Abstract
Background
The incidence and mortality of kidney cancer have steadily increased by 2%- 3% per decade worldwide, and an increased risk of kidney cancer has been observed in many Asian countries. The information on the incidence and mortality of a disease and its distribution is essential for better planning for prevention and further studies.
Objectives
This study aimed to assess the incidence and mortality of kidney cancer and their correlation with the human development index (HDI) in Asia.
Materials and Methods
This ecological study was based on GLOBOCAN data Asia for assessment the correlation between age-specific incidence rate (ASIR) and age-specific mortality rate (ASMR) with HDI and its details that include life expectancy at birth, mean years of schooling and gross national income (GNI) per capita. We use of correlation bivariate method for assessment the correlation between ASIR and ASMR with HDI and its components.
Results
A total of 121 099 kidney cancer cases were recorded in Asian countries in 2012.Overall, 80 080 cases (66.12%) were males. Sex ratio was 1.95. The three countries with the highest number of new patients were china (66 466 cases), Japan (16 830 cases), India(9658 cases), respectively. Positive correlation were seen between HDI and ASIR of kidney cancer 0.655 (P = 0.001), and HDI and ASMR of kidney cancer 0.285 (P = 0.055).
Conclusions
A positive relationship between ASIR and the HDI was seen. The relationship is due to risk factors in countries with high development such as older age, smoking, hypertension, obesity, and diet. However, ASMR showed no significant relationship with HDI.
Keywords: Kidney cancer, Human development index, Incidence, Mortality, Asia
Implication for health policy/practice/research/medical education:
The result of this article can help policy-makers and health managers to find the cause of the incidence and mortality of kidney cancer in Asia. This study provides information on the incidence and mortality of the cancer and its distribution in terms of geographical areas, so it provides information for better planning for prevention and further studies. Increasing public awareness of the cancer risk factors is a high priority in low development country.
1. Background
It was estimated that 14.9 million incidence cases of cancer and 8.2 million deaths from cancer with 196.3 million disability-adjusted life years (DALYs) in 2013(1). Kidney cancer was the 13th most common cancer worldwide in both sexes (2,3), and the 9th and the 14th type of cancer in men and women, respectively, and the 16th cause of death from the disease in 2012 (4). In the world, age-specific incidence rate (ASIR) of kidney cancer was 4.4, mortality of kidney cancer was 1.8, and its 5-year prevalence was 17.5 in 2013 (1).
A difference is observed in the incidence of kidney cancer by 15 times around the world (5). Therefore, the highest incidence and mortality rates are attributable to Europe, North America, and Australia, while the lowest to Asia and Africa (2,6-8). In 2006, there were approximately 209000 new cases and 102000 deaths from renal cell carcinoma (RCC) worldwide, of which 39000 new cases and 13000 deaths occurred in the United States (9-12). In 2002, the incidence rates in Asian countries such as Japan and Republic Korea were 2509 and 481 cases per 100000, respectively, while 8567 and 901 cases were seen in Canada and United States, respectively (13). In 2012, the incidence rates per 100000 person-years in nine Asian countries were 4.6 in men and 3.1 in women, respectively. Given that the incidence and mortality have steadily increased by 2%-3% per decade worldwide (10,14-24), reduction of mortality has been reported from many developed European countries since the 1990s (19,20).
An increased risk of kidney cancer was observed in many Asian countries such as Korea, China, Hong Kong, Singapore, and Japan (25). A significant increase in the incidence and mortality rates in Asian countries occurred along with remarkable changes in food supply and diet (26). It appears that lifestyle plays an important role in the development of RCC. However, the cause of the differences cannot be understood, it is assumed that different trends are related to early detection, improved access to health care, complex diagnostic imaging, and treatment availability (27,28).
According to studies conducted in Asian countries, a number of common risk factors for RCC include sex, hypertension, diabetes mellitus, high body mass index (BMI), a medical history of kidney disease, smoking, low physical activity, and Western diet (25,29,30), all of which were related to India (30), Japan (29) and Malaysia (31). Other environmental, genetic and hormonal factors were studied, but no definitive conclusions. The cancer primarily affects men and women in the fifth and sixth decades of their life, and one of the specific characteristics of the disease is asymptomatic (19,20,32).
Deciding factors for diagnosis and treatment of patients with locally and advanced RCC are sex, race, the income level and social economic status, which influence the provider and the recipient of health care and decision-making on the disease (33). Studies showed that the incidence of kidney cancer is affected by economic and social disparities (18,19). It reflects the regional disparities in human development. To study the trend and its relationship with risk factors, the human development index (HDI) is useful. The index to classify cancer for globalization is beneficial because it considers education, life expectancy and the national income (34-37). The assumption is that the populations of developing countries have higher mortality rates than the incidence compared with developed countries (38,39). After several decades of increasing trend in the incidence and mortality of kidney cancer, rates are stable, or are started to decrease in many Western countries (40). It seems it is due to reducing the prevalence of tobacco consumption, and improving health professional than decades ago because the prevalence of smoking is higher among people who live below the poverty line (31.5%) than those at the top of this level (19.6%) (7,40).
2. Objectives
The information on the incidence and mortality of a disease and its distribution in terms of geographical areas is essential for better planning for prevention and further studies. There is, probably, a relationship between the development and cancer incidence and mortality. Considering lack of a study to investigate kidney cancer incidence and deaths in Asia, this study aimed to assess the incidence and mortality of kidney cancer and their correlation with the HDI in Asia.
3. Materials and Methods
This study was an ecologic study in Asia for assessment of the correlation between age-specific incidence rate (ASIR) and age-specific mortality rate (ASMR) with HDI and its details that include mean years of schooling, life expectancy at birth and gross national income (GNI) per capita. Data about the ASIR and ASMR for every Asian country for the year 2012 get from global cancer project (http://globocan.iarc.fr/Default.aspx) (41). HDI extracted from Human Development Report 2013 included information about HDI and its details for every country in the word for year 2012 (42).
Method of estimation of the age-specific incidence and mortality rates in the global cancer project by international agency for research on cancer is as follows:
3.1. Age-specific incidence rate
The methods of estimation are country specific and the quality of the estimation depends upon the quality and on the amount of the information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system, which independently describes the availability of incidence and mortality data, has been established at the country level. The combined score is presented together with the estimates for each country with an aim of providing a broad indication of the robustness of the estimation. More details about the GLOBOCAN project were previously published (4,43,44).
3.2. Age-specific mortality rate
Depending on the degree of detail and accuracy of the national mortality data, six methods have been utilized in the following order of priority: 1-Rates projected to 2012 (69 countries), 2) Most recent rates applied to 2012 population (26 countries), 3) Estimated as the weighted average of regional rates (1 country), 4) Estimated from national incidence estimates by modelling, using country-specific survival (2 countries), 5) Estimated from national incidence estimates using modelled survival (83 countries), 6) The rates are those of neighboring countries or registries in the same area (3 countries( (4,44,45).
3.3. Human development index
HDI, a composite measurement of indicators along three dimensions: life expectancy, educational attainment and command over the resources needed for a decent living. All groups and regions have seen notable improvement in all HDI components, with faster progress in low and medium HDI countries. On this basis, the world is becoming less unequal. Nevertheless, national averages hide large variations in human experience. Wide disparities remain within countries of both the North and the South, and income inequality within and between many countries has been rising (42).
3.4. Ethical issues
The research followed the tenets of the Declaration of Helsinki.
3.5. Statistical analysis
In this study, we use correlation of coefficient for assessment of the correlation between ASIR and ASMR with HDI and its details. All reported P values are two-sided, and statistical significance was assumed if P<0.05. Statistical analyses were performed using SPSS (version 15.0, SPSS Inc.).
4. Results
A total of 121099 kidney cancer cases were recorded in Asian countries in 2012. Overall, 80080 cases (66.12%) were males and 41019 cases (33.87%) females. Sex ratio in Asia was 1.95. The five countries with the highest number of new patients were china (66466 cases), Japan (16830 cases), India (9658 cases), Republic Korea (5651 cases), and Turkey (3992 cases), respectively.
Among Asian countries, five countries with the highest standardized incidence rates of the cancer were Republic Korea with 8 per 100000, Turkey with 5.6 per 100000, Japan with 5.3 per 100000, Singapore with 5.2 per 100000, and Korea, Democratic Republic of 4.3 per 100000, respectively. Five countries with the lowest standardized incidence rates of the cancer were Maldives with 0 per 100000, Bhutan with 0.6 per 100000, Yemen with 0.6 per 100000, Bangladesh with 0.8 per 100000, and Sri Lanka with 0.9 per 100000, respectively. The number, crude and standardized incidence rates of the cancer in Asian countries based on sex are presented in Table 1. The countries with the highest and lowest ASIR in both sexes are observable in Table 1, Figure 1 and Figure 2.
Table 1. Proportion, crude, and ASIR of kidney cancer in Asian countries in 2012 .
| Kidney - Estimated incidence, all ages: both sexes | Kidney - Estimated incidence, all ages: male | Kidney - Estimated incidence, all ages: female | |||||||||
| Population | Number | Crude rate | ASR (W) | Population | Number | Crude rate | ASR (W) | Population | Numbers | Crude rate | ASR (W) |
| Korea, Republic of | 5651 | 11.6 | 8.0 | Korea, Republic of | 1763 | 7.2 | 4.7 | Korea, Republic of | 3888 | 16.1 | 11.7 |
| Turkey | 3992 | 5.4 | 5.6 | Turkey | 1656 | 4.4 | 4.4 | Japan | 11141 | 18.1 | 7.8 |
| Japan | 16830 | 13.3 | 5.3 | Mongolia | 37 | 2.6 | 3.3 | Singapore | 272 | 10.3 | 7.4 |
| Singapore | 401 | 7.6 | 5.2 | Singapore | 129 | 4.9 | 3.2 | Turkey | 2336 | 6.3 | 6.8 |
| Korea, Democratic Republic of | 1318 | 5.4 | 4.3 | Korea, Democratic Republic of | 566 | 4.5 | 3.1 | Korea, Democratic Republic of | 752 | 6.2 | 5.9 |
| China | 66466 | 4.9 | 3.8 | Japan | 5689 | 8.8 | 3.0 | China | 44372 | 6.3 | 5.1 |
| Qatar | 33 | 1.7 | 3.5 | Bahrain | 9 | 1.8 | 2.5 | Lebanon | 100 | 4.8 | 4.8 |
| Jordan | 129 | 2.0 | 3.2 | China | 22094 | 3.4 | 2.5 | State of Palestine | 49 | 2.3 | 4.5 |
| Lebanon | 142 | 3.3 | 3.2 | Iraq | 262 | 1.6 | 2.3 | Kazakhstan | 314 | 4.0 | 4.4 |
| Mongolia | 66 | 2.3 | 3.1 | Syrian Arab Republic | 171 | 1.6 | 2.2 | Qatar | 27 | 1.8 | 4.4 |
| Syrian Arab Republic | 467 | 2.2 | 3.1 | Iran | 660 | 1.8 | 2.1 | Georgia | 123 | 6.1 | 4.4 |
| State of Palestine | 71 | 1.7 | 3.1 | Oman | 15 | 1.3 | 2.1 | Jordan | 87 | 2.6 | 4.3 |
| Kazakhstan | 491 | 3.0 | 2.9 | Jordan | 42 | 1.3 | 2.0 | Syrian Arab Republic | 296 | 2.8 | 4.0 |
| Iraq | 581 | 1.7 | 2.9 | Saudi Arabia | 166 | 1.3 | 1.8 | Iraq | 319 | 1.9 | 3.7 |
| Georgia | 167 | 3.9 | 2.7 | Kazakhstan | 177 | 2.1 | 1.8 | Timor-Leste | 9 | 1.5 | 3.4 |
| Bahrain | 23 | 1.7 | 2.6 | Lebanon | 42 | 1.9 | 1.8 | Armenia | 57 | 3.9 | 3.4 |
| Iran | 1641 | 2.2 | 2.6 | United Arab Emirates | 22 | 0.9 | 1.8 | Malaysia | 415 | 2.8 | 3.3 |
| Malaysia | 611 | 2.1 | 2.4 | State of Palestine | 22 | 1.0 | 1.6 | Kyrgyzstan | 57 | 2.1 | 3.2 |
| Saudi Arabia | 454 | 1.6 | 2.3 | Qatar | 6 | 1.3 | 1.5 | Iran | 981 | 2.6 | 3.0 |
| United Arab Emirates | 64 | 0.8 | 2.3 | Malaysia | 196 | 1.4 | 1.5 | Mongolia | 29 | 2.1 | 3.0 |
| Kyrgyzstan | 91 | 1.7 | 2.2 | Kuwait | 9 | 0.8 | 1.5 | Saudi Arabia | 288 | 1.8 | 2.8 |
| Kuwait | 34 | 1.2 | 2.2 | Brunei | 3 | 1.5 | 1.4 | Bahrain | 14 | 1.6 | 2.7 |
| Timor-Leste | 14 | 1.2 | 2.1 | Kyrgyzstan | 34 | 1.2 | 1.4 | United Arab Emirates | 42 | 0.7 | 2.6 |
| Oman | 36 | 1.2 | 2.1 | Georgia | 44 | 1.9 | 1.3 | Kuwait | 25 | 1.5 | 2.6 |
| Armenia | 78 | 2.5 | 1.9 | Turkmenistan | 26 | 1.0 | 1.2 | Turkmenistan | 49 | 1.9 | 2.5 |
| Turkmenistan | 75 | 1.5 | 1.8 | Indonesia | 1132 | 0.9 | 1.0 | Oman | 21 | 1.2 | 2.1 |
| Brunei | 6 | 1.5 | 1.8 | Philippines | 367 | 0.8 | 1.0 | Indonesia | 2093 | 1.7 | 2.0 |
| Indonesia | 3225 | 1.3 | 1.5 | Timor-Leste | 5 | 0.9 | 1.0 | Azerbaijan | 96 | 2.1 | 2.0 |
| Philippines | 1008 | 1.0 | 1.4 | Lao PDR | 24 | 0.8 | 0.9 | Philippines | 641 | 1.3 | 2.0 |
| Tajikistan | 63 | 0.9 | 1.4 | Uzbekistan | 107 | 0.8 | 0.9 | Tajikistan | 42 | 1.2 | 2.0 |
| Afghanistan | 237 | 0.7 | 1.3 | Pakistan | 575 | 0.6 | 0.9 | Brunei | 3 | 1.4 | 1.9 |
| Azerbaijan | 135 | 1.4 | 1.3 | Afghanistan | 77 | 0.5 | 0.9 | Afghanistan | 160 | 0.9 | 1.8 |
| Pakistan | 1646 | 0.9 | 1.3 | Tajikistan | 21 | 0.6 | 0.9 | Pakistan | 1071 | 1.2 | 1.7 |
| Uzbekistan | 283 | 1.0 | 1.2 | Myanmar | 194 | 0.8 | 0.8 | Uzbekistan | 176 | 1.3 | 1.6 |
| Thailand | 1017 | 1.5 | 1.2 | Thailand | 373 | 1.0 | 0.8 | Thailand | 644 | 1.9 | 1.6 |
| Myanmar | 476 | 1.0 | 1.1 | Bhutan | 2 | 0.6 | 0.8 | Nepal | 155 | 1.0 | 1.6 |
| Lao PDR | 52 | 0.8 | 1.1 | Armenia | 21 | 1.3 | 0.8 | Myanmar | 282 | 1.2 | 1.4 |
| Nepal | 218 | 0.7 | 1.0 | Azerbaijan | 39 | 0.8 | 0.8 | Cambodia | 65 | 0.9 | 1.4 |
| India | 9658 | 0.8 | 0.9 | Viet Nam | 352 | 0.8 | 0.7 | Sri Lanka | 160 | 1.5 | 1.3 |
| Cambodia | 101 | 0.7 | 0.9 | Cambodia | 36 | 0.5 | 0.6 | India | 6620 | 1.0 | 1.3 |
| Viet Nam | 810 | 0.9 | 0.9 | India | 3038 | 0.5 | 0.6 | Lao PDR | 28 | 0.9 | 1.3 |
| Sri Lanka | 221 | 1.0 | 0.9 | Nepal | 63 | 0.4 | 0.5 | Bangladesh | 620 | 0.8 | 1.1 |
| Bangladesh | 900 | 0.6 | 0.8 | Sri Lanka | 61 | 0.6 | 0.5 | Viet Nam | 458 | 1.0 | 1.1 |
| Yemen | 112 | 0.4 | 0.6 | Yemen | 46 | 0.4 | 0.4 | Yemen | 66 | 0.5 | 0.8 |
| Bhutan | 3 | 0.4 | 0.6 | Bangladesh | 280 | 0.4 | 0.4 | Bhutan | 1 | 0.3 | 0.4 |
| Maldives | 0 | 0.0 | 0.0 | Maldives | 0 | 0.0 | 0.0 | Maldives | 0 | 0.0 | 0.0 |
Figure 1.

ASIR and ASMR from kidney cancer in Asia in 2012.
Figure 2.
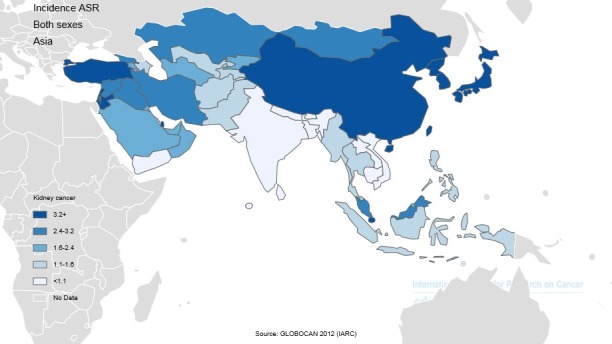
Distribution of the ASIR of kidney cancer in Asia in 2012
However, in 2012, in Asia, the number of deaths due to kidney cancer was 26102 cases, 36224 cases in men and 19878 cases in women. The sex ratio (male to female) of mortality was equal to 1.82. The five countries with the highest number of deaths were china (25583 cases), Japan (8124 cases), India (5973 cases), Turkey (2656 cases), and Indonesia (2459 cases), respectively. The countries included a total of 44795 cases (89.78%) of the total mortality in Asia.
In Asian countries, 5 countries with the highest standardized mortality rates from the cancer were Turkey with 3.8 per 100000, Republic Korea 2.5 per 100000, Palestine with 2.4 per 100000, Syria with 2.3 per 100000, and Iraq with 2.3 per 100000, respectively. Five countries with the lowest standardized mortality rates from the cancer were Brunei with 0 per 100000, Maldives with 0 per 100000, Yemen with 0.5 per 100000, India with 0.6 per 100000, and Sri Lanka with 0.6 per 100000, respectively. The number, crude, and standardized incidence rates of the cancer in Asian countries based on sex are presented in Table 2. The countries with the highest and lowest ASIR are observable in both sexes in Table 2, Figure 1 and Figure 4.
Table 2. Proportion, crude, and ASMR of kidney cancer in Asian countries in 2012 .
| Kidney - Estimated mortality, all ages: both sexes | Kidney- Estimated mortality, all ages: female | Kidney- Estimated mortality, all ages: male | |||||||||
| Population | Numbers | CrudeRate | ASR(W) | Population | Number | Crude rate | ASR(W) | Population | Number | Crude rate | ASR(W) |
| Turkey | 2656 | 3.6 | 3.8 | Turkey | 1094 | 2.9 | 2.9 | Turkey | 1562 | 4.2 | 4.7 |
| Korea, Democratic Republic of | 794 | 3.2 | 2.5 | Mongolia | 21 | 1.5 | 2.1 | State of Palestine | 35 | 1.6 | 3.4 |
| State of Palestine | 52 | 1.2 | 2.4 | Korea, Democratic Republic of | 381 | 3.0 | 1.9 | Korea, Democratic Republic of | 413 | 3.4 | 3.4 |
| Syrian Arab Republic | 345 | 1.6 | 2.3 | Iraq | 206 | 1.2 | 1.8 | Singapore | 125 | 4.7 | 3.3 |
| Iraq | 462 | 1.4 | 2.3 | Syrian Arab Republic | 126 | 1.2 | 1.6 | Syrian Arab Republic | 219 | 2.1 | 3.1 |
| Singapore | 175 | 3.3 | 2.2 | Oman | 9 | 0.8 | 1.5 | Iraq | 256 | 1.5 | 3.1 |
| Jordan | 86 | 1.3 | 2.2 | Iran, Islamic Republic of | 432 | 1.2 | 1.4 | Timor-Leste | 8 | 1.3 | 3.1 |
| Qatar | 15 | 0.8 | 2.2 | Jordan | 28 | 0.9 | 1.4 | Lebanon | 62 | 3.0 | 3.0 |
| Mongolia | 39 | 1.4 | 2.0 | State of Palestine | 17 | 0.8 | 1.3 | Japan | 5177 | 8.4 | 2.9 |
| Lebanon | 88 | 2.1 | 2.0 | Singapore | 50 | 1.9 | 1.1 | Jordan | 58 | 1.7 | 2.9 |
| Japan | 8124 | 6.4 | 1.9 | Kyrgyzstan | 25 | 0.9 | 1.1 | Kazakhstan | 189 | 2.4 | 2.9 |
| Timor-Leste | 12 | 1.0 | 1.8 | Lebanon | 26 | 1.2 | 1.1 | Qatar | 12 | 0.8 | 2.8 |
| Kyrgyzstan | 69 | 1.3 | 1.8 | Japan | 2947 | 4.5 | 1.1 | Kyrgyzstan | 44 | 1.6 | 2.7 |
| Kazakhstan | 296 | 1.8 | 1.8 | Saudi Arabia | 94 | 0.7 | 1.1 | Georgia | 77 | 3.8 | 2.6 |
| Iran, Islamic Republic of | 1071 | 1.4 | 1.7 | Kazakhstan | 107 | 1.3 | 1.1 | Korea, Republic of | 850 | 3.5 | 2.4 |
| Georgia | 104 | 2.4 | 1.6 | China | 8871 | 1.4 | 0.9 | Armenia | 38 | 2.6 | 2.1 |
| Korea, Republic of | 1264 | 2.6 | 1.6 | Qatar | 3 | 0.6 | 0.8 | Iran, Islamic Republic of | 639 | 1.7 | 2.0 |
| Oman | 21 | 0.7 | 1.4 | Korea, Republic of | 414 | 1.7 | 0.8 | Mongolia | 18 | 1.3 | 1.9 |
| Saudi Arabia | 257 | 0.9 | 1.4 | Turkmenistan | 17 | 0.6 | 0.8 | China | 16712 | 2.4 | 1.9 |
| China | 25583 | 1.9 | 1.4 | Bhutan | 2 | 0.6 | 0.8 | Turkmenistan | 33 | 1.3 | 1.9 |
| Turkmenistan | 50 | 1.0 | 1.3 | Afghanistan | 68 | 0.4 | 0.8 | Afghanistan | 140 | 0.8 | 1.8 |
| United Arab Emirates | 25 | 0.3 | 1.3 | Indonesia | 866 | 0.7 | 0.8 | Saudi Arabia | 163 | 1.0 | 1.8 |
| Afghanistan | 208 | 0.6 | 1.3 | Georgia | 27 | 1.2 | 0.8 | Tajikistan | 32 | 0.9 | 1.7 |
| Armenia | 53 | 1.7 | 1.2 | Lao PDR | 19 | 0.6 | 0.8 | United Arab Emirates | 18 | 0.3 | 1.6 |
| Tajikistan | 49 | 0.7 | 1.2 | Pakistan | 483 | 0.5 | 0.8 | Indonesia | 1593 | 1.3 | 1.6 |
| Indonesia | 2459 | 1.0 | 1.2 | Myanmar | 167 | 0.7 | 0.8 | Malaysia | 184 | 1.2 | 1.6 |
| Pakistan | 1374 | 0.8 | 1.1 | Tajikistan | 17 | 0.5 | 0.7 | Oman | 12 | 0.7 | 1.5 |
| Malaysia | 255 | 0.9 | 1.0 | United Arab Emirates | 7 | 0.3 | 0.7 | Bahrain | 5 | 0.6 | 1.5 |
| Myanmar | 413 | 0.8 | 1.0 | Timor-Leste | 4 | 0.7 | 0.7 | Azerbaijan | 66 | 1.4 | 1.5 |
| Kuwait | 14 | 0.5 | 1.0 | Uzbekistan | 75 | 0.5 | 0.7 | Pakistan | 891 | 1.0 | 1.4 |
| Uzbekistan | 205 | 0.7 | 1.0 | Philippines | 221 | 0.5 | 0.6 | Nepal | 133 | 0.9 | 1.4 |
| Azerbaijan | 92 | 1.0 | 1.0 | Viet Nam | 274 | 0.6 | 0.6 | Uzbekistan | 130 | 0.9 | 1.3 |
| Bahrain | 7 | 0.5 | 1.0 | Kuwait | 3 | 0.3 | 0.6 | Myanmar | 246 | 1.0 | 1.3 |
| Philippines | 600 | 0.6 | 0.9 | Malaysia | 71 | 0.5 | 0.6 | Philippines | 379 | 0.8 | 1.3 |
| Nepal | 187 | 0.6 | 0.9 | Azerbaijan | 26 | 0.5 | 0.5 | Kuwait | 11 | 0.6 | 1.2 |
| Lao PDR | 40 | 0.6 | 0.9 | Armenia | 15 | 0.9 | 0.5 | Cambodia | 52 | 0.7 | 1.2 |
| Cambodia | 80 | 0.6 | 0.7 | Bahrain | 2 | 0.4 | 0.5 | Lao PDR | 21 | 0.7 | 1.0 |
| Viet Nam | 630 | 0.7 | 0.7 | Thailand | 233 | 0.7 | 0.5 | Thailand | 399 | 1.2 | 1.0 |
| Thailand | 632 | 0.9 | 0.7 | Cambodia | 28 | 0.4 | 0.5 | Bangladesh | 513 | 0.7 | 0.9 |
| Bangladesh | 776 | 0.5 | 0.7 | Nepal | 54 | 0.3 | 0.4 | Viet Nam | 356 | 0.8 | 0.9 |
| Bhutan | 3 | 0.4 | 0.6 | Bangladesh | 263 | 0.3 | 0.4 | Sri Lanka | 108 | 1.0 | 0.9 |
| Sri Lanka | 150 | 0.7 | 0.6 | Yemen | 40 | 0.3 | 0.4 | India | 4054 | 0.6 | 0.8 |
| India | 5973 | 0.5 | 0.6 | India | 1919 | 0.3 | 0.3 | Yemen | 57 | 0.4 | 0.7 |
| Yemen | 97 | 0.4 | 0.5 | Sri Lanka | 42 | 0.4 | 0.3 | Bhutan | 1 | 0.3 | 0.4 |
| Maldives | 0 | 0.0 | 0.0 | Brunei | 0 | 0.0 | 0.0 | Brunei | 0 | 0.0 | 0.0 |
| Brunei | 0 | 0.0 | 0.0 | Maldives | 0 | 0.0 | 0.0 | Maldives | 0 | 0.0 | 0.0 |
Figure 4.
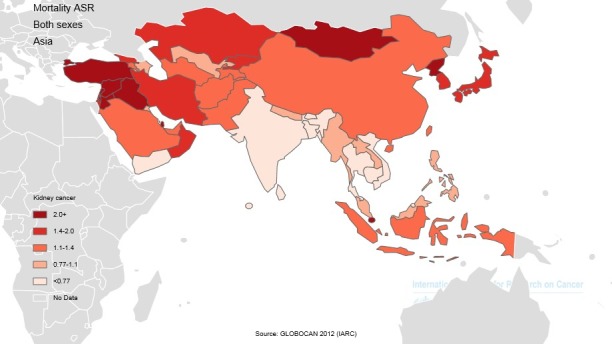
Distribution of ASMR of kidney cancer in Asia in 2012.
In Table 3, amounts related to HDI and its components for each of the Asian countries (sorted based on HDI) is shown. Accordingly, Asian countries are classified according to HDI as follows: three countries in the very high category, four countries in high, 35 countries in the middle category, three countries in low, and one in the unknown category.
Table 3. HDI in Asian countries in 2012 .
| HDI status | Population | HDI | Life expectancy at birth | Mean Year of schooling | GNI per capita |
| Very high | Japan | 0.912 | 83.6 | 11.6 | 32545 |
| Korea, Republic of | 0.909 | 80.7 | 11.6 | 28231 | |
| High | Singapore | 0.895 | 81.2 | 10.1 | 52613 |
| Brunei | 0.855 | 78.1 | 8.6 | 45690 | |
| Qatar | 0.834 | 78.5 | 7.3 | 87478 | |
| United Arab Emirates | 0.818 | 76.7 | 8.9 | 42716 | |
| Average | Bahrain | 0.796 | 75.2 | 9.4 | 19154 |
| Kuwait | 0.79 | 74.7 | 6.1 | 52793 | |
| Saudi Arabia | 0.782 | 74.1 | 7.8 | 22616 | |
| Malaysia | 0.769 | 74.5 | 9.5 | 13676 | |
| Kazakhstan | 0.754 | 67.4 | 10.4 | 10451 | |
| Georgia | 0.745 | 73.9 | 12.1 | 5005 | |
| Lebanon | 0.745 | 72.8 | 7.9 | 12364 | |
| Iran | 0.742 | 73.2 | 7.8 | 10695 | |
| Azerbaijan | 0.734 | 70.9 | 11.2 | 8153 | |
| Oman | 0.731 | 73.2 | 5.5 | 24092 | |
| Armenia | 0.729 | 74.4 | 10.8 | 5540 | |
| Turkey | 0.722 | 74.2 | 6.5 | 13710 | |
| Sri Lanka | 0.715 | 75.1 | 9.3 | 5170 | |
| Jordan | 0.7 | 73.5 | 8.6 | 5272 | |
| China | 0.699 | 73.7 | 7.5 | 7945 | |
| Turkmenistan | 0.698 | 65.2 | 9.9 | 7782 | |
| Thailand | 0.69 | 74.3 | 6.6 | 7722 | |
| Maldives | 0.688 | 77.1 | 5.8 | 7478 | |
| Mongolia | 0.675 | 68.8 | 8.3 | 4245 | |
| State of Palestine | 0.67 | 73 | 8 | 3359 | |
| Philippines | 0.654 | 69 | 8.9 | 3752 | |
| Uzbekistan | 0.654 | 68.6 | 10 | 3201 | |
| Syrian Arab Republic | 0.648 | 76 | 5.7 | 4674 | |
| Indonesia | 0.629 | 69.8 | 5.8 | 4154 | |
| Kyrgyzstan | 0.622 | 68 | 9.3 | 2009 | |
| Tajikistan | 0.622 | 67.8 | 9.8 | 2119 | |
| Viet Nam | 0.617 | 75.4 | 5.5 | 2970 | |
| Iraq | 0.59 | 69.6 | 5.6 | 3557 | |
| Timor-Leste | 0.576 | 62.9 | 4.4 | 5446 | |
| India | 0.554 | 65.8 | 4.4 | 3285 | |
| Cambodia | 0.543 | 63.6 | 5.8 | 2095 | |
| Lao PDR | 0.543 | 67.8 | 4.6 | 2435 | |
| Bhutan | 0.538 | 67.6 | 2.3 | 5246 | |
| Bangladesh | 0.515 | 69.2 | 4.8 | 1785 | |
| Pakistan | 0.515 | 65.7 | 4.9 | 2566 | |
| Low | Myanmar | 0.498 | 65.7 | 3.9 | 1 817 |
| Nepal | 0.463 | 69.1 | 3.2 | 1137 | |
| Yemen | 0.458 | 65.9 | 5.3 | 928 | |
| Afghanistan | 0.374 | 49.1 | 3.1 | 1000 | |
| Unknown | Korea, Democratic Republic of | - | - | - | - |
Abbreviations: GNI, gross national income (GNI); HDI, human development index.
4.1. ASIR and HDI
A positive correlation was seen between the ASIR of kidney cancer and HDI about 0.655. This association was statistically significant (P=0.001). There was a positive correlation between the ASIR and life expectancy at birth about 0.558 (P=0.001), positive correlation between the ASIR and mean years of schooling about 0.523 (P=0.001), and positive correlation between the level of income per each person of the population and the ASIR equal to 0.409 (P=0.005) (Figure 3).
Figure 3.
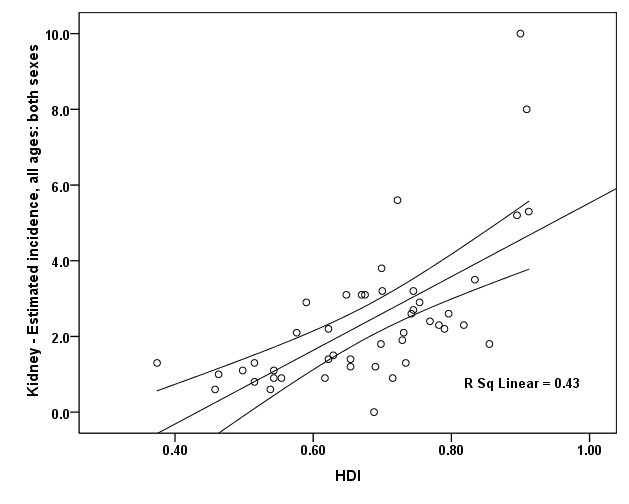
Correlation between HDI and ASIR of kidney cancer in Asia in 2012
In men, a positive correlation of 0.637 was observed between the ASIR of kidney cancer and HDI. It was statistically significant (P=0.001). There was a positive correlation between the ASIR and life expectancy at birth about 0.539 (P=0.001), positive correlation between mean years of schooling and the ASIR about 0.557 (P=0.001), and positive correlation between the level of income per each person of the population and the ASIR equal to 0.366 (P=0.012).
In women, a positive correlation of 0.612 was observed between the ASIR of kidney cancer and HDI. It was statistically significant (P=0.001). There was a positive correlation between the ASIR and life expectancy at birth about 0.509 (P=0.001), positive correlation between mean years of schooling and the ASIR about 0.448 (P=0.002), and positive correlation between the level of income per each person of the population and the ASIR equal to 0.347 (P=0.018).
4.2. ASMR and HDI
There was between the ASMR for kidney cancer and HDI a positive correlation of 0.285 (P=0.055), expectancy at birth a positive correlation of 0.183 (P=0.222), mean years of schooling a positive correlation equal to 0.226 (P=0.132), and the level of income per each person of population a positive correlation of 0.174 (P=0.248; Figure 3).
In men, there was between the ASMR for kidney cancer and HDI a positive correlation of 0.314 (P=0.033), expectancy at birth a positive correlation of 0.187 (P=0.212), mean years of schooling a positive correlation equal to 0.23 (P=0.029), and the level of income per each person of population a positive correlation of 0.152 (P=0.314).
In women, there was between the ASMR for kidney cancer and HDI a positive correlation of 0.131 (P=0.386), expectancy at birth a positive correlation of 0.076 (P=0.616), mean years of schooling a positive correlation equal to 0.045 (P=0.768), and the level of income per each person of population a negative correlation of 0.017 (P=0.913).
5. Discussion
Considering that close to 60% of the world’s population live in Asia, paying attention to causes of incidence and mortality from the cancer is significant in the continent (46). Lifestyle changes in Asian countries could be predisposing factor for the cancer (47,48). In Asia, 56% of incidence cases, 62% of deaths, 70% of DALYs occurred in 2013 worldwide. In Asian countries, ASIRs of incidence, mortality, and 5-year prevalence of kidney cancer in this year were 2.8, 1.3, and 9.4, respectively (1).
Kidney cancer incidence and mortality is different in various countries. This difference in incidence between countries is because of the accumulation of risk factors (49), including smoking, obesity, hypertension, age, and diet in countries with high incidence. According to studies conducted in Asian countries, age-standardized incidence rates per 100000 and the proportion of deaths to incidence per 100000 were in Central and South Asia 1 and 0.7, in Southeast Asia, 1.9 and 0.68, in West Asia 2.3 and 0.62, and in East Asia 2.4 and 0.36, while in the United States 11.8 and 2.2. Given the under-reporting in developing countries (50), the United States with a HDI has mortality rates a declining trend, but in Asian countries the rates are stable or increasing. Based the results of the this study and other studies, incidence and mortality rates of all cancers are different in the world and in various socioeconomic levels due to early detection, improved access to health care, complex diagnostic imaging, and treatment availability.
The incidence of kidney cancer has increased in many Asian countries, so that men, specifically in China, and Asian women in India have a significant increase. In a country like Singapore, trends in mortality have remained constant, but Japan has been a clear decline (46). In this study, the sex ratio (male to female) was 1.95, which is in line with other studies. This may be due to higher exposure to risk factors such as smoking and obesity (2,19,20,25,27,28,32).
According to the findings of this study, the incidence of kidney cancer in Asia is related to the HDI. It seems that among Asian countries, the high incidence of kidney cancer has been associated with increasing HDI. It can be attributed to a decrease in other diseases and control of infectious diseases as well as aging in the countries. Aging is one of the most important risk factors for this cancer. According to statistics published by GLOBOCAN 2015, the cancer generally has increased between 1990 and 2013. In other words, the incidence rate for both sexes in terms of ASIR increased 23% (from 3.82 to 4.7), 34% in developing countries (from 1.96 to 2.27) and 36 percent in developed countries (from 7.15 to 9.71) (1).
Our results showed that there was no relationship between the HDI and kidney cancer mortality rate in Asia. Of the characteristics of kidney cancer is no clinical signs warning, and RCC has been difficult malignancy in diagnose and treat. As a result, early diagnosis of the cancer is not different by the development in Asian countries.
Life expectancy at birth is one of components of human development. Our findings showed that there was a relationship between the ASIR and life expectancy at birth. Kidney cancer is a disease that is typically detected between the fifth and seventh life. In another study, incidence in Europe and the United States is constantly increasing, with a smooth trend in 70 to 75 years (32,51-53). Today, the average life expectancy for a child born in the United States is about 78 years, while a child in a country in Sub-Saharan Africa with an average life expectancy of between 39.6 to 65.9 years (33). Standard age distribution is various in different geographical areas. ASMR is similar to the ASIR, so that the highest in Europe and North America (3.1 and 2.6 per 100000) and the lowest in Asia and Africa (0.6 and 1.5 per 100000). With the increasing development of countries, aging of populations and reducing non-communicable diseases, rates of chronic disease such as cancer increasing. (54).
Access to knowledge is another component. Our study found a positive correlation between the standardized incidence and the level of education. Also, in United States, in the lowest level of education in the population, kidney cancer mortality rate is 2.6 times higher than the highest level of education (55). It was shown that risk of kidney cancer in men is inversely related to higher education levels (56).
In this study, there was a positive correlation between standardized incidence of the cancer and income levels per one in community. An ecological study has also reported that per capita daily intake of fat and protein is positively correlated with the incidence of kidney cancer in women and men (26). These findings are justified with aging (52) and an increase in cumulative effects of risk factors. The annual economic burden of kidney cancer in the United States in 2009 is estimated about $5.2 billion (43). About 85% of health care dollars is spent caring for kidney cancer inpatients (23). It can be concluded that the incidence of kidney cancer can also affect the HDI because it imposes economic costs on health systems and poverty in people (13).
Conclusions
The ASIR and ASMR of kidney cancer in countries with higher development is more. There was a positive and significant relationship between the ASIR of kidney cancer and HDI and HDI components (life expectancy at birth, the average years of schooling, and the level of income for each one of the country’s population). The relationship is due to risk factors in countries with high development such as older age, smoking, hypertension, obesity, and diet. However there was a positive, but no significant relationship between the ASMR of kidney cancer and HDI and HDI components.
Limitations of the study
Our study was an ecological study and special limitations of this study include ecological misleading and lack of relation of group results with individuals.
Authors’ contribution
All authors contributed to the design of the research. AMH, MG, FD and FT collected the data. MG, MA and HS conducted analysis and interpretation of data. All authors drafted the first version. HS, KV and AMH edited the first draft. All authors reviewed and commented on final draft.
Acknowledgements
Hereby we appreciate of the cooperation of all employees involved in data collection in the GLOBOCAN project and World Bank.
Conflicts of interest
The authors declare no conflict of interests.
Funding/Support
None.
Please cite this paper as: Arabsalmani M, Mohammadian-Hafshejani A, Ghoncheh M, Hadadian F, Towhidi F, Vafaee K, et al. Incidence and mortality of kidney cancers, and human development index in Asia; a matter of concern. J Nephropathol. 2017;6(1):30-42. DOI: 10.15171/jnp.2017.06.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M. et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(2):1893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Mirzaei M, Pournamdar Z, Salehiniya H. Epidemiology and trends in incidence of kidney cancer in Iran. Asian Pac J Cancer Prev. 2015;16(14):5859–61. doi: 10.7314/APJCP.2015.16.14.5859. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Bosetti C, Bertuccio P, Chatenoud L, Negri E, La Vecchia C, Levi F. Trends in mortality from urologic cancers in Europe, 1970–2008. Eur Urol. 2011;60(1):1–15. doi: 10.1016/j.eururo.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Parkin D, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J cancer. 2010;46(4):765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults--United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(45):1221–6. [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C. et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 10.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 11. American Cancer Society. Cancer facts & figures. American Cancer Society; 2008.
- 12.Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998;352(9141):1691–6. doi: 10.1016/S0140-6736(98)01041-1. [DOI] [PubMed] [Google Scholar]
- 13.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 15.Hock LM, Lynch J, Balaji K. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167(1):57–60. doi: 10.1016/S0022-5347(05)65382-7. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 17.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–5. doi: 10.1016/S0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 18.Lindblad P. Epidemiology of renal cell carcinoma. Scand J Surg. 2003;93(2):88–96. doi: 10.1177/145749690409300202. [DOI] [PubMed] [Google Scholar]
- 19.Murai M, Oya M. Renal cell carcinoma: etiology, incidence and epidemiology. Curr Opin Urol. 2004;14(4):229–33. doi: 10.1097/01.mou.0000135078.04721.f5. [DOI] [PubMed] [Google Scholar]
- 20.Rathmell WK, Godley PA. Renal cell carcinoma. Curr Opin Urol. 2004;16(3):247–52. doi: 10.1097/00001622-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Rathmell WK, Godley PA, Rini BI. Renal cell carcinoma. Curr Opin Urol. 2005;17(3):261–7. doi: 10.1097/01.cco.0000155007.51495.d6. [DOI] [PubMed] [Google Scholar]
- 22.Rini BI, Campbell SC, Rathmell WK. Renal cell carcinoma. Curr Opin Urol. 2006;18(3):289–96. doi: 10.1097/01.cco.0000219260.60714.c4. [DOI] [PubMed] [Google Scholar]
- 23. Wallen E, Pruthi R, editors. The burden of kidney cancer in America: the urologic diseases in America Project. JOURNAL OF UROLOGY; 2006: LIPPINCOTT WILLIAMS & WILKINS 530 WALNUT ST, PHILADELPHIA, PA 19106-3621 USA.
- 24.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 25.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15(4):617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 27.Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol. 2009;1:33. doi: 10.2147/clep.s4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hébert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA. et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115(11):2539–52. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washio M, Mori M, Mikami K, Miki T, Watanabe Y, Nakao M. et al. Risk factors for renal cell carcinoma in a Japanes e population. Asian Pac J Cancer Prev. 2014;15:9065–70. doi: 10.7314/apjcp.2014.15.21.9065. [DOI] [PubMed] [Google Scholar]
- 30.Sivaramakrishna B, Gupta NP, Wadhwa P, Hemal AK, Dogra PN, Seth A. et al. Pattern of metastases in renal cell carcinoma: a single institution study. Indian J Cancer. 2005;42(4):173. [PubMed] [Google Scholar]
- 31.Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR. et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60(1):39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23(3):202–12. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 33.Hellenthal NJ, Bermejo CE, editors editors. The role of socioeconomic status in renal cell carcinoma. Urol Oncol. 2012;30(1):89–94. doi: 10.1016/j.urolonc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac J Cancer Prev. 2016;17(1):381–6. doi: 10.7314/APJCP.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 35.Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of lung cancer and their relationship to development in Asia. Transl Lung Cancer Res. 2015;4(6):763–74. doi: 10.3978/j.issn.2218-6751.2015.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoncheh M, Mohammadian-Hafshejani A, Salehiniya H. Incidence and mortality of breast cancer and their relationship to development in Asia. Asian Pac J Cancer Prev. 2015;16(14):6081–7. doi: 10.7314/APJCP.2015.16.14.6081. [DOI] [PubMed] [Google Scholar]
- 37.Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate. Int doi: 10.1016/j.prnil.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghoncheh M, Mirzaei M, Salehiniya H. Incidence and mortality of breast cancer and their relationship with the human development index (HDI) in the world in 2012. Asian Pac J Cancer Prev. 2016;16(18):8439–43. doi: 10.7314/APJCP.2015.16.18.XXXX. [DOI] [PubMed] [Google Scholar]
- 39.Pakzad R, Mohammadian-Hafshejani A, Mohammadian M, Pakzad I, Safiri S, Khazaei S. et al. Incidence and mortality of bladder cancer and their relationship with development in Asia. Asian Pac J Cancer Prev. 2015;16(16):7365–74. doi: 10.7314/APJCP.2015.16.16.7365. [DOI] [PubMed] [Google Scholar]
- 40.Ljungberg B, Campbell SC, Cho HY, Jacqmin D, Lee JE, Weikert S. et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 41. Ferlay J S, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed June 7, 2015.
- 42. Malik K. Human development report 2013. The rise of the South: Human progress in a diverse world. The Rise of the South: Human Progress in a Diverse World (March 15, 2013) UNDP-HDRO Human Development Reports; 2013.
- 43.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 44.Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6(8):603–12. doi: 10.1038/nrc1948. [DOI] [PubMed] [Google Scholar]
- 45.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 46.Kamarudin R, Shah SA, Hidayah N. Lifestyle factors and breast cancer: a case-control study in Kuala Lumpur, Malaysia. Asian Pac J Cancer Prev. 2006;7(1):51. [PubMed] [Google Scholar]
- 47.Almasi Z, Rafiemanesh H, Salehiniya H. Epidemiology characteristics and trends of incidence and morphology of stomach cancer in Iran. Asian Pac J Cancer Prev. 2015;16(7):2757–61. doi: 10.7314/APJCP.2015.16.7.2757. [DOI] [PubMed] [Google Scholar]
- 48.Keyghobadi N, Rafiemanesh H, Mohammadian-Hafshejani A, Enayatrad M, Salehiniya H. Epidemiology and trend of cancers in the province of Kerman: southeast of Iran. Asian Pac J Cancer Prev. 2015;16(4):1409–13. doi: 10.7314/APJCP.2015.16.4.1409. [DOI] [PubMed] [Google Scholar]
- 49.de Andrade LO, Pellegrini Filho A, Solar O, Rígoli F, de Salazar LM, Serrate PC. et al. Social determinants of health, universal health coverage, and sustainable development: case studies from Latin American countries. Lancet. 2015;385(9975):1343–51. doi: 10.1016/S0140-6736(14)61494-X. [DOI] [PubMed] [Google Scholar]
- 50.Corrao MA, Guindon GE, Cokkinides V, Sharma N. Building the evidence base for global tobacco control. Bull World Health Organ. 2000;78(7):884–90. doi: 10.1590/S0042-96862000000700005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsui KH, Shvarts O, Smith RB, Figlin R, De Kernion JB, Belldegrun A. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163(2):426–30. doi: 10.1016/S0022-5347(05)67892-5. [DOI] [PubMed] [Google Scholar]
- 52.Alkhateeb SS, Alkhateeb JM, Alrashidi EA. Increasing trends in kidney cancer over the last 2 decades in Saudi Arabia. Saudi Med J. 2015;36(6):698–703. doi: 10.15537/smj.2015.6.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2007;14(5):288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferlay J, Héry C, Autier P, Sankaranarayanan R. Global burden of breast cancer. In: Li C, ed. Breast Cancer Epidemiology. New York: Springer; 2010. p. 1-19. doi:10.1007/978-1-4419-0685-4_1.
- 55.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 56.Smailyte G, Jasilionis D, Vincerzevskiene I, Krilaviciute A, Ambrozaitiene D, Stankuniene V. et al. Educational differences in incidence of cancer in Lithuania, 2001–2009: evidence from census-linked cancer registry data. Eur J Cancer Prev. 2015;24(3):261–6. doi: 10.1097/CEJ.0000000000000036. [DOI] [PubMed] [Google Scholar]


