 (.02) | .06 (.02) |
| BL Cognition → Early ΔGait | .07 (.02) | .06 (.02) | .09 (.02) | .09 (.02) |
| BL Gait→ Later Cognition | .21 (.04) | .21 (.04) | .20 (.04) | .19 (.04) |
| BL Cognition → Later ΔGait | .08 (.04) | .08 (.04) | .08 (.04) | .08 (.04) |
| Early ΔGait→ Later Cognition | .10 (.03) | .10 (.04) | .16 (.04) | .16 (.04) |
| Early Cognition → Later ΔGait | .08 (.03) | .07 (.03) | .06 (.03) | .06 (.03) |
| Residual correlations | ||||
| Early ΔGait ←→ Early ΔCognition | .13 (.02) | .13 (.02) | .19 (.02) | .19 (.02) |
| Late ΔGait ←→ Late ΔCognition | .17 (.03) | .17 (.03) | .23 (.03) | .23 (.03) |
Notes: Standardized estimates (and standard errors) are shown. BL = baseline; 3MS = Modified Mini-Mental State Examination; DSST = digit symbol substitution test; PA = physical activity as indexed by self-reported walking (min/week).
*Covariates include clinical site, education, age, race, baseline BMI, gender, baseline smoking and drinking status, and prevalent diabetes, cardiovascular disease, and cerebrovascular disease.
Table 4.
Comparison of Predictive Associations Between Women and Men.
| Path | Standardized Estimate for Men (n = 1,377) | Standardized Estimate for Women (n = 1,499) | p Value for Comparison of Men Versus Women |
|---|---|---|---|
| Cognition measured by 3MS | |||
| BL Gait→ Early Δ3MS | .09 (.03) | .08 (.03) | .838 |
| BL 3MS→ Early ΔGait | .08 (.03) | .05 (.03) | .283 |
| BL Gait→ Later Δ3MS | .25 (.06) | .17 (.05) | .476 |
| BL 3MS→ Later ΔGait | .10 (.05) | .07 (.05) | .617 |
| Early ΔGait→ Later Δ3MS | .12 (.05) | .09 (.05) | .838 |
| Early Δ3MS→ Later ΔGait | .13 (.05) | .03 (.04) | .099 |
| Early ΔGait ←→ Early Δ3MS | .14 (.03) | .11 (.03) | .728 |
| Late ΔGait ←→ Late Δ3MS | .18 (.04) | .17 (.04) | .773 |
| Cognition measured by DSST | |||
| BL Gait→ Early ΔDSST | .07 (.03) | .05 (.03) | .774 |
| BL DSST→ Early ΔGait | .07 (.03) | .10 (.03) | .790 |
| BL Gait→ Later ΔDSST | .21 (.06) | .19 (.05) | .836 |
| BL DSST→ Later ΔGait | .06 (.05) | .10 (.05) | .667 |
| Early ΔGait→ Later ΔDSST | .17 (.05) | .15 (.05) | .917 |
| Early ΔDSST→ Later ΔGait | .11 (.05) | .04 (.05) | .229 |
| Early ΔGait ←→ Early ΔDSST | .16 (.03) | .21 (.03) | .283 |
| Late ΔGait ←→ Late ΔDSST | .24 (.04) | .24 (.05) | .917 |
Notes: Standardized beta values (and standard errors) are presented. BL = baseline; 3MS = Modified Mini-Mental State Examination; DSST = digit symbol substitution test.
Discussion
Among older community-dwelling men and women, individuals who showed faster rates of gait speed decline over the first 4 years of the study had faster rates of cognitive decline during the following 5 years of the study. In contrast, early cognitive decline did not significantly predict later gait speed decline. Contrary to expectations, these predictive associations could not be accounted for by changes in PA. Thus, while PA can be an important contributor to maintaining cognition in older age (30), it does not appear to explain the link between gait speed decline and cognitive decline. Instead, these findings support a global age-related biologic degeneration that affects physical performance and cognition (3). Interestingly, our findings also suggest that this degeneration may manifest as a deficit primarily in physical performance early on, and then later, cognitive performance.
An important contribution of the current work is the comparison of two distinct cognitive measures—a general cognitive assessment used to screen for dementia (3MS) and a specific measure of executive functioning and psychomotor processing (DSST). Several findings were consistent for both tasks. Using either measure, early change in gait speed predicted later change in cognition and the correlated change in gait speed and cognition could not be accounted for by PA decline. However, there were also noteworthy differences. Foremost, gait speed decline was more strongly related to decline in DSST than to decline in 3MS performance, with standardized coefficients roughly 50–75% larger for the former than the latter. This finding supports the conclusions of a 2013 meta-analysis (3), and might provide insight into the nature of age-related degeneration that afflicts gait speed and cognition. Previously, it had been suggested that degeneration in regions of the brain important for motor control, executive functioning, and attention could negatively impact gait speed because gait relies on both motor and cognitive processes, including executive function and attention (31). Our findings support this proposition given that the DSST assesses these cognitive and motor control processes more so than the 3MS, a more generalized cognitive assessment of orientation, language, and memory performance. Recent studies demonstrated that macro-structural disruptions in white matter were associated with poorer DSST performance and impaired gait (32,33). This included lesions in fiber tracts related to motor control, processing speed and executive functioning, including the frontal portion of the corpus callosum and the anterior thalamic radiation (32). A more recent study extended this finding by suggesting that white matter lesions can especially impact gait speed when paired with decreased microstructural integrity of normal-appearing white matter (34). White matter lesions are common in older adults (33), and conceivably, their accumulation over time might partly underlie the directional associations between age-related decline in gait speed and cognition observed herein. In light of these associations, interventions that slow the progression of white matter damage—eg, by addressing the underlying cardio-metabolic risk factors for such damage (35)—might have downstream benefits to mobility and to cognition.
A second important contribution is the formal testing of whether observed associations differed between women and men, which we found did not. This is informative because previous studies either examined women and men together or included only men or women in the cohort (eg, the Honolulu Heart Program or the Women’s Health Initiative Memory Study).
Although we observed that early change in gait speed was a stronger predict of later change in cognition, rather than vice versa, our findings do not suggest that the association is strictly unidirectional in this population or that early examination of cognition is unimportant. Both baseline measures of cognition predicted early changes in gait speed. Moreover, the residuals between gait speed and cognition were consistently correlated with one another during each phase of the study. Thus, in line with a previous study using a similar analytic framework in a smaller female cohort, there appears to be some degree of bidirectionality in these associations between cognition and physical performance (14). Furthermore, when assessing an older adult’s risk for dementia, it may be important to consider gait speed along with cognitive functioning, as suggested by the notion of a motoric cognitive risk syndrome (36,37).
The SEM approach used herein has advantages over more commonly used linear mixed modeling (LMM) of longitudinal data (eg, 9,12). As noted by Krall and colleagues (14), SEM better captures the dynamic, bidirectional associations between cognition and physical performance over time. In contrast, in LMM one of the time-varying variables must be specified as the dependent variable and the other as a time-varying predictor variable. Presupposing a specific directionality may not be warranted and this does not allow for the simultaneous modeling of bidirectional relations. However, there are instances in which LMM would be preferred, eg, to examine the shape of group-level change over time (25) or to determine individual-level points of acceleration in decline, as has been done to identify the onset of terminal decline (38). In another recent example, LMM was used to show that cognitive decline accelerated after, rather than before, the onset of functional limitations in strength and basic mobility (39). These findings are consistent with the findings herein as they suggest that decline in physical performance might precede cognitive decline. Thus, SEM and LMM should be considered complementary approaches to examine longitudinal associations between cognition and physical performance.
This study has limitations. One limitation is that PA was measured via a self-report measure of time spent walking. This introduces issues such as inaccurate recall due to cognitive limitations and social desirability, as well as does not capture the full range of activities that older adults might engage in. However, walking is the most common PA (40) and was the one form of PA assessed repeatedly over time in this cohort, which was required for the analyses conducted for this study. It is possible that the use of objective PA measurement might lead to different conclusions than those herein. Beyond the PA assessment, there might be measurement error associated with the other assessments—especially the cognitive assessments—that might influence the nature of these results. The availability of only three time points during which all primary measures were collected prohibits us from determining whether changes in gait speed continue to predict subsequent changes in cognition over longer periods of time. Another consideration is that the standardized coefficients, though highly significant in most instances and independent of various covariates, represent small effects based on the convention that standardized estimates of .10 are small and .30 are moderate in size (41). Thus, although we demonstrated an independent relationship between gait speed decline and subsequent cognitive decline, our results suggest that the contribution is small, which implies that there are likely various other contributors to cognitive decline worthy of study. A final limitation is that study sample was quite healthy at study entry. This raises questions regarding the generalizability of our findings; eg, it should not be assumed that the directionality we observed would also be evident among older adults who have significant physical and cognitive impairment at study entry.
Conclusions
The major strength of this study is the use of multivariate longitudinal modeling to examine the course of changes in gait speed and cognition. We found consistent evidence that gait speed decline predicts future cognitive decline, independent of PA and several demographic variables, health behaviors, and chronic disease conditions. This predictive effect was evident for both general cognitive decline and specific psychomotor/executive functioning decline, but was stronger for the latter. The reciprocal association—that cognitive decline predicts future gait speed decline—was consistently weaker and non-significant. We also found that the longitudinal associations between cognition and gait speed were similar in older women and men. Thus, routine measurement of usual gait speed appears to be an important—and simple—clinical tool to be used along with measures of cognition to identify heightened risk for future cognitive decline among initially healthy older women and men.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Additional sources of funding: J.R.B. is a Canadian Institutes of Health Research and Michael Smith Foundation of Health Research Post-Doctoral Fellow. T.L-A. is a Canada Research Chair (Tier II) in Physical Activity, Mobility, and Cognitive Neuroscience.
Conflict of Interest
All authors have no conflict of interest and nothing to disclose.
Supplementary Material
Acknowledgments
We thank Dr Matteo Cesari for helpful comments on an earlier draft of this manuscript.
References
- 1. Salthouse TA. Correlates of cognitive change. J Exp Psychol Gen. 2014;143:1026–1048. doi:10.1037/a0034847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a Mobility Working Group recommendation. J Am Med Assoc. 2014;311:2061–2062. doi:10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clouston SA, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. doi:10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gothe NP, Fanning J, Awick E, et al. Executive function processes predict mobility outcomes in older adults. J Am Geriatr Soc. 2014;62:285–290. doi:10.1111/jgs.12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. [DOI] [PubMed] [Google Scholar]
- 6. Watson NL, Rosano C, Boudreau RM, et al. ; Health ABC Study. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65:1093–1100. doi:10.1093/gerona/glq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soumaré A, Tavernier B, Alpérovitch A, Tzourio C, Elbaz A. A cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J Gerontol A Biol Sci Med Sci. 2009;64:1058–1065. doi:10.1093/gerona/glp077 [DOI] [PubMed] [Google Scholar]
- 8. Holtzer R, Wang C, Lipton R, Verghese J. The protective effects of executive functions and episodic memory on gait speed decline in aging defined in the context of cognitive reserve. J Am Geriatr Soc. 2012;60:2093–2098. doi:10.1111/j.1532-5415.2012.04193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the women’s health initiative memory study. J Gerontol A Biol Sci Med Sci. 2010;65:300–306. doi:10.1093/gerona/glp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivera JA, Fried LP, Weiss CO, Simonsick EM. At the tipping point: predicting severe mobility difficulty in vulnerable older women. J Am Geriatr Soc. 2008;56:1417–1423. doi:10.1111/j.1532-5415.2008.01819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi:10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi:10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Best JR, Davis JC, Liu-Ambrose T. Longitudinal analysis of physical performance, functional status, physical activity, and mood in relation to executive function in older adults who fall. J Am Geriatr Soc. 2015;63:1112–1120. doi:10.1111/jgs.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krall JR, Carlson MC, Fried LP, Xue QL. Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. Am J Epidemiol. 2014;180:838–846. doi:10.1093/aje/kwu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hillman CH, Erickson K, Kramer A. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi:10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- 16. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi:10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spreen O, Strauss E. A Compendium of Neurological Tests. 2nd ed. New York: Oxford University Press, Inc, 1998. [Google Scholar]
- 18. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 19. Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi:10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 20. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi:10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 22. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muthén LK, Muthén BO Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 1998–2014. [Google Scholar]
- 24. Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. doi:10.1080/10705519909540118 [Google Scholar]
- 25. Selig JP, Little TD. Autoregressive and cross-lagged panel analysis for longitudinal data. In: Laursen B, Little TD, Card NA, eds. Handbook of Developmental Research Methods. New York, NY: The Guilford Press; 2012:265–278. [Google Scholar]
- 26. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, 1987. [Google Scholar]
- 27. Schafer JL. Analysis of Incomplete Multivariate Data. London, UK: Chapman & Hall; 1997. [Google Scholar]
- 28. Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70:319–324. doi:10.1093/gerona/glu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi:10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 30. Yaffe K, Barnes D, Nevitt M, Lui L, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch Inter Med. 2001;161:1703–1708. doi:10.1001/archinte.161.14.1703 [DOI] [PubMed] [Google Scholar]
- 31. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi:10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolandzadeh N, Liu-Ambrose T, Aizenstein H, et al. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage. 2014;99:7–13. doi:10.1016/j.neuroimage.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith EE, O’Donnell M, Dagenais G, et al. ; PURE Investigators Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol. 2015;77:251–261. doi:10.1002/ana.24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosario BL, Rosso AL, Aizenstein HJ, et al. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J Am Geriatr Soc. 2015;63:2052–2060. doi:10.1111/jgs.13644 [DOI] [PubMed] [Google Scholar]
- 36. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi:10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi:http://dx.doi.org/10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piccinin AM, Muniz G, Matthews FE, Johansson B. Terminal decline from within- and between-person perspectives, accounting for incident dementia. J Gerontol B Psychol Sci Soc Sci. 2011;66:391–401. doi:10.1093/geronb/gbr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rajan KB, Hebert LE, Scherr PA, Mendes de Leon CF, Evans DA. Rate of cognitive decline before and after the onset of functional limitations in older persons. J Gerontol A Biol Sci Med Sci. 2015;70:1221–1225. doi:10.1093/gerona/glv062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siegel PZ, Brackbill RM, Heath GW. The epidemiology of walking for exercise: implications for promoting activity among sedentary groups. Am J Public Health. 1995;85:706–710. doi:10.2105/AJPH.85.5.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen J. A power primer. Psychol Bull. 1992;112:155. doi:http://dx.doi.org/10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.