Abstract
Background:
Nonhuman studies suggest a protective effect of caffeine on cognition. Although human literature remains less consistent, reviews suggest a possible favorable relationship between caffeine consumption and cognitive impairment or dementia. We investigated the relationship between caffeine intake and incidence of cognitive impairment or probable dementia in women aged 65 and older from the Women’s Health Initiative Memory Study.
Methods:
All women with self-reported caffeine consumption at enrollment were included (N = 6,467). In 10 years or less of follow-up with annual assessments of cognitive function, 388 of these women received a diagnosis of probable dementia based on a 4-phase protocol that included central adjudication. We used proportional hazards regression to assess differences in the distributions of times until incidence of probable dementia or composite cognitive impairment among women grouped by baseline level of caffeine intake, adjusting for risk factors (hormone therapy, age, race, education, body mass index, sleep quality, depression, hypertension, prior cardiovascular disease, diabetes, smoking, and alcohol consumption).
Results:
Women consuming above median levels (mean intake = 261mg) of caffeine intake for this group were less likely to develop incident dementia (hazard ratio = 0.74, 95% confidence interval [0.56, 0.99], p = .04) or any cognitive impairment (hazard ratio = 0.74, confidence interval [0.60, 0.91], p = .005) compared to those consuming below median amounts (mean intake = 64mg) of caffeine for this group.
Conclusion:
Our findings suggest lower odds of probable dementia or cognitive impairment in older women whose caffeine consumption was above median for this group and are consistent with the existing literature showing an inverse association between caffeine intake and age-related cognitive impairment.
Key Words: Dementia, Aging, Hormone therapy, Cognitive impairment, Cognitive function
Mounting evidence from nonhuman animal studies suggests a protective effect of caffeine (1–3) and other bioactive components of coffee (4) on cognition. In vitro and preclinical studies underscore some mechanisms by which caffeine may exert protective effects against cognitive impairment and Alzheimer’s disease (AD) (2,5). AD, an age-related neurodegenerative disorder and the most common cause of dementia, is characterized by a progressive cognitive impairment, memory loss being a hallmark, and represents the culmination of neuropathological changes (amyloid-β plaques and neurofibrillary tangles deposition) thought to evolve over several decades (6). Moreover, in animal models, caffeine seems to have a positive effect not only on behavior but also on certain aspects of AD-related neuropathology, such as beta-amyloid levels (1,7,8).
The potential protective effect of caffeine is thought to occur primarily through the blockade of adenosine A2A receptors (ARs), whose expression and function become aberrant with both normal aging and age-related pathology (9). Adenosine acts by facilitating A2A and acting on the inhibitory A1 receptors to integrate dopamine, glutamate, and brain-derived neurotrophic factor signaling, thereby modulating synaptic plasticity in regions relevant to learning and memory and providing the molecular and cellular bases for AR role in modulating cognition (10). Furthermore, ARs are increasingly recognized as potential targets for reversing cognitive impairment within the context of both normal and pathological aging, and there is support for AR activity-related reversal of cognitive impairment in animal models of AD, Parkinson’s disease, Huntington’s disease, and schizophrenia (11).
Lastly, epidemiological studies, although less consistent (12–16), generally report an inverse association of caffeine intake with age-related cognitive impairment and AD (13,17,18). Moreover, it has been reported that baseline plasma caffeine levels are lower by 50% in individuals with mild cognitive impairment (MCI) who progressed to dementia 2–4 years later compared to those who remained stable (19). It is well known that age increases the risk of developing AD. With mean population age rising steadily and some predictions indicating that the AD prevalence will quadruple by 2050 (20), there is an alarming need for effective treatments that prevent, delay or slow the disease that is already the sixth leading cause of death in the developed world (21).
We investigated the relationship between caffeine intake and overall incidence of probable dementia (PD) or global cognitive impairment in postmenopausal women aged 65 and older from the Women’s Health Initiative Memory Study (WHIMS), a randomized controlled clinical trial of postmenopausal hormone therapy (22). WHIMS offers an unprecedented opportunity to examine these relationships in a large and well-defined, prospectively studied cohort of women who were free of dementia at baseline. Our aim is to characterize the relationship between caffeine consumption and the incidence of PD or global cognitive impairment.
Methods
Participants
All women who provided self-reported caffeine data within 6 months of WHIMS enrollment, nested within the Women’s Health Initiative (WHI) randomized clinical trial of hormone therapy, and had at least one follow-up cognitive assessment (N = 6,467 of 7,497 enrolled in WHIMS) (22), were included. WHIMS examined the relative effect of 0.625mg/d conjugated equine estrogens (CEE) alone and in combination with 2.5mg/d medroxyprogesterone acetate (CEE + MPA) on the incidence of global cognitive decline and PD (23). Participants were randomly assigned, through the WHI hormone trials, with equal probability to active therapy (CEE if prior hysterectomy; CEE + MPA if no prior hysterectomy) or matching placebo. WHIMS enrollment spanned 1995–1999. Through 2007, global cognitive function was assessed annually by centrally trained, masked, and certified technicians and interviewers using the 100-point Modified Mini Mental State (3MS) exam (24). Subsequently, during the Women’s Health Initiative Memory Study of the Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), annual assessment was based on the 40-point Telephone Interview for Cognitive Status-modified (TICSm) (25,26). All participants provided written informed consent. Studies were approved by the National Institutes of Health and the Institutional Review Boards of participating institutions.
The WHIMS clinical trial design, eligibility criteria, recruitment procedures, and a detailed description of the protocol for detecting PD and MCI have been published (22). The central adjudication committee consists of three board-certified specialists (two neurologists and one geriatric psychiatrist) with extensive experience in dementia, who reviewed medical records to inform the diagnosis. All cases judged as PD by the local physician specialists were independently reviewed by the central adjudicators, as well as 50% of MCI cases and 10% of cases without dementia. In WHIMS-ECHO, when women screened positive for cognitive impairment (TICSm < 31), a reliable and preidentified informant was interviewed by telephone using the validated Dementia Questionnaire (27) to assesses the history of cognitive and behavioral changes, functional impairments, and health events that can affect cognitive functioning (eg, stroke). If MCI case progressed to PD, PD was used as the outcome for that patient.
Caffeine intake was based on self-report at baseline using the Food Frequency Questionnaire (FFQ) (28). Caffeine intake was estimated from FFQ questions about coffee, tea, and cola beverage intake, including frequency and serving size. The FFQ does not have separate questions about caffeinated and decaffeinated beverages; hence, it was assumed that all intake was of caffeinated forms of these beverages. Personal Habits Questionnaire queried specifically about drinking caffeinated coffee each day. Those who reported drinking coffee each day were asked “How many cups of regular (non-decaf) coffee do you usually drink each day?” (if none, mark “None”; potential answers: None, 1, 2, 3, 4, 5, and 6 or more). Potential confounders (eg, age, education, body mass index, hypertension, and prior cardiovascular disease) were collected at WHI enrollment via self-report and standardized assessments at WHI enrollment (23). The caffeine intake for the main analyses purposes was based on the FFQ, and only related to the cups of coffee obtained via Personal Habits Questionnaire as we could not otherwise discern the source caffeine in our sample as the FFQ does not have separate questions about caffeinated and decaffeinated beverages, hence it was presumed that all intake was of caffeinated forms of these beverages.
Statistical Analyses
Participants were grouped according to self-reported caffeine intake at baseline to examine differences among groups with respect to risk factors for PD and baseline global cognitive impairment (3MS) in WHIMS. We examined relationships that baseline caffeine intake (grouped into quartiles) had with risk factors for PD alone or in combination with MCI (composite cognitive impairment) or global cognitive performance (3MS) using analysis of variance and chi-square tests. Composite cognitive impairment (MCI + PD) has been historically reported in previously published WHIMS manuscripts, given that originally the MCIs were a few, and hence were grouped together with PD as a cognitively impaired; we include it here for comparison purposes.
We used proportional hazards regression to assess differences in the distributions of times until incidence of PD or composite cognitive impairment among women grouped by baseline level of caffeine intake. Follow-up was censored at the last cognitive assessment or 10-year postrandomization. General linear models were used to examine relationships that caffeine intake has with global cognitive function over time based on 3MS scores or PD. All models included hormone therapy assignment, age, race/ethnicity, and education as covariates. Additional models adjusted for risk factors that are related to caffeine intake (body mass index, sleep quality, depressive symptoms, hypertension, prior cardiovascular disease, diabetes, smoking, alcohol consumption, and 3MS scores at baseline).
Results
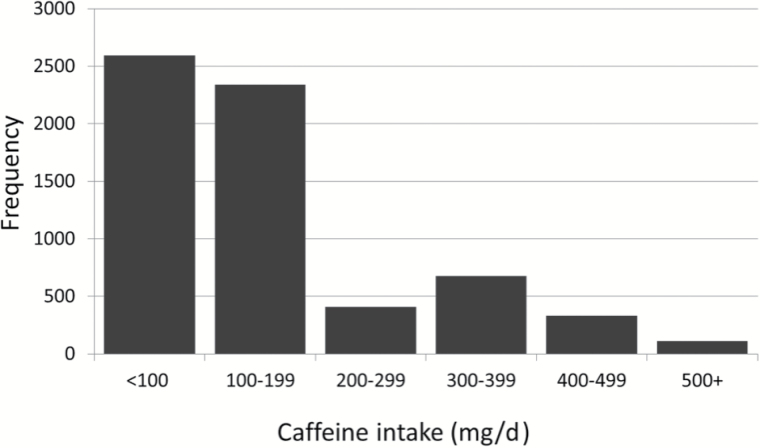
Sample characteristics of women at WHI enrollment are presented in Table 1. The sample was predominantly Caucasian (87%) and women were between 65 and 80 years of age at enrollment. Most women had either some college education or a college degree; fewer than 8% had not completed high school. Most women had no prior history of cardiovascular disease (89%) or diabetes (92%). About 5% of the women were still working and the distribution of women currently working was similar across caffeine intake. The distribution of self-reported daily caffeine intake (mg/d) at baseline is presented in Figure 1; mean (SD) self-reported daily caffeine intake at baseline in this sample of women with follow-up cognitive data in WHIMS was 172 (134) mg/d. To help provide perspective, an 8-ounce cup of brewed coffee contains 95mg of caffeine, 8-ounces of brewed black tea contains 47mg and a 12-ounce can of carbonated cola contains 33mg. Across 10 years of follow-up, caffeine intake was queried two additional times. The Spearman correlation of self-reported intake over time ranged from .60 to .63.
Table 1.
Characteristics of Participants at WHI Enrollment Grouped by Quartile of Self-reported Caffeine Intake
| Characteristic | Daily Caffeine Intake (mg) | p Value | |||
|---|---|---|---|---|---|
| <75 mg | 75–174 mg | 175–189 mg | ≥190 mg | ||
| N (%) | N (%) | N (%) | N (%) | ||
| Age (y) | |||||
| 65–69 | 584 (44.6) | 696 (43.0) | 849 (47.3) | 883 (50.5) | |
| 70–74 | 457 (34.9) | 612 (37.8) | 624 (34.8) | 601 (34.4) | <.001 |
| 75–80 | 267 (20.4) | 310 (19.2) | 321 (17.9) | 263 (15.0) | |
| Education, missing = 9 | |||||
| Less than HS graduation | 164 (12.6) | 121 (7.5) | 95 (5.3) | 107 (6.1) | |
| HS graduation | 286 (21.9) | 369 (22.8) | 394 (22.0) | 401 (23.0) | <.001 |
| Some college | 498 (38.2) | 631 (39.0) | 729 (40.7) | 742 (42.6) | |
| College graduation | 357 (27.4) | 495 (30.6) | 575 (32.1) | 494 (28.3) | |
| Race/ethnicity | |||||
| American Indian | 4 (0.3) | 4 (0.2) | 7 (0.4) | 7 (0.4) | |
| Asian | 36 (2.8) | 41 (2.5) | 22 (1.2) | 13 (0.7) | <.001 |
| African American | 213 (16.3) | 123 (7.6) | 68 (3.8) | 55 (3.2) | |
| Hispanic | 55 (4.2) | 43 (2.7) | 31 (1.7) | 26 (1.5) | |
| Non-Hispanic white | 979 (74.8) | 1,382 (85.4) | 1,645 (91.7) | 1,620 (92.7) | |
| Other | 21 (1.6) | 25 (1.6) | 21 (1.2) | 26 (1.5) | |
| Currently employed, missing = 22 | |||||
| No | 1,210 (92.9) | 1,530 (95.0) | 1,693 (94.6) | 1,649 (94.7) | .07 |
| Yes | 93 (7.1) | 81 (5.0) | 96 (5.4) | 93 (5.3) | |
| BMI (kg/m2), missing = 37 | |||||
| <25 | 330 (25.4) | 496 (30.8) | 537 (30.0) | 498 (28.8) | |
| 25–29 | 454 (34.9) | 560 (34.8) | 652 (36.4) | 645 (37.2) | .003 |
| 30+ | 516 (39.7) | 553 (34.4) | 600 (33.5) | 589 (34.0) | |
| Smoking, missing = 89 | |||||
| Never | 842 (65.1) | 891 (55.9) | 888 (50.2) | 784 (45.5) | |
| Former | 403 (31.2) | 618 (38.8) | 762 (43.1) | 754 (43.8) | <.001 |
| Current | 48 (3.7) | 86 (5.4) | 117 (6.6) | 185 (10.7) | |
| Alcohol intake (drinks/d), missing = 7 | |||||
| None | 812 (62.1) | 773 (47.8) | 686 (38.3) | 753 (43.2) | <.001 |
| <2/d | 466 (35.7) | 790 (48.8) | 1,012 (56.5) | 908 (52.0) | |
| 2+/d | 28 (2.1) | 55 (3.4) | 93 (5.2) | 84 (4.8) | |
| Sleep quality index | |||||
| ≥ Median | 725 (55.4) | 910 (56.2) | 986 (55.0) | 928 (53.1) | .31 |
| < Median | 583 (44.6) | 708 (43.8) | 808 (45.0) | 819 (46.9) | |
| Depression, Burnam score | |||||
| <0.06 | 1,192 (91.1) | 1,480 (91.5) | 1,680 (93.6) | 1,614 (92.4) | .04 |
| ≥0.06 | 116 (8.9) | 138 (8.5) | 114 (6.4) | 133 (7.6) | |
| Hypertension | |||||
| No | 588 (45.0) | 794 (49.1) | 919 (51.2) | 925 (53.0) | <.001 |
| Yes | 719 (55.0) | 824 (50.9) | 875 (48.8) | 822 (47.0) | |
| Diabetes | |||||
| No | 1,172 (89.6) | 1,489 (92.0) | 1,634 (91.1) | 1,646 (94.2) | <.001 |
| Yes | 136 (10.4) | 129 (8.0) | 160 (8.9) | 101 (5.8) | |
| Prior CVD | |||||
| No | 1,149 (87.8) | 1,417 (87.6) | 1,616 (90.1) | 1,596 (91.4) | <.001 |
| Yes | 159 (12.2) | 201 (12.4) | 178 (9.9) | 151 (8.6) | |
| Drinks of coffee every day, missing = 45 | |||||
| No | 829 (64.1) | 254 (15.8) | 155 (8.7) | 197 (11.3) | <.001 |
| Yes | 464 (35.9) | 1,354 (84.2) | 1,629 (91.3) | 1,540 (88.7) | |
| Baseline 3MS score | |||||
| <90 | 209 (16.0) | 168 (10.4) | 122 (6.8) | 127 (7.3) | |
| 90–94 | 323 (24.7) | 417 (25.8) | 369 (20.6) | 379 (21.7) | <.001 |
| 95–100 | 776 (59.3) | 1,033 (63.8) | 1,303 (72.6) | 1,241 (71.0) | |
Notes: 3MS = Modified Mini Mental State; BMI = body mass index; CVD = cardiovascular disease; HS = high school; WHI = Women’s Health Initiative.
Figure 1.
Histogram of the distribution of self-reported caffeine intake at baseline for the Women’s Health Initiative Memory Study cohort.
Of the women included in this study during their initial 10 years of follow-up, 209 women received a classification of PD and 388 received a classification of any impairment (composite cognitive impairment = MCI + PD) based on a standard four-phase protocol that included central adjudication.
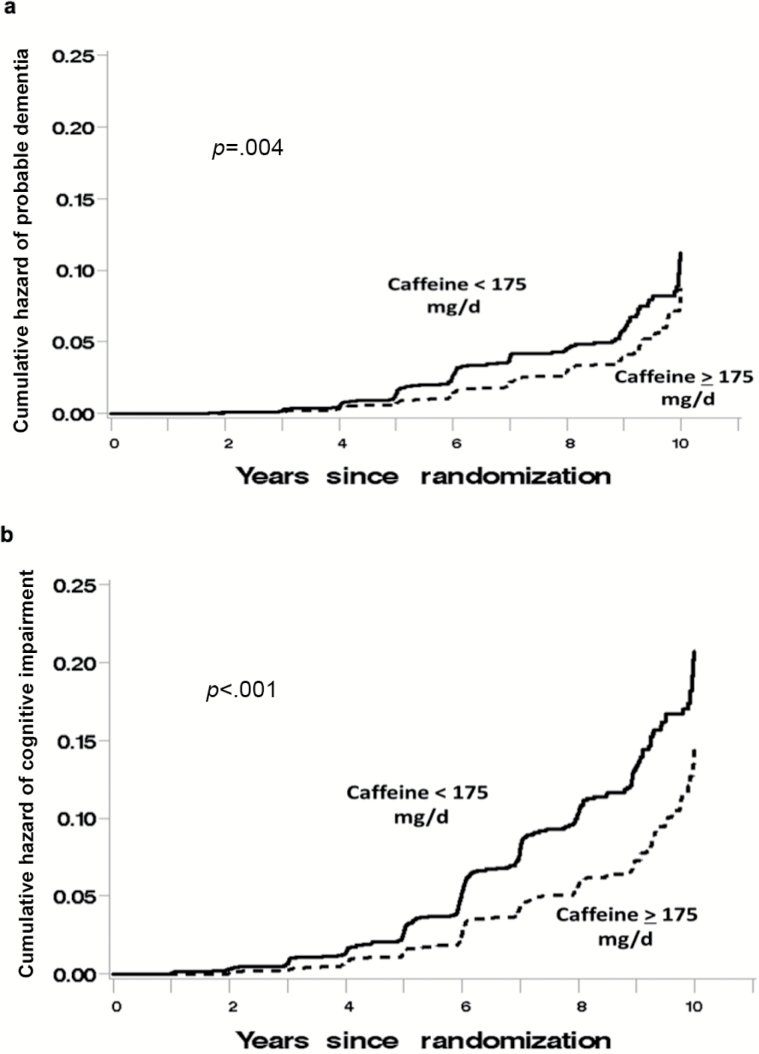
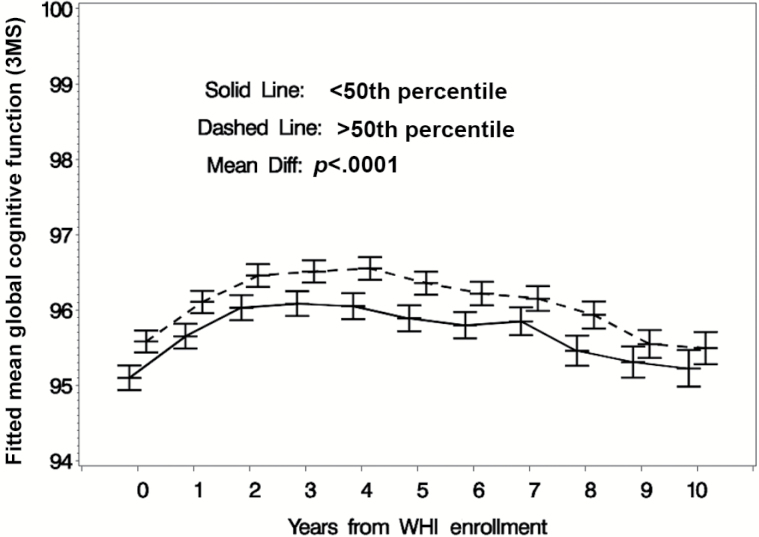
Mean (SD) follow-up times for surveillance for cognitive impairment (ie, until an event or censoring) were slightly longer for women consuming above the median caffeine intake at baseline compared to others: 7.2 (2.3) versus 6.9 (2.5) years. In our sample, greater levels of caffeine intake at baseline were associated with a reduced incidence of both PD (Figure 2a) and composite cognitive impairment (Figure 2b). Those women consuming above median levels of caffeine intake for this group were less likely to develop incident PD (adjusted hazard ratio [HR] = 0.74, 95% confidence interval [CI] [0.56, 0.99], p = .04) or composite cognitive impairment (adjusted HR = 0.74, 95% CI [0.60, 0.91], p = 0.005) compared to those women consuming below median amounts of caffeine (Table 2). Similarly, women consuming more caffeine had higher levels of global cognitive functioning as indexed by higher 3MS scores both with and without full covariate adjustment (p < 0.001; Table 3) compared to women who consumed below median amounts of caffeine for this group (Figure 3), and covariate adjustment for baseline 3MS dampened the association between caffeine intake and incidence of cognitive impairment (adjusted HR [PD alone] = 0.80, 95% CI [0.60, 1.06], p = 0.12; adjusted HR [PD + MCI composite] = 0.81, 95% CI [0.65, 1.00], p = 0.05).
Figure 2.
Kaplan–Meier curve denoting the association that baseline self-reported caffeine intake has with the distribution of times until (a) probable dementia and (b) composite cognitive impairment (mild cognitive impairment + dementia).
Table 2.
Results of Proportional Hazards Regression to Examine Relationships That Baseline Caffeine Intake Has With Probable Dementia and Composite Cognitive Impairment (MCI + Probable Dementia) With Varying Levels of Covariate Adjustment for Other Risk Factors
| Caffeine Intake (mg/d) | Probable Dementia | Composite Cognitive Impairment | ||
|---|---|---|---|---|
| Hazard Ratio (SE) | Hazard Ratio (SE) | |||
| N = 209 Cases | N = 388 Cases | |||
| Limited Covariate Adjustment* | Full Covariate Adjustment† | Limited Covariate Adjustment | Full Covariate Adjustment | |
| <175 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥175 | 0.74 (0.56, 0.98) | 0.74 (0.56, 0.99) | 0.73 (0.60, 0.90) | 0.74 (0.60, 0.91) |
| p = .038 | p = .040 | p = .0032 | p = .0046 | |
Notes: BMI = body mass index; CVD = cardiovascular disease; HT = hormone therapy; MCI = mild cognitive impairment; SE = standard error; WHI = Women’s Health Initiative.
*Age, education, WHI HT assignment, and race/ethnicity.
†All above and BMI, smoking, alcohol intake, depressive symptoms, hypertension, diabetes, and history of CVD.
Table 3.
Mean 3MS Scores for Women Grouped by Baseline Caffeine Intake
| Daily Caffeine Intake at Baseline (mg) | Mean (SE) 3MS Score Over Follow-up | ||
|---|---|---|---|
| Adjustment for Age, Education, Race/Ethnicity, and WHI HT Assignment | Additional Adjustment for BMI, Smoking, Alcohol Intake, Depression Symptoms, Hypertension, Diabetes, and Prior CVD | All Adjustments + Occupation Category | |
| <75 | 95.51 (0.10) | 95.56 (0.10) | 95.68 (0.09) |
| 75–174 | 95.76 (0.09) | 95.78 (0.09) | 95.84 (0.08) |
| 175–189 | 96.20 (0.08) | 96.18 (0.08) | 96.24 (0.07) |
| ≥190 | 96.08 (0.08) | 96.07 (0.08) | 96.15 (0.07) |
| p < .0001 | p < .001 | p < .001 | |
Notes: 3MS = Modified Mini Mental State; BMI = body mass index; CVD = cardiovascular disease; HT = hormone therapy; WHI = Women’s Health Initiative.
Figure 3.
Mean (95% confidence interval) Modified Mini Mental State scores with full covariate adjustment for women grouped by baseline daily caffeine intake: below or above the 50th percentile (175mg).
There was a significant relationship between dietary caffeine and cups of caffeinated coffee consumed per day in this sample (r = .44, p < .0001). Furthermore, the results of proportional hazards regressions with full covariate adjustments that examined the relationships between dietary caffeine and both composite cognitive impairment (HR = 0.68, 95% CI [0.53, 0.88], p = 0.003) or PD (HR = 0.64, 95% CI [0.46, 0.90], p = 0.01) remained significant even after adjusting for cups of caffeinated coffee per day.
Discussion
Above median (for this group) baseline caffeine intake level (mean intake for the upper 50% = 261mg; mean intake for lower 50% = 64mg) is associated with lower incidence of both PD and global cognitive impairment in this sample of generally healthy, community-dwelling, postmenopausal women. Covariate adjustment for baseline 3MS dampened the association between caffeine intake and incident global cognitive impairment, suggesting that the examined associations may be partly mediated by the baseline level of cognitive functioning. This is not entirely surprising for the WHI sample, given the initial reports whereby generally adverse effects of hormone therapy on cognition were exacerbated in women with lower baseline cognitive function (29).
Our findings suggesting lower risk of PD incidence in women with higher caffeine consumption are generally consistent with the literature. Although we cannot generalize to men, our findings are in agreement with Ritchie and colleagues (17), who reported a protective relationship between caffeine and cognition in women, but not men. A study of European men did report, however, that the men who consumed three cups of coffee per day had the lowest cognitive decline over a 10-year period (30). Our study is also in general agreement with the literature review, which reports a modest reduction in rates of cognitive decline across six studies over a follow-up ranging from 1.3 to 10 years (31).
This study comes with some inherent limitations that may limit generalizability. The sample is not population-based, is relatively highly educated and some biases may have arisen due to differential survivorship. All participants are older, postmenopausal, and female. The limitations, however, should not undermine the many unique aspects of the study, including the large number of extensively screened and characterized community-dwelling, older women through detailed prospective follow-up and relatively short intervals between assessments. As is the case with most studies to date reporting lower incidence of cognitive impairment in caffeine consumers, we are also unable to show clear dose response with cups of caffeinated coffee per day. Still, it is likely that caffeine consumption is underestimated as we are not able to discern potential dietary sources of caffeine other than coffee or tea and the FFQ does not have separate questions about caffeinated and decaffeinated beverages; hence, it was assumed that all intake was of caffeinated forms of these beverages. Furthermore, the source of caffeine may be an important consideration for future research. Nonetheless, dietary caffeine was significantly correlated with cups of caffeinated coffee in our sample. Further research is needed in order to assess or confirm the exposure through more objective, biological assays compared to self-reported caffeine intake, and also to isolate potential acute effects that caffeine may have on cognitive performance.
The mounting evidence of caffeine consumption as a potential protective factor against cognitive impairment is exciting given that caffeine is also an easily modifiable dietary factor with very few contraindications. The literature suggests several possible mechanisms that may provide clues to the causal pathways. At normal daily consumption range per person, which is 2–4 cups of coffee (2.4–4.0mg/kg) (32), the primary action of caffeine is that of a nonselective adenosine receptor antagonist (see a review by Rosso and colleagues (33)). The physiologic role for adenosine and its receptors have been widely studied in recent years and implicated in a range of neurological properties, including regulation of sleep, anxiety, memory, and cognitive performance (34). Further research is needed in order to determine whether coffee consumption may have a major effect on the incidence of dementia and age-related cognitive impairment in general, as well as the mechanisms that may lend themselves to new preventative strategies and pharmacologic avenues of research. There is a convergence of molecular studies revealing ARs as molecular targets for integrating neurotransmitter signaling and controlling synaptic plasticity. Studies of cognition under normal and pathological aging will stimulate the necessary translational investigations of adenosine and AR antagonists, such as caffeine, as novel strategies to modulate cognitive impairments.
We report a lower risk of PD or global cognitive impairment incidence in women with higher caffeine consumption, which are generally consistent with the literature. Although more studies are needed to verify the consistency of reports (35), given that caffeine intake is easily modifiable, it is important to quantify its relationship with cognitive health outcomes not only from preventative stand point but also to better understand the underlying mechanisms and their involvement in dementia and cognitive impairment. Given that AD prevalence is expected to quadruple by 2050 (10), even a small reduction in age-related cognitive impairment or dementia burden would thereby have significant public health implications.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Acknowledgments
Program office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and academic centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) J.E.M.; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) S.A.S.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) S.A.S.
Trial registration clinicaltrials.gov Identifier: NCT00685009.
References
- 1. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20(suppl 1):S117–S126. doi:10.3233/JAD-2010-091249 [DOI] [PubMed] [Google Scholar]
- 2. Cao C, Wang L, Lin X, et al. Caffeine synergizes with another coffee component to increase plasma GCSF: linkage to cognitive benefits in Alzheimer’s mice. J Alzheimers Dis. 2011;25:323–335. doi:10.3233/JAD-2011-110110 [DOI] [PubMed] [Google Scholar]
- 3. Chu YF, Chang WH, Black RM, et al. Crude caffeine reduces memory impairment and amyloid β(1-42) levels in an Alzheimer’s mouse model. Food Chem. 2012;135:2095–2102. doi:10.1016/j.foodchem.2012.04.148 [DOI] [PubMed] [Google Scholar]
- 4. Basurto-Islas G, Blanchard J, Tung YC, et al. Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2701–2712. doi:10.1016/j.neurobiolaging.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu GS, Chatterjee D, Darmody PT, et al. Hypoxia/reoxygenation impairs memory formation via adenosine-dependent activation of caspase 1. J Neurosci. 2012;32:13945–13955. doi:10.1523/JNEUROSCI.0704-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thal DR, Braak H. Post-mortem diagnosis of Alzheimer’s disease. Pathologe. 2005;26:201–213. [DOI] [PubMed] [Google Scholar]
- 7. Arendash GW, Schleif W, Rezai-Zadeh K, et al. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. [DOI] [PubMed] [Google Scholar]
- 8. Arendash GW, Mori T, Cao C, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J Alzheimers Dis. 2009;17:661–680. doi:10.3233/JAD-2009-1087 [DOI] [PubMed] [Google Scholar]
- 9. Marques S, Batalha VL, Lopes LV, Outeiro TF. Modulating Alzheimer’s disease through caffeine: a putative link to epigenetics. J Alzheimers Dis. 2011;24(suppl 2):161–171. doi:10.3233/JAD-2011-110032 [DOI] [PubMed] [Google Scholar]
- 10. Chen JF. Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol. 2014;119:257–307. doi:10.1016/B978-0-12-801022-8.00012-X [DOI] [PubMed] [Google Scholar]
- 11. Carman AJ, Dacks PA, Lane RF, Shineman DW, Fillit HM. Current evidence for the use of coffee and caffeine to prevent age-related cognitive decline and Alzheimer’s disease. J Nutr Health Aging. 2014;18:383–392. doi:10.1007/s12603-014-0021-7 [DOI] [PubMed] [Google Scholar]
- 12. Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi:10.3233/JAD-2009-0920 [DOI] [PubMed] [Google Scholar]
- 13. Flaten V, Laurent C, Coelho JE, et al. From epidemiology to pathophysiology: what about caffeine in Alzheimer’s disease? Biochem Soc Trans. 2014;42:587–592. doi:10.1042/BST20130229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis. 2011;23:607–615. doi:10.3233/JAD-2010-101428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritchie K, Carrière I, de Mendonca A, et al. The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology. 2007;69:536–545. doi:10.1212/01.wnl.0000266670.35219.0c [DOI] [PubMed] [Google Scholar]
- 16. Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(suppl 1):S187–S204. doi:10.3233/JAD-2010-091387 [DOI] [PubMed] [Google Scholar]
- 17. Biessels GJ. Caffeine, diabetes, cognition, and dementia. J Alzheimers Dis. 2010;20(suppl 1):S143–S150. doi:10.3233/JAD-2010-091228 [DOI] [PubMed] [Google Scholar]
- 18. Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J Alzheimers Dis. 2010;20(suppl 1):S167–S174. doi:10.3233/JAD-2010-1404 [DOI] [PubMed] [Google Scholar]
- 19. Cao C, Loewenstein DA, Lin X, et al. High blood caffeine levels in MCI linked to lack of progression to dementia. J Alzheimers Dis. 2012;30:559–572. doi:10.3233/JAD-2012-111781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi:10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy SL, Xu JQ, Kochanek KD. Deaths: Final Data for 2010. National Vital Statistics Reports. Vol. 61, No. 4. Hyattsville, MD: National Center for Health Statistics; 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. Accessed December 15, 2014. [PubMed] [Google Scholar]
- 22. Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. [DOI] [PubMed] [Google Scholar]
- 23. The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 24. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25. Rapp SR, Legault C, Espeland MA, et al. ; CAT Study Group Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc. 2012;60:1616–1623. doi:10.1111/j.1532-5415.2012.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatr Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 27. Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the dementia questionnaire. Arch Neurol. 1994;51:901–906. [DOI] [PubMed] [Google Scholar]
- 28. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. [DOI] [PubMed] [Google Scholar]
- 29. Espeland MA, Rapp SR, Shumaker SA, et al. ; Women’s Health Initiative Memory Study Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. [DOI] [PubMed] [Google Scholar]
- 30. van Gelder BM, Buijsse B, Tijhuis M, et al. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr. 2007;61:226–232. [DOI] [PubMed] [Google Scholar]
- 31. Arab L, Khan F, Lam H. Epidemiologic evidence of a relationship between tea, coffee, or caffeine consumption and cognitive decline. Adv Nutr. 2013;4:115–122. doi:10.3945/an.112.002717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 33. Rosso A, Mossey J, Lippa CF. Caffeine: neuroprotective functions in cognition and Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2008;23:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ribeiro JA, Sebastiao AM, de Mendonca A. Participation of adenosine receptors in neuroprotection. Drug News Perspect. 2003;16:80–86. [DOI] [PubMed] [Google Scholar]
- 35. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643 doi:10.1186/1471-2458-14-643 [DOI] [PMC free article] [PubMed] [Google Scholar]