Abstract
Background:
Clinical cognitive impairment and physical frailty often co-occur. However, it is unclear whether preclinical impairment or decline in cognitive domains are associated with onset of physical frailty. We tested this hypothesis and further hypothesized that preclinical impairment and decline in executive functioning are more strongly associated with frailty onset than memory or general cognitive performance.
Methods:
We used 9 years of data from the Women’s Health and Aging Study II (six visits) that longitudinally measured psychomotor speed and executive functioning using the Trail Making Test, parts A and B, respectively, and immediate and delayed word-list recall from the Hopkins Verbal Learning Test. We used Cox proportional hazards models to regress time to frailty on indicators for impairment on these cognitive tests and on rates of change of the tests. Models adjusted for depressive symptoms, age, years of education, and race.
Results:
Of the 331 women initially free of dementia and frailty, 44 (13%) developed frailty. A binary indicator of impaired executive functioning (Trail Making Test, part B [TMT-B]) was most strongly associated with hazard, or risk, of frailty onset (hazard ratio [HR] = 3.3, 95% confidence interval [CI] = 1.4, 7.6) after adjustment for covariates and other tests. Adjusting for baseline cognitive performance, faster deterioration on TMT-B (HR = 0.6, 95% CI = 0.4, 1.0) was additionally associated with hazard of frailty onset.
Conclusions:
Findings inform the association of executive functioning with transitions to frailty, suggesting both impairments in and declines in executive functioning are associated with risk of frailty onset. It remains to be determined whether these associations are causal or whether shared aging related or other mechanisms are involved.
Key Words: Cognition, Cognitive aging, Frailty, Epidemiology
There is emerging recognition that older adults with physical frailty, a common but potentially preventable condition, may also exhibit poorer cognitive performance and steeper cognitive decline than older adults without frailty (1–4). Physical frailty is manifested as a medical syndrome (5) comprising slowness, weakness, reduced activity, weight loss, and exhaustion, which at the clinical level occurs in between 10% and 15% of community-living older adults (6,7), and is associated with elevated risk for adverse outcomes including hospitalization, falls, delirium, future disability, and mortality (5).
Prospective studies demonstrate that people with cognitive impairment or dementia are at elevated risk for physical frailty (1,8–10). Early impairment in global cognitive performance has been shown to be associated with elevated risk of onset of physical frailty (11). In addition to the associations of the level of cognitive impairment with frailty and its markers, other studies show that accelerated age-related cognitive decline, measured mostly via global cognitive measures (2,12–14), is related to physical frailty (15–17). Findings with respect to early preclinical impairments in cognitive performance are sparse and mixed, with one study reporting an association (14) and another not (12). It is possible that the preclinical progressions of these two processes either are causally related or are driven by shared underlying age-related processes. Thus, an important gap in research is whether preclinical, age-related cognitive impairment and decline are associated with onset of physical frailty.
Findings regarding the association of cognition with frailty could differ according to the stage of preclinical cognitive decline and impairment: in other words, associations with physical frailty may differ for persons at preclinical cognitive levels versus clinical cognitive impairment. Further, it is possible that global cognitive measures may not be as sensitive to physical frailty interventions as are domain-specific measures. These observations underscore the importance of determining the associations between domain-specific preclinical cognitive declines and impairment and the onset of physical frailty.
Few studies have examined the association of specific cognitive domains with physical frailty (1). Declines in executive functioning often precede declines in memory (18,19), and executive functioning is related to instrumental activities of daily living (20,21) and deterioration in mobility and balance (22,23), thus executive functioning may be related to physical frailty among older adults without clinical cognitive impairment. Indeed, some previous research suggests that physical frailty is associated with higher level executive functions including set-shifting ability, judgment of external and internal cues, and other cognitive factors important to gait and physical functioning (24,25). However, the association of frailty with specific cognitive domains is understudied (1).
In this study, we extend the previous findings by examining associations of impairments and changes in cognitive domains important to independent function with subsequent onset of physical frailty. We used longitudinal data spanning up to 9 years from a well-characterized sample of initially high-functioning women aged 70–79 at baseline. Based on prior work, identifying age-related vulnerability in executive functions (18,20), we hypothesized that deficits and declines in executive functioning are more strongly associated with the onset of physical frailty than measures of memory or general cognitive functioning.
Methods
Participants
We used longitudinal data from the Women’s Health and Aging Study II. Details of the sample and recruitment strategies are described elsewhere (18,26). Participants (N = 436) were initially selected from Health Care Financing Administration Medicare eligibility lists and represented the two thirds highest functioning community-living older women in Baltimore, Maryland. Exclusion criteria included scoring <24 on the Mini-Mental State Examination and difficulty in more than one functional domain (upper extremity function, exercise and mobility tolerance, and instrumental activities of daily living) (13,27). Participants were interviewed at baseline and 1.5, 3, 6, 7.5, and 9 years afterward. We excluded participants with frailty at baseline (n = 12) and who developed dementia during the study (n = 93) to isolate a sample free of known neuropathologies (16). Participants excluded due to dementia during follow-up or frailty at baseline (n = 105) had lower cognitive scores at baseline and were older, less white, and reported fewer years of education (ps < .03). Although the latter exclusion criterion potentially tilts the analytic sample to people with later onset of impairment or cognitive decline, it purifies the sample of persons with dementia pathologies and we note that the original sample was also selected for higher than average physical functioning. The final sample size was N = 331. The study was approved by the Institutional Review Board at Johns Hopkins.
Physical frailty
Frailty was the primary study outcome (frail vs. pre-frail or robust). It was assessed using the medical syndrome model based on Fried/Cardiovascular Health Study criteria that operationalizes frailty as the confluence of three or more of the following: weak grip strength, slow gait, low levels of physical activity, self-reported exhaustion, and unintentional weight loss (5). This syndrome is a manifestation of physiologic dysregulation of multiple physiologic systems and the complex adaptive system that maintains a resilient human organism (28). We chose not to study associations of cognition with components of the frailty phenotype because determining whether or not one component drives the association does not inform the unique associations between cognition and frailty, an outcome identified as encompassing more than the sum of its parts (29).
Cognitive tests
Scores for psychomotor speed, executive functioning, and immediate and delayed episodic memory were obtained from a standardized neuropsychological test battery administered at each Women’s Health and Aging Study II visit. The Trail Making Test, part A is a test of psychomotor speed that involves connecting randomly placed numbers on a page. The Trail Making Test, part B (TMT-B) is a test of psychomotor speed and set-shifting ability that involves alternating between randomly placed numbers and letters (30). Participants were allowed 240 seconds and 420 seconds on Trails A and B, respectively; scores were converted to speed by dividing (60 seconds)/time so that higher scores reflect better performance (line drawings per second) (20). The Hopkins Verbal Learning Test (HVLT) (31) is a four-trial word list learning test of 12 semantically related words. The sum of words recalled across the first three successive immediate recall trials of the HVLT represents immediate recall (possible range: 0, 36 words). The fourth trial was a 20-minute delayed recall trial (possible range: 0, 12 words).
Indicators for mild (preclinical) domain-specific cognitive impairment were derived for each test (Trails A ≥81 seconds, Trails B ≥225 seconds, HVLT immediate recall ≤16 words, and HVLT delayed recall ≤4 words) based on external cutoffs using age- and education-matched published norms (18,31). Cognitive tests were treated continuously in analyses of longitudinal change. A summary factor representing general cognitive performance was derived as a factor score from a single-factor analysis of the four tests; the factor analysis used a maximum likelihood estimator (32).
Analysis plan
We characterized the sample using means and percentages and evaluated differences between frail and non-frail groups using t tests for continuous variables and χ2 tests for categorical variables. To model time to onset of frailty as a function of domain-specific cognitive impairment, we used discrete-time proportional hazards survival models with domain-specific cognitive impairment as a time-varying exposure (33). We evaluated the proportional hazards assumption by including interactions between cognitive impairment indicators and time; none were statistically significant. To model time to onset of physical frailty as a function of cognitive change, we used similar models but with model-estimated slopes of cognitive change as independent variables. Slopes were estimated from linear mixed effects models with random effects for people and time. Higher scores on cognitive tests indicate better performance, and steeper (eg, less negative) slopes represent less cognitive decline.
We conducted sensitivity analyses by substituting the difference between Trails B and A for Trails B performance and rerunning all primary analyses using this score; impairment on Trails B–A was based on the 20th percentile of the empirical distribution. We did not use the difference score in the main analysis because of the lack of available preclinical cutoffs. We conducted two additional sets of sensitivity analyses to evaluate the robustness of our findings. The first assumed participants who dropped out before the final study visit developed frailty after they went missing. The second sensitivity analysis assumed participants with missing cognitive testing had cognitive impairment at that visit. We reran all analyses under these extreme assumptions.
Statistical models were controlled for depressive symptoms based on the Geriatric Depression Scale score (34), age, race (white, non-white), and years of education. Previous studies have reported neurological deficits related to falls (35). We did not adjust for falls because they were relatively rare in this cohort.
Results
The sample of N = 331 women were, on average, 73.6 years of age at baseline; 44 (13%) were non-white, with mean years of education 13.0 years (Table 1). The analysis included 2,046 person-years, with mean follow-up time of 6.8 years (median 9 years). Thirteen percent (44/331) developed frailty over 9 years and 46% (132/284) developed cognitive impairment in one or more domains. Compared with participants who experienced incident cognitive impairment during follow-up (N = 132), participants without incident impairment tended to be younger by 1.0 years, more educated by 1.2 years, and reported fewer depressive symptoms (difference of 0.8 points in the Geriatric Depression Scale score; p values < .05). Simultaneous onset of impairment in a given cognitive domain and frailty at a given visit was observed in between 36% and 62% of participants (Table 2, last row). Onset of any cognitive impairment more often preceded onset of physical frailty among 38% (n = 11/29) of participants who developed both, whereas onset of physical frailty preceded onset of any cognitive impairment in just 17% (n = 5/29) of participants who developed any cognitive impairment (Table 2). Five percent (15/331) of the sample developed frailty without cognitive impairment. Across cognitive domains, impairment in executive functioning was most likely to precede frailty (Table 2).
Table 1.
Baseline Characteristics of the WHAS II Sample (N = 331)
| Characteristic | Mean (SD) | Range |
|---|---|---|
| Age | 73.6 (2.7) | 70.0, 80.0 |
| Years of education | 13.0 (3.2) | 2.0, 18.0 |
| Black, n (%) | 44 (13.3) | |
| Geriatric Depression Scale | 3.6 (3.2) | 0.0, 16.0 |
| Number of comorbidities (of 14) | 1.5 (1.0) | 0.0, 5.0 |
| Coronary artery disease, n (%) | 53 (16.0) | |
| Congested heart failure, n (%) | 6 (1.8) | |
| Peripheral artery disease, n (%) | 10 (3.0) | |
| Stroke, n (%) | 3 (0.9) | |
| Pulmonary diseases, n (%) | 72 (21.8) | |
| Hip fracture, n (%) | 5 (1.5) | |
| Diabetes, n (%) | 24 (7.3) | |
| Body mass index | 26.8 (5.3) | 15.8, 47.1 |
| High blood pressure, n (%) | 170 (51.4) | |
| Baseline smoking status, n (%) | ||
| Nonsmoker | 176 (53.7) | |
| Former | 118 (36.0) | |
| Current | 34 (10.4) | |
| Baseline activity status, n (%) | ||
| Inactive (0min/wk) | 23 (7.0) | |
| Insufficient (1–149min/wk) | 112 (34.1) | |
| Recommended (≥150min/wk) | 193 (58.8) | |
| Cognitive tests | ||
| Trail Making Test, part A | 1.6 (0.5) | 0.5, 3.5 |
| Trail Making Test, part B | 0.6 (0.2) | 0.1, 1.4 |
| HVLT immediate recall | 23.4 (5.2) | 7.0, 35.0 |
| HVLT delayed recall | 8.6 (2.4) | 0.0, 12.0 |
| General cognitive performance factor | 0.3 (0.8) | −2.4, 1.9 |
Notes: HVLT = Hopkins Verbal Learning Test; WHAS II = Women’s Health and Aging Study II.
Table 2.
Prevalence and Onset of Impairment in Each Cognitive Test Over 9 y: Results From WHAS II (N = 331)
| Executive Functioning (Trails A) | Executive Functioning (Trails B) | Memory (HVLT immediate recall) | Memory (HVLT delayed recall) | Any Cognitive Impairment Indicated | |
|---|---|---|---|---|---|
| Prevalent at baseline | 12 (4%) | 21 (6%) | 24 (7%) | 16 (5%) | 47 (14%) |
| Not prevalent at baseline | 319 (96%) | 310 (94%) | 307 (93%) | 315 (95%) | 284 (86%) |
| Incidence (9 y) | 51 (16%) | 94 (30%) | 65 (21%) | 53 (17%) | 132 (46%) |
| Incidence of 2+ cognitive impairments together | 23 (7%) | 32 (10%) | 44 (14%) | 36 (11%) | NA |
| Developed neither frailty nor cognitive impairment | 253 (79%) | 218 (70%) | 238 (78%) | 247 (78%) | 184 (65%) |
| Developed only frailty | 27 (8%) | 19 (6%) | 28 (9%) | 31 (10%) | 15 (5%) |
| Developed only cognitive impairment | 34 (11%) | 69 (22%) | 49 (16%) | 40 (13%) | 103 (36%) |
| Developed both | 17 (5%) | 25 (8%) | 16 (5%) | 13 (4%) | 29 (10%) |
| Cognition first | 4 (24%) | 10 (40%) | 5 (31%) | 3 (23%) | 11 (38%) |
| Frailty first | 5 (29%) | 6 (24%) | 4 (25%) | 2 (15%) | 5 (17%) |
| Co-occurring with frailty | 8 (47%) | 9 (36%) | 7 (44%) | 8 (62%) | 13 (45%) |
Notes: HVLT: Hopkins Verbal Learning Test; NA = not applicable; WHAS II = Women’s Health and Aging Study II. Percentages in the first and second rows were calculated based on the full sample of 331. Percentages in the third, fourth, fifth, sixth, seventh, and eighth rows were calculated based on the sample size without prevalent impairment at baseline (second row). Denominators for percentages in the final three rows are the eighth row.
Cognitive Impairment and Time to Onset of Frailty
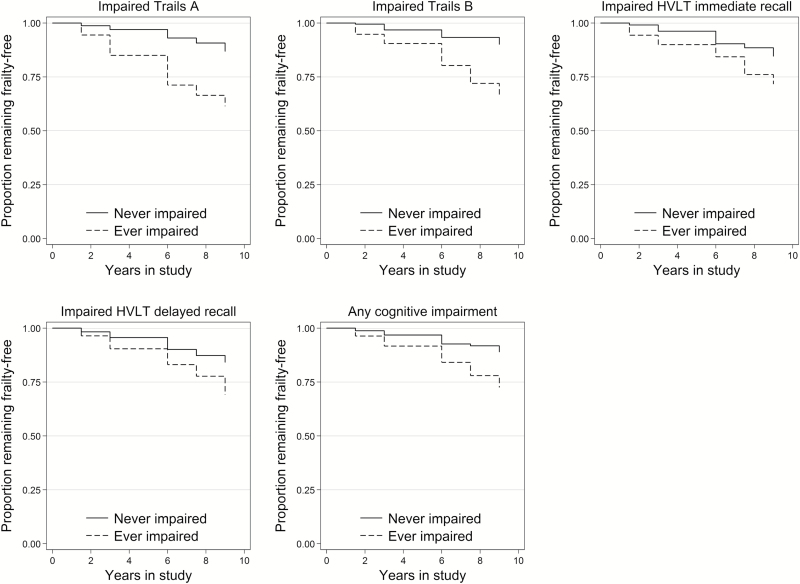
Figure 1 and Table 3 show hazard ratios (HR) for the association of preclinical impairment in each test with time to onset of frailty. Impairment on each cognitive test was predictive of frailty onset, adjusting for background characteristics. Impairment in executive functioning (TMT-B) was most strongly associated with onset of frailty (HR = 4.4, 95% confidence interval [CI] = 2.2, 9.0) after adjusting for Trail Making Test, part A. In a model adjusted for impairment in all cognitive tests together, only executive functioning (TMT-B) was associated with frailty onset (HR = 3.3, 95% CI = 1.4, 7.6; Table 3).
Figure 1.
Kaplan–Meier plots of time to onset of frailty by cognitive impairment: results from WHAS II (N = 331). WHAS II = Women’s Health and Aging Study II.
Table 3.
Associations of Cognitive Impairment With Time to Onset of Frailty in WHAS II (N = 331)
| Cognitive Impairment Independent Variable | Associations With Time to Frailty, Unadjusted for Other Domains | Associations With Time to Frailty, Adjusted for Other Domains |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Psychomotor speed (TMT-A) | 4.1* (1.8, 9.3) | 2.0 (0.8, 5.3) |
| Executive functioning (TMT-B) | 4.4* (2.2, 9.0) | 3.3* (1.4, 7.6) |
| Memory (HVLT immediate recall) | 2.5* (1.1, 5.7) | 1.0 (0.3, 3.4) |
| Delayed memory (HVLT delayed recall) | 2.9* (1.3, 6.5) | 1.8 (0.5, 5.6) |
| Any impairment | 3.7* (2.0, 7.2) | — |
Notes: CI = confidence interval; HR = hazard ratios; HVLT: Hopkins Verbal Learning Test; TMT-A = Trail Making Test, part A; TMT-B = Trail Making Test, part B; WHAS II = Women’s Health and Aging Study II. HR represent the factor by which the hazard, or risk, of frailty onset differs between those with and without cognitive impairment. The first column shows HR for cognitive variables and time to frailty onset, adjusting for depressive symptoms, age, race, and years of education. The second column shows HR for cognitive tests and time to frailty onset, additionally adjusting for other cognitive tests.
*p < .05.
Cognitive Change and Time to Onset of Frailty
Table 4 shows HR for the association of baseline levels and changes in each cognitive test with time to onset of frailty. To enable direct comparisons of associations across cognitive tests, we standardized each cognitive variable representing levels and rates of change to mean 0 (SD 1), thus placing all HRs on a common scale. The standardized mean rate of change was −0.38 SD units for Trail Making Test, part A, −0.83 SD units for TMT-B, −0.17 SD units for HVLT immediate recall, −0.34 SD units for HVLT delayed recall, and −0.38 SD units for general cognitive performance. Better baseline performance on psychomotor speed (Trail Making Test, part A; HR = 0.5, 95% CI = 0.3, 0.7), executive functioning (TMT-B; HR = 0.2, 95% CI = 0.1, 0.5), delayed memory (HVLT delayed recall; HR = 0.7, 95% CI = 0.4, 1.0), and general cognitive performance (HR = 0.6, 95% CI = 0.4, 0.9) were associated with lower risk of onset of frailty. Slower rate of annual decline in executive functioning (TMT-B) was associated with a 40% reduction in risk of frailty onset (HR = 0.6, 95% CI = 0.4, 1.0) and was the only cognitive test whose trajectory was associated with lower risk of frailty onset (Table 4).
Table 4.
Associations of Cognitive Trajectory With Time to Onset of Frailty in WHAS II (N = 331)
| Cognitive Independent Variable | Associations of Baseline Cognition With Time to Onset of Frailty | Associations of Annual Rate of Change With Time to Onset of Frailty |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Psychomotor speed (TMT-A) | 0.5* (0.3, 0.7) | 0.8 (0.6, 1.1) |
| Executive functioning (TMT-B) | 0.2* (0.1, 0.5) | 0.6* (0.4, 1.0) |
| Memory (HVLT immediate recall) | 0.8 (0.5, 1.2) | 0.7 (0.5, 1.0) |
| Delayed memory (HVLT delayed recall) | 0.7* (0.4, 1.0) | 0.9 (0.7, 1.2) |
| General cognitive performance | 0.6* (0.4, 0.9) | 0.8 (0.6, 1.0) |
Notes: CI = confidence interval; HR = hazard ratios; HVLT: Hopkins Verbal Learning Test; TMT-A = Trail Making Test, part A; TMT-B = Trail Making Test, part B; WHAS II = Women’s Health and Aging Study II. The first column of HR represents the factor by which the hazard, or risk, of frailty onset differs for each standard deviation unit increase in cognitive performance. The second column of HR represents the factor by which the hazard of frailty onset differs for each standard deviation unit shallower rate of cognitive decline. Higher scores on cognitive tests indicate better performance. Steeper, or less negative, slopes indicate less cognitive decline. All models are adjusted for depressive symptoms, age, race, and years of education.
*p < .05.
Sensitivity Analyses
Preclinical impairment in the difference between Trails B and A was associated with an HR = 2.5 (95% CI = 1.2, 5.0) elevated risk of onset of physical frailty.
To account for potential underascertainment of physical frailty, we assumed everyone who dropped out before the end of the study developed frailty during their next visit following dropout. No inferences changed. Cognitive tests were missing in 4.5%–5.9% of visits. To accommodate the potential for selective attrition among more cognitively impaired participants, we set missing indicators of cognitive impairment to impaired if they were missing and reran analyses. Inferences did not change.
Discussion
In this longitudinal study of initially healthy older women who were free of physical frailty and cognitive impairment at baseline, impairments and declines in executive functioning were associated with elevated risk of frailty onset. Cognitive impairment, defined according to cut points for preclinical impairment, preceded physical frailty in most cases. Preclinical cognitive impairment was associated with earlier onset of frailty, and executive functioning was a stronger predictor than memory, as hypothesized. Further, after adjusting for baseline cognitive performance, age, and other characteristics, faster deterioration in executive functioning was additionally associated with earlier frailty onset. Overall, these findings inform the role of specific domains of cognitive functioning in transitions to clinical frailty. Repeated measures of multiple domains of cognition in relation to incident physical frailty over a 9-year study period improved our ability to specify these associations as older adults enter an age of elevated risk for physical frailty.
The present findings demonstrate that the cognition–frailty association exists among those with preclinical, specific cognitive impairment and appears to be strongest for the domain of executive functioning. Executive functions, important to the maintenance of independent functioning, deteriorate prior to memory in community-dwelling older adults and thus may be more sensitive than other cognitive domains to dysregulated brain processes at the preclinical stage (18,19). Impaired executive functioning is found in vascular dementia. Episodic memory, measured by the HVLT, is a hallmark of early Alzheimer’s disease. Thus, because domain-specific changes and impairment in executive functioning was more strongly associated with frailty than memory, our results suggest impairments underlying prodromal Alzheimer’s disease and physical frailty may be distinct. In other studies, physical frailty has been shown to be associated in diverse samples with incidence of dementia, particularly vascular dementia as opposed to Alzheimer’s disease (8–10).
Mechanistic implications of this study require further investigation. Prefrontally mediated executive functions are related to mobility changes (24,36) and other features important in physical frailty, but whether there is a causal relationship between cognition and frailty or whether they are two manifestations of common underlying systemic physiologic alterations is unclear from our study alone. Common physiologic systems underlying cognitive declines and physical frailty may include inflammatory pathways (37,38), energy dysregulation, dysregulation of the hypothalamic–pituitary–adrenal axis (39), and cerebrovascular disease (40). In particular, low-grade inflammation has been found to be related to poorer executive functioning in older adults (39,41). More generally, a growing body of research has uncovered novel mechanisms through which biological alterations affect brain aging and higher level cognitive functions related to executive functioning (42,43). Subclinical deterioration in subcortical networks of the brain, in particular, the prefrontal cortex, basal ganglia, and medial temporal lobe, is associated cross-sectionally and longitudinally with slowed gait speed (44). Other research suggests control of gait speed is related to integrity of both white and gray matter (45).
Although not all persons with cognitive impairment become frail and not all persons with frailty become demented (46), the two co-occur frequently and according to our data may begin around the same time. Cognitive impairment and frailty have common risk factors, antecedents, and consequences (17). Common antecedents include cardiovascular risk factors, physical activity, and poor nutrition. For example, cardiovascular risk factors and physical activity are each associated with frailty and cognitive impairment (17). A large proportion of diagnosed cases of dementia have substantial cerebrovascular disease observed at autopsy, suggesting commonality in etiology of all of these conditions (47). Nutrition is another potential common link: diet has both behavioral (eg, proper diet and meal planning) and biological contributors (eg, oxidative stress and muscle weakness) (17). Adherence to a high-antioxidant Mediterranean diet is protective against cognitive impairment and physical frailty (48).
Our findings are consistent with previous work. Other studies have suggested frailty is most strongly linked to executive functioning (1,25), although we are aware of no studies that have previously examined longitudinal changes in executive functioning. Cognitive change is a more informative phenotype than is a one-time measure of cognitive level, because the latter is strongly influenced by education and other early life experiences and thus does not reflect in an unbiased fashion cognitive abilities in later life (49).
Several limitations should be mentioned. First, frailty may lead to selective attrition, mortality, and other competing events that prevent us from observing frailty. Dropout due to frailty is more likely than dropout due to cognitive impairment. Because of this, we conducted a sensitivity analysis assuming persons who dropped out of the study developed frailty; this did not change inferences. A second limitation is that Women’s Health and Aging Study II is not a representative sample; participants were selected for high functioning at baseline and were demographically restricted to women. The high function of the sample is exemplified by the low incidence of frailty (13%) in our sample over 9 years, which was lower than another study’s 26% over 8 years (50). This is also a strength: we were able to observe the natural association of early, domain-specific cognitive impairment with onset of frailty in a sample relatively free of disability at baseline, strengthening causal inferences. Regarding generalizability to men, frailty and clinical cognitive impairment (dementia) are more prevalent among women (51), placing them at elevated risk for the convergence of both outcomes. However, there are no data examining the associations between frailty and cognition by sex. Other research has found no evidence for sex differences in associations between physical function and cognition in older adults (52). A third limitation is that our study alone cannot infer causation in the relationship between frailty and domain-specific cognitive impairment and decline; the associations may be attributable to shared common precursors such as cardiovascular risk factors (17,47), low physical activity, and poor nutrition (48).
These findings inform our understanding of cognitive domain-specific associations in the etiology of physical frailty and transitions to clinical frailty. Our study is consistent with the notion that older adults with impaired executive function at preclinical levels should be more closely monitored for the onset of frailty. It would be of public health importance to examine in future research whether both impaired executive functioning and frailty share causal etiologies, and if they are jointly associated with elevated risk of adverse outcomes (eg, falls, hospitalization, and delirium) relative to either alone. Further, the results have implications for selecting cognitive tests to assess the risk of frailty: executive functioning tasks appear more sensitive than others to frailty in community-living older adults.
Funding
The WHAS II study was supported by R01 AG11703-01A1 from the National Institute on Aging (NIA). A.L.G. was supported by a Research Career Development Core Award (PI: Gross) from the Johns Hopkins Claude D. Pepper Older Americans Independence Center (P30 AG021334). M.M.D. was supported by NIA K01 AG043501 (PI: McAdams DeMarco). K.B.R. was supported by the Johns Hopkins Older Americans Independence Center (P30 AG021334) from the NIA and the Alzheimer’s Disease Research Center (P50 AG005146). Q.L.X. was supported by the Johns Hopkins Older Americans Independence Center (P30 AG021334) from the NIA. L.P.F. was supported by a MERIT award from the NIA. M.C.C. was supported by the WHAS II Cognitive Pathways study (RO1 AG11703-01A1) supported by NIA. The contents do not necessarily represent views of the funding entities. Funders had no deciding roles in the design and conduct of the study.
Acknowledgments
This work was supported by a Research Career Development Core Award (PI: A. L. Gross) from the Johns Hopkins Claude D. Pepper Older Americans Independence Center. The contents do not necessarily represent views of the funding entities. Funders had no deciding roles in the design and conduct of the study.
References
- 1. Canevelli M, Cesari M, van Kan GA. Frailty and cognitive decline: how do they relate? Curr Opin Clin Nutr Metab Care. 2015;18:43–50. doi:10.1097/mco.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 2. Nishiguchi S, Yamada M, Fukutani N, et al. Differential association of frailty with cognitive decline and sarcopenia in community-dwelling older adults. J Am Med Dir Assoc. 2015;16:120–124. doi:10.1016/j.jamda.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 3. Panza F, Solfrizzi V, Barulli MR, et al. Cognitive frailty—epidemiological and neurobiological evidence of an age-related clinical condition: a systematic review. Rejuvenation Res. 2015. doi:10.1089/rej.2014.1637 [DOI] [PubMed] [Google Scholar]
- 4. Gill TM, Williams CS, Richardson ED, Tinetti ME. Impairments in physical performance and cognitive status as predisposing factors for functional dependence among nondisabled older persons. J Gerontol A Biol Sci Med Sci. 1996;51:M283–M288. doi:10.1093/gerona/51a.6.m283 [DOI] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 6. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi:10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi:10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avila-Funes JA, Carcaillon L, Helmer C, et al. Is frailty a prodromal stage of vascular dementia? Results from the Three-City Study. J Am Geriatr Soc. 2012;60:1708–1712. doi:10.1111/j.1532-5415.2012.04142.x [DOI] [PubMed] [Google Scholar]
- 9. Gray SL, Anderson ML, Hubbard RA, et al. Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 2013;68:1083–1090. doi:10.1093/gerona/glt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solfrizzi V, Scafato E, Frisardi V. Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimers Dement. 2013;9:113–122. doi:10.1093/gerona/glt013 [DOI] [PubMed] [Google Scholar]
- 11. Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65:1228–1234. doi:10.1093/gerona/glq12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alencar MA, Dias JM, Figueiredo LC, Dias RC. Frailty and cognitive impairment among community-dwelling elderly. Arq Neuropsiquiatr. 2013;71:362–367. doi:10.1590/0004-282x20130039 [DOI] [PubMed] [Google Scholar]
- 13. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–255. doi:10.1111/j.1532-5415.2009.02671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–489. doi:10.1097/psy.0b013e318068de1d [DOI] [PubMed] [Google Scholar]
- 15. Halil M, Cemal Kizilarslanoglu M, Emin Kuyumcu M, Yesil Y, Cruz Jentoft AJ. Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015;19:276–283. doi:10.1007/s12603-014-0535-z [DOI] [PubMed] [Google Scholar]
- 16. Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–734. doi:10.1007/s12603-013-0367-2 [DOI] [PubMed] [Google Scholar]
- 17. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Research Reviews. 2013;12:840–851. doi:10.1016/j.arr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 18. Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2009;64:110–117. doi:10.1093/gerona/gln008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrington MG, Chiang J, Pogoda JM, et al. Executive function changes before memory in preclinical Alzheimer’s pathology: a prospective, cross-sectional, case control study. PLoS One. 2013;8:e79378. doi:10.1371/journal.pone.0079378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlson MC, Fried LP, Xue QL, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Soc Sci. 1999;54B:S262–S270. doi:10.1093/geronb/54b.5.s262 [DOI] [PubMed] [Google Scholar]
- 21. Royall DR, Lauterbach EC, Kaufer D, et al. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19(3):249–265. doi:10.1176/appi.neuropsych.19.3.249 [DOI] [PubMed] [Google Scholar]
- 22. Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi:10.1093/ageing/afl107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Killane I, Donoghue OA, Savva GM, Cronin H, Kenny RA, Reilly RB. Relative association of processing speed, short-term memory and sustained attention with task on gait speed: a study of community-dwelling people 50 years and older. J Gerontol A Biol Sci Med Sci. 2014;69:1407–1414. doi:10.1093/gerona/glu140 [DOI] [PubMed] [Google Scholar]
- 24. Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov Disord. 2013;28:1520–1533. doi:10.1002/mds.25674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langlois F, Vu TT, Kergoat MJ, Chassé K, Dupuis G, Bherer L. The multiple dimensions of frailty: physical capacity, cognition, and quality of life. Int Psychogeriatr. 2012;24:1429–1436. doi:10.1017/s1041610212000634 [DOI] [PubMed] [Google Scholar]
- 26. Fried LP, Bandeen-Roche K, Chaves PHM, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol Med Sci. 2000;55:M43–M52. doi:10.1093/gerona/55.1.m43 [DOI] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 28. Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi:10.1093/gerona/glp076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi:10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 30. Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. doi:10.2466/pms.1958.8.3.271 [Google Scholar]
- 31. Brandt J. Benedict RHB Hopkins Verbal Learning Test–Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 32. Jones RN, Rudolph JL, Inouye SK, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol. 2010;32:1041–1049. doi:10.1080/13803391003662728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. doi:10.2307/2529588 [PubMed] [Google Scholar]
- 34. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 35. Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: a systematic review. Dement Geriatr Cogn Disord. 2013;36:20–35. doi:10.1159/000350031 [DOI] [PubMed] [Google Scholar]
- 36. Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. doi:10.1093/gerona/63.12.1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cappola AR, Xue QL, et al. DHEAS levels and mortality in disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2006;61(9):957–962. doi:10.1093/gerona/61.9.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC. Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2014. pii: glu132. doi:10.1093/gerona/glu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agbedia OO, Varma VR, Seplaki CL, et al. Blunted diurnal decline of cortisol among older adults with low socioeconomic status. Ann New York Acad Sci. 2011;1231:56–64. doi:10.1111/j.1749-6632.2011.06151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43:2526–2534. doi:10.1161/strokeaha.112.655803 [DOI] [PubMed] [Google Scholar]
- 41. Heringa SM, van den Berg E, Reijmer YD, et al. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population—the Hoorn Study. Psychoneuroendocrinology. 2014;40:108–118. doi:10.1016/j.psyneuen.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 42. Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol A Biol Sci Med Sci. 2014;69:1389–1398. doi:10.1093/gerona/glt207 [DOI] [PubMed] [Google Scholar]
- 43. Ungvari Z, Sonntag WE. Brain and cerebrovascular aging–new mechanisms and insights. J Gerontol A Biol Sci Med Sci. 2014;69:1307–1310. doi:10.1093/gerona/glu187 [DOI] [PubMed] [Google Scholar]
- 44. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi:10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi:10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimada H, Makizako H, Doi T, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc. 2013;14:518–524. doi:10.1016/j.jamda.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 47. Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi:10.1212/01.wnl.0000149519.47454.f2 [DOI] [PubMed] [Google Scholar]
- 48. Talegawkar SA, Bandinelli S, Bandeen-Roche K, et al. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr. 2012;142:2161–2166. doi:10.3945/jn.112.165498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi:10.1093/aje/kwi187 [DOI] [PubMed] [Google Scholar]
- 50. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi:10.1001/archinte.166.4.418 [DOI] [PubMed] [Google Scholar]
- 51. Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi:10.1111/j.1532-5415.2005.53008.x [DOI] [PubMed] [Google Scholar]
- 52. Garrett SL, Sawyer P, Kennedy RE, et al. Racial and sex differences in associations between activities of daily living and cognition in community-dwelling older adults. J Am Geriatr Soc. 2013;61(12):2174–2180. doi:10.1111/jgs.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]