Abstract
The αvβ3 integrin is known to be highly up-regulated during cancer progression and promotes a migratory and metastatic phenotype in many types of tumors. We hypothesized that the αvβ3 integrin is transferred through exosomes and, upon transfer, has the ability to support functional aberrations in recipient cells. Here, for the first time, it is demonstrated that αvβ3 is present in exosomes released from metastatic PC3 and CWR22Pc prostate cancer cells. Exosomal αvβ3 is transferred as a protein from donor to non-tumorigenic and tumorigenic cells since the β3 protein or mRNA levels remain unaffected upon transcription and translation inhibition in recipient cells. Furthermore, it is shown that upon exosome uptake, de novo expression of αvβ3 increases adhesion and migration of recipient cells on αvβ3 ligand, vitronectin. To evaluate the relevance of these findings, exosomes were purified from the blood of TRAMP mice carrying tumors where the expression of αvβ3 is found higher than in exosomes from wild-type mice. In addition, it is demonstrated that αvβ3 is co-expressed with synaptophysin, a biomarker for aggressive neuroendocrine prostate cancer.
Implications
Overall this study reveals that the αvβ3 integrin is transferred from tumorigenic to non-tumorigenic and cancer cells via exosomes, and its de novo expression in recipient cells promotes cell migration on its ligand. The increased expression of αvβ3 in exosomes from mice bearing tumors points to its clinical relevance and potential use as a biomarker.
Keywords: TRAMP, cell migration, exosome, integrin, prostate cancer
Introduction
Prostate cancer is the second leading cause of cancer related deaths among men in the United States. According to the NCI, there will be 180,890 estimated new cases and 26,120 estimated deaths in 2016. Although, there have been continuous advances in the understanding of diagnosis and treatment of locally advanced and metastatic prostate cancer, the current therapies only provide partial disease stabilization. Androgen receptor (AR) targeted therapies are the standard of care for prostate cancer (1) but androgen-independent or AR-negative forms of cancer such as the neuroendocrine (NE) cancer are very challenging to treat (2–4).
Integrins are transmembrane receptors that comprise an α and a β subunit, known to be deregulated as prostate cancer progresses to advanced stages (5, 6). Compelling evidence indicates that signals originating from integrin ligand binding orchestrate key mechanisms of tumor progression, including cell survival, adhesion, proliferation, gene expression and modulation of the migratory/invasive phenotypes (5, 7–9). There is a strong interplay between integrins, extracellular cell matrix (ECM), cancer cells and their microenvironment as prostate cancer progresses towards a metastatic stage with bone being one of the major sites for metastasis (5, 10). Prostate tumors are highly heterogenous (11) making it challenging to generate novel treatment strategies and effective drugs. The αvβ3 integrin is present at very low levels in normal human prostate but is highly up-regulated in primary cultures of epithelial cells from human prostate adenocarcinoma and promotes adhesion and invasion of cancer cells to ECM proteins such as vitronectin (VN) (12, 13). The αvβ3 integrin promotes tumor growth within the bone, plays a role in the formation of osteoblastic lesions mediated by prostate cancer cells (14) and is often overexpressed in melanoma and metastatic colorectal cancer (15). Given its wide spread distribution in advanced cancer, many therapeutic approaches have been used to target αvβ3 such as inhibitory monoclonal antibodies (mAbs) and small molecules (16, 17).
Exosomes (Exo) are small (30–150 nm) extracellular vesicles (EV) found in a number of biological fluids like blood, urine, amniotic fluid and cell culture media (18, 19). There has been debate about the nomenclature for EVs and recently, a new term “ small EVs” has been introduced by Kowal et al (20) for EVs that are isolated by high-speed ultracentrifugation methods. However, the term Exo will be used for this study until further analysis is performed in our laboratory. Exo are enriched in tetraspanins such as CD63 and CD81. CD63 is mostly present in the compartments of the endosomal/lysosomal system and binds to many adaptor proteins (21). CD63 has been shown to bind to syntenin-1 which plays a role in Exo biogenesis (22). CD81 is another tetraspanin which not only has multiple binding partners that play a role in interaction with cytoskeletal proteins but it has also been shown to bind to the GTPase Rac, thus regulating tumor cell migration (21). More importantly, CD81 is known to regulate outside-in signaling for integrins (23). Exo and larger vesicles including oncosomes play a role in cancer progression by horizontally transferring bioactive molecules to recipient cells (24). Specifically, transfer of proteins and mRNAs to non-tumorigenic and tumorigenic cells has been shown. Regarding Exo transfer to non-tumorigenic cells, Mian He et al have demonstrated that hepatocellular carcinoma-derived Exo transfer pro-tumorigenic RNAs and proteins to immortalized hepatocytes, thereby, inducing motility of these cells (25). It has also been reported that prostate cancer Exo containing H-ras and K-ras transcripts cause neoplastic reprogramming of adipose stem cells in vivo (26) and that Exo purified from breast cancer patient sera are able to induce normal epithelial cells to form tumors in a dicer-dependent fashion (27). In contrast, for Exo transfer to tumorigenic cells, Tauro et al have shown that Exo obtained from H-ras transformed MDCK cells contain integrins which may induce EMT of recipient cells (28). Exo containing different tumor-derived integrins have also been shown to prepare a fertile microenvironment for organ-specific cancer metastasis (29). We have recently demonstrated that the αvβ6 integrin is expressed in Exo from prostate cancer cells and is transferred via Exo; however, only transfer of the αvβ6 integrin among cancer cells was shown (30).
It is evident that Exo play a major role in cell-cell communication and several studies have shown that Exo promote cancer progression (27, 29, 30). The protein content of Exo is of great interest and in this study, we investigated whether the αvβ3 integrin is expressed in Exo from prostate cancer cells and is transferred from tumorigenic to non-tumorigenic cells.
We demonstrate for the first time that exosomal αvβ3 integrin is transferred from tumorigenic to non-tumorigenic and cancer cells leading to functional changes in recipient cells such as increase in cell adhesion and migration. We also show higher αvβ3 expression in Exo from tumor-bearing mice indicating that αvβ3 integrin is a potential biomarker for prostate cancer.
Materials and Methods
Cell Lines
PC3, C4–2B, BPH-1 cell lines and culture conditions have been previously described (31). CWR22Pc cells were cultured as previously described (32).
Antibodies
The following antibodies (Abs) were used for immunoblotting (IB): mouse mAbs to CD63 (#ab 8219), CD81 (#ab 23505), or rabbit polyclonal Abs (pAbs) to FLOTILLIN-1 (FLOT-1) (#ab 41927) from Abcam; mouse mAb to ubiquitin (sc-8017) or rabbit pAbs to ERK (#sc 93), AKT (#sc 8312), CALNEXIN (CANX) (#sc 11397), rat mAb to CD9 (#sc 18869) from Santa Cruz; rabbit pAb to ACTIN (#A2066) from Sigma and rabbit pAb to SYNAPTOPHYSIN (SYN) (#180130) from Invitrogen; rabbit pAb serum against the cytoplasmic domain of human β3 has been described (12). A rabbit mAb against β3 (#ab 75872) from Abcam was used in immunofluorescence (IF). The AP3 mAb against β3 (ATCC) was used for FACS analysis. A mouse anti-human αvβ3 integrin (VN receptor) mAb LM609 (#MAB1976) from Millipore and an isotype negative control Ab were used in adhesion and migration assays.
Exosome Isolation and Analysis
Exo were isolated from culture supernatant (SN) collected 48 hours after starvation by differential ultracentrifugation (19). Briefly, the SN was spun down at 10,000 x g at 4°C for 35 minutes. The SN was collected in a fresh ultracentrifuge tube without disturbing the pellet. The collected SN was then spun at 100,000 x g at 4°C for 1 hour, the pellet was washed in PBS followed by a second spin at 100,000 x g for 1 hour at 4°C. The final Exo pellet was resuspended in PBS. Proteins were extracted from Exo and lysates were prepared. Equal amounts of proteins were separated by SDS-PAGE and analyzed by IB as described before (30). Chemiluminescence kits from Thermo scientific and Bioexpress were used for visualization. Most of the pelleting material at 10,000 x g has been shown to have a mean size of 200 nm by Nanoparticle Tracking Analysis (NTA) by Kowal et al (20). We also performed NTA analysis on the 10,000 x g pellet and saw similar results (data not shown) suggesting that microvesicles were removed.
Nanoparticle Tracking Analysis
NTA was used to determine the size distribution and concentration of Exo released from both PC3 and CWR22Pc cells. Exo from plasma of tumor-bearing TRAMP and wild-type Non-TRAMP mice were also analyzed. Exo were resuspended in PBS and diluted 1:1000. The samples were loaded in the instrument manually and analysis performed according to the manufacturer’s instructions using the NTA software (NS300, Malvern Instruments, MA). The temperature for all experiments was 25°C.
Sucrose Gradient
Exo analysis was performed using a 0.25 M (top) – 2.0 M (bottom) continuous sucrose gradient as described before (19). A dual piston gradient maker (Jule Biotechnologies, Inc) was used. Briefly, the Exo pellet was resuspended in 2.5 M sucrose, loaded at the bottom of the ultracentrifuge tube and centrifuged overnight at 210,000 x g at 4°C (Sorvall, SW41, Swinging – bucket Ultracentrifuge rotor). One mL fractions were collected and resuspended in 20 mM HEPES solution. This was followed by centrifugation at 110,000 x g at 4°C for 1 hour (TLA-100.2 rotor). The final Exo pellet was resuspended in RIPA buffer. The density of each fraction was determined using refractive index with ABBE-3L refractometer (Fisher Scientific).
Analysis of Exosome-mediated αvβ3 transfer via FACS and Immunoblotting
BPH-1 and C4-2B cells were serum-starved for 24 hours and then incubated with 20 μg/mL of PC3 Exo. After 24 hours, the cells were trypsinized, washed with PBS and subsequently stained with the AP-3 Ab specific to β3. Samples were then incubated with Alexa 488 rabbit anti-mouse Ab (Molecular Probes), washed in PBS and the data were analyzed using the FACS Calibur flow cytometer (BD Biosciences). The β3 subunit transfer to BPH-1 and C4-2B recipient cells was also analyzed through IB. In some experiments, the recipient cells that were incubated with PC3 Exo for 24 hours were also subjected to acid wash treatment before cell lysis as described previously (30).
Analysis of Exosome-mediated αvβ3 transfer upon Actinomycin D or Cycloheximide treatment
BPH-1 and C4-2B cells were serum starved for 24 hours. C4-2B cells were either treated with Actinomycin D (Act D) (10 μg/mL) (Acros Organics) or Cycloheximide (CHX) (10 μg/mL) (33) (Sigma) followed by incubation with or without PC3 Exo (15 μg/mL). BPH-1 cells were treated with CHX (1 μg/mL) (34) followed by incubation with or without PC3 Exo (18 μg/mL). After 24 hours, cells were lysed and analyzed by IB as described above.
Quantitative Real Time PCR
Quantitative Real Time PCR (qRT-PCR) analysis was performed as described earlier (35). The following primers were used: β3 F 5′-ACTTCTCCTGTGTCCGCTACAAG-3′ and β3 R 5′-GGTGTCAGTACGCGTGGTACA-3′ (36) or GAPDH F 5′-GGGAAGGTGAAGGTCGGAGT-3′ and GAPDH R 5′-GTTCTCAGCCTTGACGGTGC-3′. GAPDH was used as a housekeeping gene for normalization. Each reaction was carried out at least in triplicates and the fold differences in the β3 expression were determined relative to GAPDH. Delta graph software was used to plot the data.
Immunofluorescence and Confocal Microscopy
Analysis of membrane protrusive events
BPH-1 cells were allowed to attach on VN coated glass coverslips for 1 hour in media without FBS. C4-2B cells were allowed to attach on VN for 1 hour in complete media followed by starvation for 1 hour. Cells were then subjected to incubation with or without PC3 Exo (20 μg/mL) for 2 hours. Cells were processed for IF as previously described (30). Cells were incubated with Alexa Fluor 488 Phalloidin (ThermoFisher Scientific) for 30 minutes to stain for F-Actin. The slides were analyzed using an inverted confocal microscope (LSM510, Carl Zeiss). A minimum of 50 cells was counted for both cell lines in each conditions to analyze membrane protrusions.
PKH26 labeled Exosome uptake and β3 expression
PC3 Exo were labeled with PKH26 dye as previously described (30). BPH-1 and C4-2B cells were serum starved followed by incubation with or without labeled PC3 Exo (40 μg/mL). After 24 hours, cells were stained using the same IF protocol as described above except that the cells were not permeabilized. Cells were incubated with mouse mAb specific to β3 for 1 hour followed by Alexa 488 rabbit anti-mouse Ab incubation for 1 hour at RT. The secondary Ab alone was used as a control for non-specific binding. Intensity analysis for β3 expression was performed on at least 35 cells in each condition using Image J software, and the ratio of β3 expression intensities was calculated.
Adhesion and Migration Assays
The top and bottom of Transwell chambers (12μm pore diameter, Millipore) were coated with VN (10 μg/mL) overnight at 4°C. BPH-1 and C4-2B cells were incubated with or without PC3 Exo (20 μg/mL). After 24 hours, recipient cells were trypsinized and either treated with GRGDSPK (RGD) (1 mg/mL) or GRGESP (RGE) peptides (Gibco BRL) (1 mg/mL) for 30 minutes at 4°C. For experiments using αvβ3 integrin blocking Ab, cells were incubated with LM609 Ab (15 μg/mL) or isotype control Ab (15 μg/mL) for 1 hour at 4°C following a 24 hours incubation with Exo. Cells were then seeded on VN coated Transwell chambers for 16 hours. After fixation with 3.7% paraformaldehyde, the cells attached on top and bottom of the filter were stained with DAPI (Sigma). Cell adhered to the top and bottom of the filter were counted and then the cells that were attached on the top of the filter were removed using a cotton swab. Cells that had migrated to the bottom of the filter were then counted (37). Migration results were analyzed as described previously (30). VN was purified from plasma by affinitity chromatography.
Generation of TRAMP mice
TRAMP mice, expressing SV40 large T antigen in the prostatic epithelium were generated and characterized as described before (38). All mice were maintained under specific pathogen-free conditions. Care and handling of animals was in compliance with IACUC experimental protocols.
Exosome Isolation from Mouse Plasma
TRAMP (n=5) and Non-TRAMP wild-type (n=5) mice (age range from 32.5 – 37.5 weeks) were subjected to intracardiac puncture immediately after euthanasia for blood withdrawal. The blood withdrawn from these mice was collected in 3.8% Na-citrate and spun down to obtain plasma. The plasma samples were processed for isolation of Exo using ExoQuick™ as per the manufacturer’s instructions (Systems Biosciences). Quantitative analysis of plasma-derived Exo was performed by determining protein concentration using BCA assay followed by IB to detect expression of the αvβ3 integrin and exosomal markers such as CD9 and FLOT-1.
Immunofluorescence of TRAMP tissues
Prostates were dissected from TRAMP mice and the tissues were preserved in formalin and embedded in paraffin. Antigen retrieval was performed on these samples by incubation in 10 mM Na-citrate buffer (pH 6.0) for 23 minutes at 95°C. The sections were blocked for 1 hour at RT with PBS/5% BSA. Staining with β3 Ab (Abcam), SYN Ab (Invitrogen) or non-immune rabbit IgG was performed by incubation of tissue samples with primary Abs (1:100) for 1 hour at RT, followed by incubation with Alexa Fluor 488-goat anti rabbit Ab (1:250) (Molecular Probes) for 20 minutes at RT. Nuclei were counterstained using DAPI. After three washes, coverslips were mounted on the sections and analyzed by confocal microscopy as described above.
Statistical Analysis
Statistical significance between datasets was calculated using Excel (Microsoft) software. Chi square tests and Student’s t-tests (2 sided) were performed. P value of < 0.05 was considered statistically significant.
Results
The αvβ3 integrin is expressed in exosomes released from prostate cancer cells
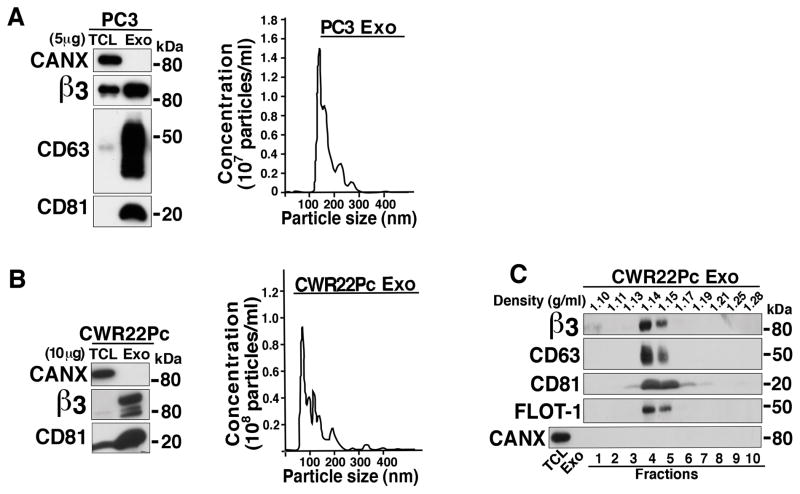
We investigated whether the αvβ3 integrin, an ECM receptor known to be up-regulated in prostate cancer (12, 13), is present in Exo secreted by different prostate cancer cells. In our experimental design, we cultured PC3 and CWR22Pc cells and isolated Exo from their supernatants by ultracentrifugation; our IB analysis shows that the αvβ3 integrin is expressed in both PC3- (Fig. 1A left panel) and CWR22Pc- (Fig. 1B left panel) derived Exo. Each Exo preparation was characterized by detection of CD63 and CD81, exosomal markers, that appear enriched in Exo lysates compared to total cell lysates (TCL). In contrast, CANX, an endoplasmic reticulum protein, is not detected in these Exo preparations (Figs. 1A and 1B left panels). The size distribution of both PC3- and CWR22Pc-derived Exo was determined using NTA. The majority of the Exo from both cell lines was in the accepted size range for Exo with peaks for particle size between 120–150 nm (18) for PC3 Exo (Fig. 1A right panel) and 70–120 nm (18) for CWR22Pc Exo (Fig. 1B Right panel). Since CWR22Pc are AR positive and PC3 are AR negative cells, we conclude that the expression of the αvβ3 integrin in Exo is not affected by the presence or absence of AR. To further characterize the fidelity and purity of Exo, a continuous sucrose gradient was performed to analyze the Exo released from CWR22Pc cells. The data show that the β3 integrin subunit expression is enriched at the expected density range between 1.13 to 1.19 g/mL (18). Expression of CD63, CD81 and FLOT-1 but not CANX, is also enriched in the same fractions as the β3 integrin subunit (Fig. 1C). This analysis was also performed using Exo from PC3 cells and similar results are observed; expression of the αv subunit is enriched in the same fractions as the β3 integrin subunit along with exosomal markers CD63 and CD81 (data not shown). Our results show that the αvβ3 integrin is expressed in Exo secreted by PC3 and CWR22Pc prostate cancer cells.
Figure 1.
The αvβ3 integrin is expressed in exosomes released by prostate cancer cells. A, Left Panel: Exo from PC3 cells were purified via ultracentrifugation; 5 μg of Exo lysate and TCL were loaded on 12.5% SDS-PAGE gel. IB analysis shows expression of β3, CANX and exosomal markers CD63 and CD81. A representative preparation of Exo out of multiple Exo preparations is shown. Right panel: Nanoparticle size distribution analysis for PC3-derived Exo (n=5). B, Left panel: IB analysis of Exo released from CWR22Pc cells. β3, CANX and CD81 expression is shown. 10 μg protein were loaded. A representative Exo preparation out of multiple Exo preparations is shown. Right panel: Nanoparticle size distribution analysis for CWR22Pc-derived Exo (n=2). A representative preparation is shown for both A and B, Right panels. C, Sucrose gradient analysis of Exo secreted by CWR22Pc cells was performed as described in the Materials and Methods. Expression of β3, CD63, CD81, FLOT-1 and CANX is shown. The expected density range for Exo is 1.13–1.19 g/mL.
Exosomal transfer of αvβ3 integrin occurs from tumorigenic prostate cells to either non-tumorigenic or tumorigenic prostate epithelial cells
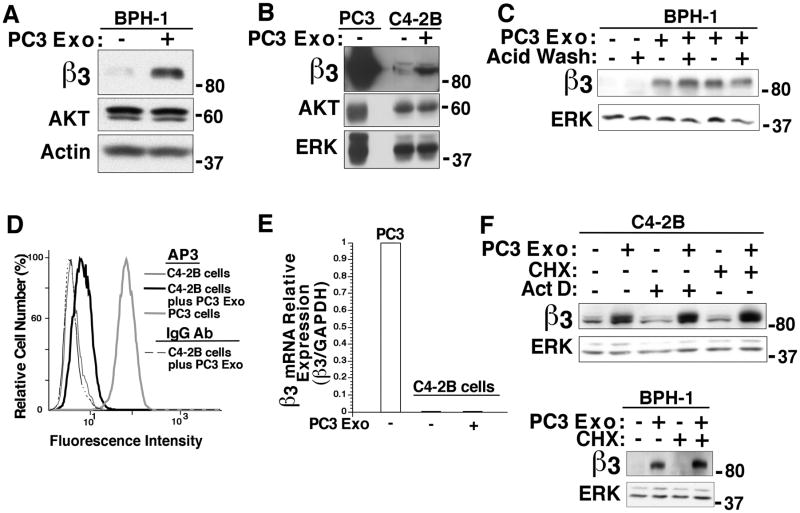
We focused our attention on whether Exo derived from prostate cancer cells that contain the αvβ3 integrin have the ability to transfer αvβ3 to non-tumorigenic BPH-1 cells or other tumorigenic cells such as C4-2B. After incubating PC3 Exo with BPH-1 cells for 24 hours, we observe using IB analysis that there is a significant increase in the αvβ3 integrin levels (Fig. 2A). In contrast, in cells incubated with PBS (vehicle) alone, there is no detectable αvβ3 expression. AKT and ACTIN are used as loading controls. Similarly, we observe an increase in αvβ3 integrin level in C4-2B cells treated with Exo (Fig. 2B). To exclude any possibility of Exo being bound externally on the surface of the recipient cells, we subjected the BPH-1 cells to acid wash treatment upon 24 hour incubation with PC3 Exo. No change in αvβ3 expression is observed after acid wash (Fig. 2C). Using FACS analysis, we showed that αvβ3 is found on the surface of C4-2B cells when treated with PC3 Exo in comparison to cells treated with vehicle (Fig. 2D). We next explored whether the αvβ3 integrin is transferred as a protein or mRNA. We performed qRT-PCR on C4-2B cells either treated with PC3 Exo or vehicle. The results reveal no change in mRNA levels of the β3 integrin subunit upon PC3 Exo treatment (Fig. 2E) and suggest that the αvβ3 integrin is not transferred as mRNA. To further exclude the possibility that exosomal β3 mRNA would transfer or cytokines may induce transcription and translation of αvβ3 in the recipient cells, C4-2B cells were treated with either a transcription inhibitor Act D or a protein biosynthesis inhibitor CHX before incubation with PC3 Exo. IB analysis shows that αvβ3 integrin levels remain unaffected by Act D or CHX treatment of C4-2B cells (Fig. 2F Upper Panel). Act D and CHX activity at the used concentrations was confirmed by evaluating their effect on ubiquitin expression levels (data not shown). Similarly, no change in αvβ3 expression is observed in BPH-1 cells upon CHX treatment (Fig. 2F Lower Panel). This shows that αvβ3 integrin is transferred as a protein via Exo. BPH-1 cells were not subjected to Act D since a concentration as low as 0.1μg/mL was toxic to these cells. Overall, these data demonstrate that extracellular αvβ3 integrin is transferred from PC3 Exo to non-tumorigenic as well as tumorigenic cells and localizes at the surface of the recipient cells.
Figure 2.
Exosome-mediated transfer of the αvβ3 integrin between non-tumorigenic and tumorigenic cells. A, Purified Exo (20 μg/mL) secreted from PC3 cells were incubated with BPH-1 cells. After 24 hours, expression levels of the αvβ3 integrin were analyzed through IB. BPH-1 cells treated with vehicle were used as a negative control. AKT and ACTIN were used as loading controls. A representative IB out of 3 experiments is shown. B, C4-2B cells were incubated with PC3 Exo and αvβ3 integrin expression was analyzed after 24 hours. AKT and ERK were used as loading controls. A representative IB out of 4 experiments is shown. C, BPH-1 cells were incubated with PC3 Exo (20 μg/mL) for 24 hours. The cells were washed with acid wash buffer twice followed by IB analysis. Expression of αvβ3 was analyzed (n=2). ERK was used as loading control. D, FACS analysis of C4-2B cells incubated with PC3 Exo for 24 hours. C4-2B cells were incubated with AP-3, a mAb specific to β3 or non-specific mIgG (negative control) (n=4). E, Quantification of mRNA levels of β3 integrin by qRT-PCR; mRNA was isolated after incubation of C4-2B recipient cells with PC3 Exo for 24 hours. β3 mRNA expression is normalized to GAPDH, P≤0.02 (n=3). F, C4-2B cells were treated with Act D (10 μg/mL) or CHX (10 μg/mL) followed by incubation with PC3 Exo (15 μg/mL) for 24 hours. BPH-1 cells were treated with CHX (1 μg/mL) and incubated with PC3 Exo (18 μg/mL) for 24 hours. αvβ3 integrin expression is shown (n=2). ERK was used as a loading control.
Higher αvβ3 integrin expression and increased membrane protrusions are observed in recipient cells upon exosome internalization
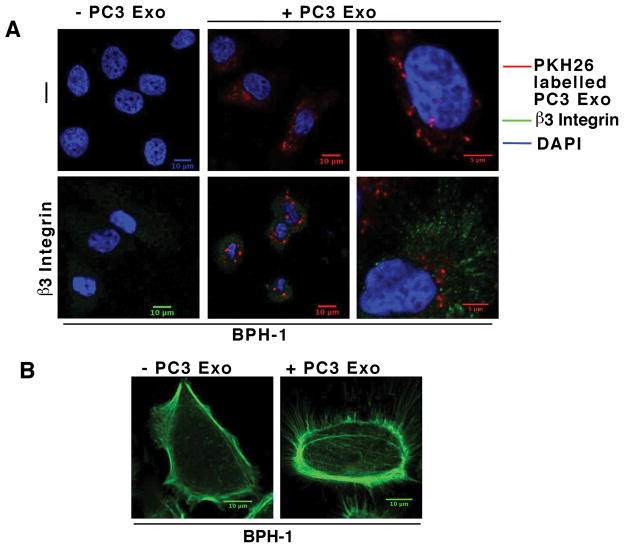
Based on the IB data shown in Figure 2 which confirmed exosomal transfer of αvβ3 integrin among cells, we performed IF experiments to show PC3 Exo internalization and changes in αvβ3 integrin expression in recipient cells. BPH-1 cells were seeded on VN coated coverslips followed by incubation with PKH26 labeled PC3 Exo for 24 hours after which the cells were processed for IF. We show that there is Exo internalization in the recipient cells (Fig. 3A Upper middle and right panels). Z-stack analysis was also performed to confirm Exo uptake (data not shown). We also show an increase in αvβ3 integrin level in BPH-1 cells upon Exo uptake (Fig. 3A Lower middle and right panels). αvβ3 integrin expression was below the level of detection in the Exo alone, however, it was increased 2.6 fold in cells that were incubated with PC3 Exo as evaluated by Image intensity analysis. Similar results were observed using C4-2B cells (data not shown). This further validates our results that the αvβ3 integrin is horizontally transferred between prostate cancer cells and non-tumorigenic cells. We then sought to investigate if Exo internalization led to any changes in recipient cells such as increased cell spreading and filopodia formation. Therefore, we incubated BPH-1 cells that were seeded on VN coated coverslips with PC3 Exo for 2 hours and processed samples for IF by staining for F-Actin filaments. We observe membrane protrusions in 81% of BPH-1 cells that were treated with Exo (Fig. 3B). About 31% of the cells that were treated with vehicle developed membrane protrusions. The experiment was also conducted in C4-2B cells and similar results were observed (data not shown). Overall, these data confirm that αvβ3 integrin is transferred to recipient cells via Exo. Our results also indicate that cells become more motile with increased membrane protrusions upon Exo internalization.
Figure 3.
Increased αvβ3 and membrane protrusions are observed upon Exosome transfer. A, BPH-1 cells were allowed to attach on VN-coated coverslips. Cells were then incubated with PKH26 labeled PC3 Exo (40 μg/mL) for 24 hours (+ PC3 Exo) or vehicle alone (− PC3 Exo). Cells were then fixed and incubated with AP3, a mAb to β3, followed by FITC-tagged anti-mouse secondary Ab (lower panels β3 integrin”) or with secondary Ab alone (upper panels “− “). PKH26-Exo signal was readily detected in confocal images taken through the interior of the recipient cells (upper panels, + PC3 Exo). A maximum projection image at higher enlargement (bottom right) revealed the presence of increased β3 integrin and PKH26-Exo signal. B, BPH-1 cells were seeded on VN-coated coverslips and attached for one hour in FBS depleted media. Cells were then incubated with PC3 Exo (20 μg/mL) for 2 hours and processed for IF as described in the Materials and Methods. F-Actin staining is shown in green.
αvβ3 integrin transferred through exosomes supports αvβ3-dependent adhesion and migration of non-tumorigenic cells
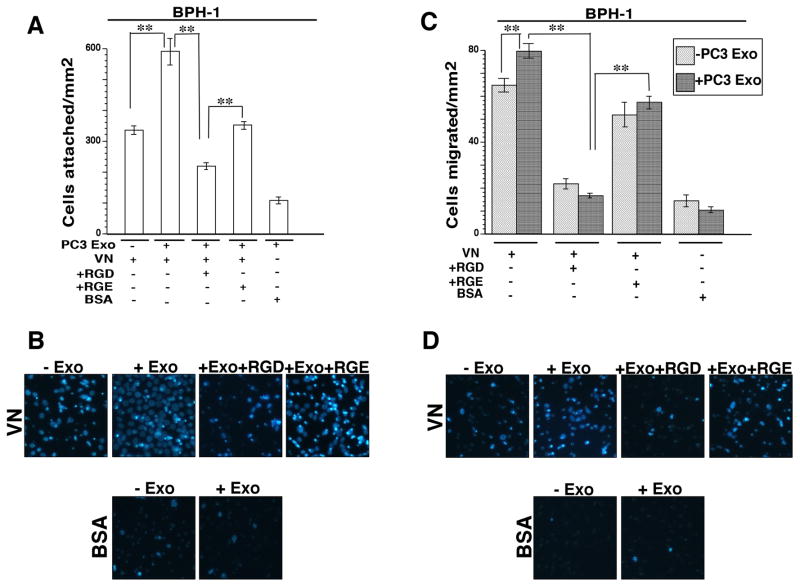
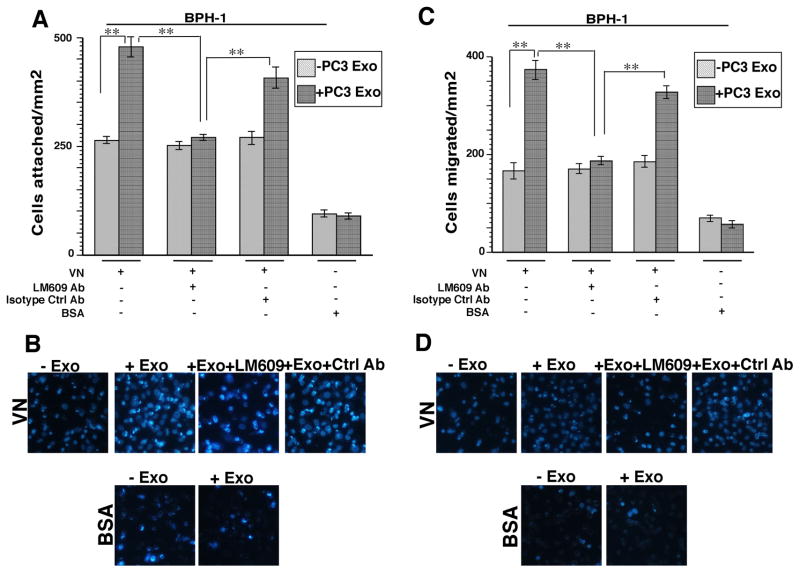
We next investigated whether the transferred αvβ3 integrin is functionally active. It is known that the αvβ3 integrin plays a role in prostate cancer cell migration (12) and modulates bone metastatic growth by mediating adhesion and migration of cancer cells to the bone (14). We asked whether non-tumorigenic cells are able to acquire an αvβ3-specific phenotype by measuring cell adhesion and migration on VN which is a substrate for this integrin. To test this hypothesis, BPH-1 cells were incubated with either PC3 Exo or vehicle. After 24 hours, these cells were incubated with GRGDSPK (RGD) peptide or GRGESP (RGE) control peptide before seeding the cells onto VN coated filters. RGD peptides have been used extensively in other studies to block the αvβ3 integrin function (39) and have been shown to enhance therapeutic efficacy in treating prostate cancer bone metastasis (40). We observe that there is a significant increase in adhesion when BPH-1 cells (Fig. 4A) were treated with Exo compared to cells treated with vehicle. Recipient cells that were preincubated with Exo and then RGD, were affected at a higher extent than control RGE-treated cells. These results show that RGD is able to block binding of the αvβ3 integrin that has been transferred from Exo, to its ligand VN. Representative images of BPH-1 cells attached to VN in the Exo treatment conditions described above are shown in Fig. 4B. Migration assays were also performed using the same treatment conditions. We observe that a higher number of BPH-1 cells incubated with PC3 Exo migrate to the bottom of the filter compared to cells treated with vehicle alone (Fig. 4C). Representative images of migrated BPH-1 cells are shown in Fig. 4D. In order to further establish that the increase in cell adhesion and migration of recipient BPH-1 cells is αvβ3 integrin specific, we treated BPH-1 cells with LM609 Ab (an αvβ3 integrin inhibitory Ab) for 1 hour after a 24 hour incubation with Exo and then the cells were seeded onto VN coated filters. We observe that there is a 50% reduction in cell attachment (Fig. 5A) and a 50% reduction in cell migration (Fig. 5C) when cells that were pretreated with Exo are incubated with LM609 Ab. Adhesion and migration are not significantly affected when BPH-1 cells that were pretreated with Exo are incubated with isotype negative control Ab. Representative images of adhered and migrated BPH-1 cells in the treatment conditions described above are shown in Fig 5B and 5D. Overall, these data show that the increase in cell adhesion and migration is dependent on the αvβ3 integrin that is transferred through Exo.
Figure 4.
Transfer of αvβ3 positive exosomes increases adhesion and migration of non-tumorigenic BPH-1 cells. A, BPH-1 cells that were incubated with or without PC3 Exo (20 μg/mL) were treated with GRGDSPK (RGD) (1 mg/mL) or GRGESP (RGE) (1 mg/mL) peptide before being added onto the filter coated with αvβ3 ligand, VN (10 μg/mL). Cells were washed, fixed and stained with DAPI; pictures of 10–15 random fields were taken. BSA was used as a non-specific substrate for cell attachment. The average number of attached cells was determined. Error bars depict SEM for 2 independent experiments **, P<0.01 compared with controls. B, Representative images of control and PC3 Exo treated BPH-1 cells attached to VN were stained with DAPI are shown. C, Migration assay of BPH-1 cells either incubated with αvβ3 positive PC3 Exo or vehicle alone. Cells were seeded for 16 hours on BSA (1%) or VN (10 μg/mL) and cell migration was evaluated as described under Materials and Methods. Cells that migrated to the bottom of the filter were counted and the average number of cells per field was determined. Error bars depict SEM for 2 independent experiments. **, P<0.01. D, Representative images of control and PC3 Exo-treated BPH-1 cells stained with DAPI in various conditions are shown.
Figure 5.
αvβ3 integrin blocking antibody inhibits adhesion and migration of recipient BPH-1 cells. A, Cell adhesion and migration assays were performed by incubating recipient cells with LM609 Ab for 1 hour after 24 hours incubation with PC3 Exo (20 μg/mL). Cells were incubated on VN-coated filters for 16 hours followed by washing, fixing and staining with DAPI. An isotype Ab was used as a negative control. A, Cell adhesion was evaluated by counting the average number of cells per field. Error bars depict SEM for 2 independent experiments. **, P<0.01 compared with controls. B, Representative images of control and PC3 Exo treated cells stained with DAPI are shown. C, The migrated cells were counted and the average cell number per field was determined. Error bars depict SEM for 2 independent experiments. **, P<0.01 D, Representative images of migrated cells.
αvβ3 integrin transferred through exosomes leads to increase in migration of tumorigenic C4-2B cells
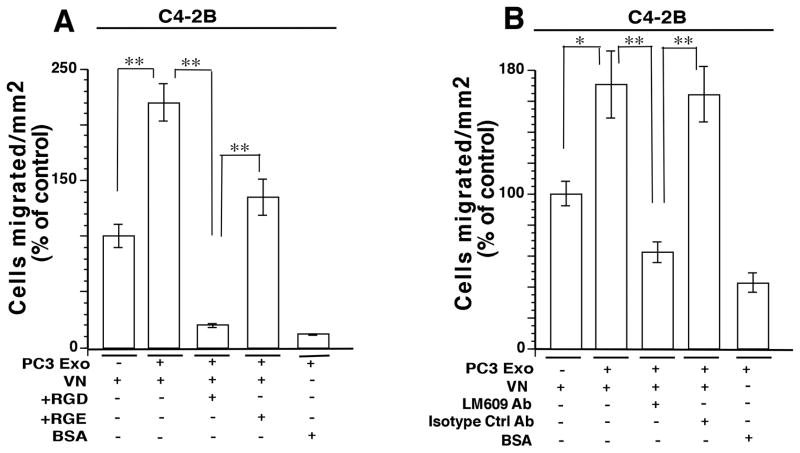
Migration assays were performed using C4-2B cells under the same treatment conditions described for BPH-1 cells. A similar trend in cell migration increase was observed when C4-2B cells were incubated with PC3 Exo (Fig. 6A). Likewise, there is a significant decrease in cell migration of C4-2B cells treated with PC3 exo and RGD versus cells treated with Exo and RGE. Migration assays were also performed using LM609 Ab as previously described. There is a significant decrease in cell migration when cells were treated with Exo and αvβ3 integrin blocking Ab versus cells treated with Exo and negative control Ab (Fig. 6B). These results demonstrate that PC3 cells are able to propagate their migratory phenotype by transferring αvβ3 integrin to recipient cells via Exo.
Figure 6.
Gain of function in prostate cancer C4-2B cells upon transfer of exosomal αvβ3. A, Cell migration assays were performed using C4-2B cells as shown in Figure 4. The average number of cells per field was counted. Error bars depict SEM for 2 independent experiments. **, P<0.01. B, Cells were treated with LM609 Ab for 1 hour after 24 hours of Exo incubation and analysis was performed after 16 hours. An isotype Ab was used as a negative control. The migrated cells were counted and the average cell number per field was determined. Error bars depict SEM for 2 independent experiments. *, P<0.05, **, P<0.01
Increased αvβ3 integrin expression is observed in exosomes derived from plasma of tumor-bearing mice
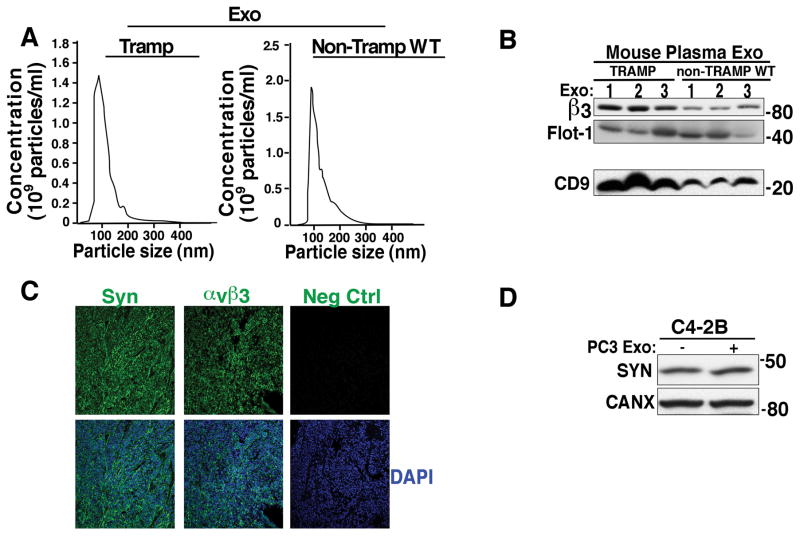
Based on the observations made thus far, we next sought to investigate whether the αvβ3 integrin is expressed in Exo derived from plasma of mice carrying tumors and whether there are any differences in its expression pattern compared to wild-type mice. We first performed NTA analysis on Exo from TRAMP and Non-TRAMP mice to show that we could obtain Exo that were within the accepted size range from both groups of mice (Fig. 7A). We then analyzed these Exo via IB and demonstrated that αvβ3 integrin is present in Exo from murine plasma and that there is a significant increase in αvβ3 in Exo from tumor-bearing TRAMP (Fig. 7B) compared to Exo from non-tumor bearing mice. Increased expression of CD9, an exosomal marker (Fig. 7B) in Exo obtained from tumor-bearing TRAMP mice compared to wild-type mice is also shown, whereas a different marker FLOT-1 is not changed.
Figure 7.
Increased αvβ3 integrin expression in exosomes from plasma of mice carrying prostate tumors. A, NTA analysis for Exo from plasma of tumor bearing TRAMP mice (n=3) and wild-type Non-TRAMP mice (n=2). B, IB evaluation of expression of the αvβ3 integrin in Exo purified using ExoQuick™ from plasma of 5 TRAMP versus 5 Non-TRAMP wild-type mice; αvβ3 integrin levels in 3 out of 5 specimens are shown. The levels of αvβ3 and FLOT-1 were evaluated in reducing conditions while CD9 was evaluated in non-reducing conditions. CD9 results were obtained from a different gel using the same mouse Exo samples. C, IF was utilized to analyze the expression of SYN and αvβ3 in serial sections of TRAMP prostate tumor tissues (n=24 biological replicates and 1 technical replicate/biological replicate). Staining with non-immune rabbit IgG was used as a negative control. Nuclei were stained with DAPI. D, Expression levels of SYN after 24 hours incubation of C4-2B cells with PC3 Exo (n=4). CANX was used a loading control.
We also observe co-expression of αvβ3 integrin and SYN in the prostate tissues from TRAMP mice (Fig. 7C). Based on this observation, we investigated if exosomal transfer of the αvβ3 integrin between prostate cancer cells leads to induction of NE phenotype. After a 24 hour incubation with PC3 Exo, SYN expression levels were unaffected compared to C4-2B cells treated with vehicle alone (Fig. 7D).
Discussion
In this study, our novel findings show that the exosomal αvβ3 integrin is transferred from tumorigenic cells to non-tumorigenic as well as cancer cells and is functionally active in recipient cells upon Exo uptake. We also show for the first time that αvβ3 expression is higher in Exo from plasma of tumor-bearing mice compared to Exo from wild-type mice. These results provide new insights into cancer cell communication with the surrounding environment.
The αvβ3 integrin is a key player in prostate cancer progression (12, 15). Given its unique functions, this integrin provides specificity in response to environmental cues and upon transfer, increases cell motility. Our study shows that the αvβ3 integrin is expressed in Exo released from prostate cancer cells. We have demonstrated that Exo uptake promotes membrane protrusions, transfers αvβ3 integrin which relocalizes to the cell surface of the recipient cells and is functionally active. Although we observe a robust increase in αvβ3 expression in our IB analysis in BPH-1 cells upon Exo incubation (Fig. 2A and C), a lower level of αvβ3 in the recipient cells was detected by FACS analysis. This suggests a possibility that either only a small amount of the integrin is loaded into the cells via Exo or even though a larger amount of exosomal αvβ3 integrin transfer may occur, only a portion of the αvβ3 receptor is exposed at the plasma membrane of the recipient cells.
It is well established now that Exo uptake by recipient cells may occur in different ways such as through filopodia clathrin-dependent endocytosis, macropinocytosis, caveolin-mediated and receptor-mediated internalization (41, 42). The αvβ3 integrin in dendritic cells has been shown to play a role in exosome uptake (43). This suggests a possibility that αvβ3 in Exo may also play a role in its uptake in recipient cells but further studies need to be performed to figure out the exact mechanism of Exo internalization and subcellular αvβ3 localization. It has also been recently shown that filopodia play a role in facilitating the Exo entry into the recipient cells by either filopodia grabbing, surfing or pulling (41). Since we observe increased membrane protrusion formation in our recipient cells upon Exo incubation, it is possible that Exo are captured by the membrane protrusions that develop, facilitating Exo uptake. It also remains to be tested whether αvβ3 travels as a unit in the Exo or β3 is transferred by itself through Exo, ultimately being able to heterodimerize with the αv subunit in the recipient cells to become functional. Further studies beyond the scope of this report will need to be performed to understand these and other aspects of this process in greater detail.
Our study also shows that the αvβ3 integrin is transferred through Exo from prostate cancer cells to non-tumorigenic or cancer cells and leads to a functional increase in adhesion and migration of recipient cells on an αvβ3 integrin ligand, VN. Recently, it has been shown that Exo from sera of breast cancer patients have the ability to induce tumorigenic properties to normal epithelial cells and lead to formation of tumors (27). Our findings are in agreement with these studies suggesting that cancer cell-derived Exo not only have the ability to communicate with other cancer cells but are also able to transfer cargo proteins to non-tumorigenic cells inducing them to migrate and develop other cancer-related phenotypes.
We have shown in our study for the first time that the αvβ3 integrin is found in Exo from plasma of mice bearing prostate tumors and is expressed at a higher level in comparison to Exo from wild-type mice. We also provide the first evidence that the αvβ3 integrin is highly expressed in the tissues of TRAMP mice with NE phenotype. The αvβ3 integrin is known to play a role in the formation of osteoblastic lesions in prostate cancer (14), and inhibition of αvβ3 substantially reduces bone destruction, tumor burden (44), adhesion and migration on components of the bone matrix (45). Our results suggest that Exo may transfer αvβ3 with its adhesive and migratory abilities to ligand-rich metastatic sites such as the bone, thereby, may offer an alternative and synergistic manner to express αvβ3. Further studies to investigate the fate of lesions in the bones of mice carrying prostate tumors upon transfer of the αvβ3 integrin will be conducted. Higher expression of the αvβ3 integrin in our tumor models implicates it as a possible biomarker but patient studies need to be performed for further validation. As an example, an integrin, α3β1 has been detected in urine Exo of metastatic prostate cancer patients and found at higher levels in these patients as compared with benign prostate hyperplasia patients (46).
Tetraspanins are known to be enriched in Exo and play an important role in Exo biogenesis as well as Exo targeting and uptake by recipient cells (21). CD9 is considered an exosomal marker and has been shown to play a role in integrin internalization as well as protein sorting into EVs (21). We observe that there is a significant increase in the expression of CD9 in Exo from plasma and sera of tumor-bearing mice compared to Exo from wild-type mice. It is possible that formation of a subset of CD9 enriched Exo occurs that also have enriched αvβ3 integrin. CD9 has also been shown to incorporate junctional adhesion molecule-A (JAM-A) in a complex with the αvβ3 integrin in endothelial cells resulting in endothelial cell migration upon basic fibroblast growth factor stimulation (47). A possible interaction between JAM-A and the αvβ3 integrin in Exo may play a role in the regulation of cell migration of recipient cells upon Exo transfer of αvβ3. Further exploration of this possible interaction between these three molecules in prostate cancer epithelial cells and Exo released by these cells may give insights into the mechanism and properties of the αvβ3 integrin both in Exo and recipient cells.
Finally, integrins are known to interact with multiple cytokine receptors to activate various signaling pathways. The αvβ3 integrin has been shown to directly bind with IGFIR (48) and PDGF receptor (49) and it is involved in both signaling pathways. Our lab has recently shown that the αvβ3 integrin does not bind to TGFβ receptor type II (TβRII) in prostate cancer cells (50). However, it does not rule out the possibility that this interaction may occur in Exo given that Exo cargo composition and interaction with other molecules may be different in the entire cell as compared to Exo. Further studies will be performed to investigate if TβRII and the αvβ3 integrin interact with each other in Exo and are able to activate downstream signaling pathways upon delivery to recipient cells.
Overall, we have shown that the αvβ3 integrin is transferred from tumorigenic to non-tumorigenic cells as well as cancer cells via Exo and its de novo expression in recipient cells promotes cell migration through interaction with its ligands. The increased expression of the αvβ3 integrin in Exo from mice bearing tumors points to its clinical relevance and potential use as a biomarker for prostate cancer.
Acknowledgments
Grant Support: This study was supported by NIH R01 CA89720 and CA109874 (to LRL), CA113580 (to MTN), P01 CA140043 (to LRL); by a Thomas Jefferson University Dean’s Transformational Science Award. This project was also funded, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.); the Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The Sidney Kimmel Cancer Center Genomics, Bioimaging and Flow Cytometry Facilities were supported by the NCI, National Institutes of Health, under Award P30CA056036. This work was also supported by a Postdoctoral Research Fellowship from the American Italian Cancer Foundation (to C.F).
We thank Dr M.J.Root for giving us access to the refractometer; Y.Covarrubias for constructive suggestions and technical support in imaging experiments; L.Yu for technical support in FACS; A. Yarmahmoodi and L. Yu for technical support for NTA experiments. Also, we are grateful to A. Sayeed, S. Kurtoglu, R. M. De Rita, D. Deming, A. N. Duffy, and L. Riddell from the Languino laboratory for constructive comments. We also thank Dr. Simon Hayward for providing BPH-1 cells.
Abbreviations
The abbreviations used are:
- Exo
Exosomes
- NE
Neuroendocrine
- VN
Vitronectin
- FLOT-1
Flotillin-1
- CANX
Calnexin
- TCL
Total Cell Lysate
- TβRII
TGFβ receptor type II
- IB
Immunoblotting
- NTA
Nanoparticle Tracking Analysis
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ contributions
Conception and design: A. Singh, L.R. Languino
Development of Methodology: A. Singh
Acquisition of data (provided animals, provided facilities, etc): A. Singh, H. Lu
Analysis and interpretation of data (e.g, statistical analysis, biostatistics, computational analysis): A. Singh, C. Fedele, H. Lu, J.H. Keen, L.R. Languino
Writing, review, and/or revision of manuscript: A. Singh, J.H. Keen, L.R. Languino
Administrative, technical, or material support (i.e, reporting or organizing databases): A. Singh, M.T. Nevalainen, J.H. Keen, L.R. Languino
Study supervision: L.R. Languino
References
- 1.Culig Z. Targeting the androgen receptor in prostate cancer. Expert Opin Pharmacother. 2014;15:1427–37. doi: 10.1517/14656566.2014.915313. [DOI] [PubMed] [Google Scholar]
- 2.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13:151–67. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 3.Hu CD, Choo R, Huang J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol. 2015;5:90. doi: 10.3389/fonc.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman-Booty LD, Knudsen KE. Models of neuroendocrine prostate cancer. Endocr Relat Cancer. 2015;22:R33–R49. doi: 10.1530/ERC-14-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- 6.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–31. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 7.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–13. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 8.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–64. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J, Vinogradova O, Plow EF. Integrin bidirectional signaling: a molecular view. PLoS Biol. 2004;2:0726–9. doi: 10.1371/journal.pbio.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sottnik JL, Dai J, Zhang H, Campbell B, Keller ET. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015;75:2151–8. doi: 10.1158/0008-5472.CAN-14-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell T, Neal DE. The genomic evolution of human prostate cancer. Br J Cancer. 2015;113:193–8. doi: 10.1038/bjc.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–64. [PubMed] [Google Scholar]
- 13.Stucci S, Tucci M, Passarelli A, Silvestris F. αvβ3 integrin: Pathogenetic role in osteotropic tumors. Crit Rev Oncol Hematol. 2015;96:183–93. doi: 10.1016/j.critrevonc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 14.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin αvβ3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–43. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teti A, Migliaccio S, Baron R. The role of the alphaVbeta3 integrin in the development of osteolytic bone metastases: a pharmacological target for alternative therapy? Calcif Tissue Int. 2002;71:293–9. doi: 10.1007/s00223-001-2071-1. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–5. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulgrew K, Kinneer K, Yao XT, Ward BK, Damschroder MM, Walsh B, et al. Direct targeting of αvβ3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther. 2006;5:3122–9. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- 18.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 19.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. John Wiley & Sons, Inc; 2006. 2008/01/30. [DOI] [PubMed] [Google Scholar]
- 20.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 23.Tejera E, Rocha-Perugini V, Lopez-Martin S, Perez-Hernandez D, Bachir AI, Horwitz AR, et al. CD81 regulates cell migration through its association with Rac GTPase. Mol Biol Cell. 2013;24:261–73. doi: 10.1091/mbc.E12-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–18. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 26.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32:983–97. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA, et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics. 2013;12:2148–59. doi: 10.1074/mcp.M112.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The αvβ6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290:4545–51. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T, et al. The αvβ6 integrin promotes an osteolytic program through upregulation of MMP2. Cancer Res. 2014;74:1598–608. doi: 10.1158/0008-5472.CAN-13-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagvadorj A, Tan SH, Liao Z, Cavalli LR, Haddad BR, Nevalainen MT. Androgen-regulated and highly tumorigenic human prostate cancer cell line established from a transplantable primary CWR22 tumor. Clin Cancer Res. 2008;14:6062–72. doi: 10.1158/1078-0432.CCR-08-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J Biol Chem. 2003;278:50402–11. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 34.Oksvold MP, Pedersen NM, Forfang L, Smeland EB. Effect of cycloheximide on epidermal growth factor receptor trafficking and signaling. FEBS Lett. 2012;586:3575–81. doi: 10.1016/j.febslet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Sayeed A, Fedele C, Trerotola M, Ganguly KK, Languino LR. IGF-IR promotes prostate cancer growth by stabilizing α5β1 integrin protein levels. PLoS One. 2013;8:e76513. doi: 10.1371/journal.pone.0076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Li T, Xia E, Yang X, Sun X, Zhou Y. Expression of integrin β3 and osteopontin in the eutopic endometrium of adenomyosis during the implantation window. Eur J Obstet Gynecol Reprod Biol. 2013;170:419–22. doi: 10.1016/j.ejogrb.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Languino LR, Gehlsen KR, Wayner E, Carter WG, Engvall E, Ruoslahti E. Endothelial cells use α2β1 integrin as a laminin receptor. J Cell Biol. 1989;109:2455–62. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan D, Li J, Cai P, Prasad P, Liu F, Rauth AM, et al. RGD-conjugated solid lipid nanoparticles inhibit adhesion and invasion of αvβ3 integrin-overexpressing breast cancer cells. Drug Deliv Transl Res. 2015;5:15–26. doi: 10.1007/s13346-014-0210-2. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Chen L, Zhang R, Chen Z, Zhu L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J Control Release. 2014;196:222–33. doi: 10.1016/j.jconrel.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213:173–84. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, et al. Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–30. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 45.Hullinger TG, McCauley LK, DeJoode ML, Somerman MJ. Effect of bone proteins on human prostate cancer cell lines in vitro. Prostate. 1998;36:14–22. doi: 10.1002/(sici)1097-0045(19980615)36:1<14::aid-pros3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJ, Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peddibhotla SS, Brinkmann BF, Kummer D, Tuncay H, Nakayama M, Adams RH, et al. Tetraspanin CD9 links junctional adhesion molecule-A to alphavbeta3 integrin to mediate basic fibroblast growth factor-specific angiogenic signaling. Mol Biol Cell. 2013;24:933–44. doi: 10.1091/mbc.E12-06-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saegusa J, Yamaji S, Ieguchi K, Wu CY, Lam KS, Liu FT, et al. The direct binding of insulin-like growth factor-1 (IGF-1) to integrin αvβ3 is involved in IGF-1 signaling. J Biol Chem. 2009;284:24106–14. doi: 10.1074/jbc.M109.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodard AS, García-Cardeña G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111:469–78. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- 50.Dutta A, Li J, Fedele C, Sayeed A, Singh A, Violette SM, et al. αvβ6 Integrin Is Required for TGFβ1-Mediated Matrix Metalloproteinase2 Expression. Biochem J. 2015;466:525–36. doi: 10.1042/BJ20140698. [DOI] [PMC free article] [PubMed] [Google Scholar]