Abstract
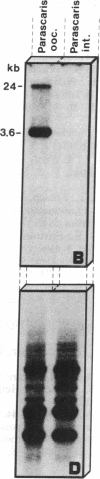
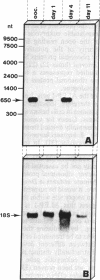
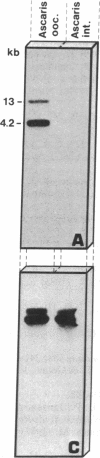
Chromatin diminution in the nematodes Parascaris equorum and Ascaris lumbricoides leads to the formation of somatic cells that contain less DNA than the germ-line cells. We present molecular evidence for the coding potential of germ-line-specific DNA. We report on a cDNA clone that codes for a putative ribosomal protein (ALEP-1, for A. lumbricoides eliminated protein 1). That the corresponding gene is located in the eliminated portion of the genome indicates a difference in germ-line and somatic ribosomes of A. lumbricoides and P. equorum. Elimination of the ALEP-1 gene from all somatic cells in its fully active state may represent an alternative way to gene regulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeby P., Spicher A., de Chastonay Y., Müller F., Tobler H. Structure and genomic organization of proretrovirus-like elements partially eliminated from the somatic genome of Ascaris lumbricoides. EMBO J. 1986 Dec 1;5(12):3353–3360. doi: 10.1002/j.1460-2075.1986.tb04650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back E., Van Meir E., Müller F., Schaller D., Neuhaus H., Aeby P., Tobler H. Intervening sequences in the ribosomal RNA genes of Ascaris lumbricoides: DNA sequences at junctions and genomic organization. EMBO J. 1984 Nov;3(11):2523–2529. doi: 10.1002/j.1460-2075.1984.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M., Bianchi M. E. A gene family for acidic ribosomal proteins in Schizosaccharomyces pombe: two essential and two nonessential genes. Mol Cell Biol. 1990 May;10(5):2341–2348. doi: 10.1128/mcb.10.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. L., Ward S. Neither a germ line-specific nor several somatically expressed genes are lost or rearranged during embryonic chromatin diminution in the nematode Ascaris lumbricoides var. suum. Dev Biol. 1986 Nov;118(1):141–147. doi: 10.1016/0012-1606(86)90081-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleavinger P. J., McDowell J. W., Bennett K. L. Transcription in nematodes: early Ascaris embryos are transcriptionally active. Dev Biol. 1989 Jun;133(2):600–604. doi: 10.1016/0012-1606(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Pearson N. J., Kim C. H., Warner J. R. The genes for fifteen ribosomal proteins of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10176–10183. [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L., Wollett G., Hollingdale M., de la Cruz V. F., Waters A. P., McCutchan T. F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- Kimura J., Arndt E., Kimura M. Primary structures of three highly acidic ribosomal proteins S6, S12 and S15 from the archaebacterium Halobacterium marismortui. FEBS Lett. 1987 Nov 16;224(1):65–70. doi: 10.1016/0014-5793(87)80423-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Molenaar C. M., Woudt L. P., Jansen A. E., Mager W. H., Planta R. J., Donovan D. M., Pearson N. J. Structure and organization of two linked ribosomal protein genes in yeast. Nucleic Acids Res. 1984 Oct 11;12(19):7345–7358. doi: 10.1093/nar/12.19.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz K. B., Roth G. E. Complexity of germline and somatic DNA in Ascaris. Nature. 1976 Jan 1;259(5538):55–57. doi: 10.1038/259055a0. [DOI] [PubMed] [Google Scholar]
- Morlé F., Lopez B., Henni T., Godet J. alpha-Thalassaemia associated with the deletion of two nucleotides at position -2 and -3 preceding the AUG codon. EMBO J. 1985 May;4(5):1245–1250. doi: 10.1002/j.1460-2075.1985.tb03767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Müller F., Walker P., Aeby P., Neuhaus H., Felder H., Back E., Tobler H. Nucleotide sequence of satellite DNA contained in the eliminated genome of Ascaris lumbricoides. Nucleic Acids Res. 1982 Dec 11;10(23):7493–7510. doi: 10.1093/nar/10.23.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natzle J. E., Fristrom D. K., Fristrom J. W. Genes expressed during imaginal disc morphogenesis: IMP-E1, a gene associated with epithelial cell rearrangement. Dev Biol. 1988 Oct;129(2):428–438. doi: 10.1016/0012-1606(88)90390-9. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Peterson R. C., Doering J. L., Brown D. D. Characterization of two xenopus somatic 5S DNAs and one minor oocyte-specific 5S DNA. Cell. 1980 May;20(1):131–141. doi: 10.1016/0092-8674(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Campioni N., Beccari E., Bozzoni I., Amaldi F. Expression of ribosomal-protein genes in Xenopus laevis development. Cell. 1982 Aug;30(1):163–171. doi: 10.1016/0092-8674(82)90022-8. [DOI] [PubMed] [Google Scholar]
- Pimpinelli S., Goday C. Unusual kinetochores and chromatin diminution in Parascaris. Trends Genet. 1989 Sep;5(9):310–315. doi: 10.1016/0168-9525(89)90114-5. [DOI] [PubMed] [Google Scholar]
- Remacha M., Santos C., Ballesta J. P. Disruption of single-copy genes encoding acidic ribosomal proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2182–2190. doi: 10.1128/mcb.10.5.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. U., Stevens J. N., Metzenberg R. L. Heterogeneity of 5S RNA in fungal ribosomes. Science. 1985 Mar 15;227(4692):1340–1343. doi: 10.1126/science.2579431. [DOI] [PubMed] [Google Scholar]
- Tobler H. The differentiation of germ and somatic cell lines in nematodes. Results Probl Cell Differ. 1986;13:1–69. doi: 10.1007/978-3-540-39838-7_1. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Hereford L. M., Rosbash M. Isolation of cloned DNA sequences containing ribosomal protein genes from Saccharomyces cerevisiae. Cell. 1979 Dec;18(4):1247–1259. doi: 10.1016/0092-8674(79)90236-8. [DOI] [PubMed] [Google Scholar]