Abstract
There is growing concern that although the more severe forms of HIV associated neurologic deficits are reduced following highly active anti-retroviral therapy (HAART), mild to moderate cognitive disorders may persist for years after HAART initiation and this may occur despite complete plasma viral suppression. According to the UNAIDS 2014 report, there were 3.2 million children living with HIV around the world at the end of 2013 and 91% of these resided in sub-Sahara Africa. In the same year only 24% of children who needed antiretroviral treatment (ART) received it and 190,000 children died of AIDS-related illnesses. We propose that behavioral interventions are needed in combination with medical treatment and care in order to fully address the needs of children and adolescents in Africa living with HIV. In early childhood, caregiver training programs to enhance the developmental milieu of the child with HIV can enhance their cognitive and social development, and that such interventions are both feasible and well-accepted by the local population. For school-age children, computerized cognitive rehabilitation training can be an entertaining and engaging way to improve attention, working memory, and problem solving skills for children with HIV. Further dissemination and implementation science work is needed for arriving at cost effective strategies for scaling up such behavioral interventions in African resource-constrained settings, given that the vast majority of HIV-affected children and youth worldwide presently live in sub-Sahara Africa.
Keywords: Pediatric HIV, Child Development, Neuropsychology, Caregiver Training, Cognitive Rehabilitation, HIV subtype
Introduction
Highly active antiretroviral therapy (HAART) and neuropsychological deficits in children
There is growing concern that although the more severe forms of HIV associated neurologic deficits are reduced following HAART, mild to moderate cognitive disorders may persist for years after HAART initiation and this may occur despite complete plasma viral suppression. In Pediatric AIDS Clinical Trials Group (PACTG) 377 and PACTG 338, treatment with protease inhibitor therapy for 48 weeks did not lead to improvement in any neuropsychological measure except a vocabulary score and even that was minimal [1]. In these cohorts, even children with good virologic response (VL < 400 copies/ml) had persistently poorer neuropsychological scores than population norms.
In Thailand, 79% of HIV infected children who had received HAART for a median 35 weeks had below normal cognitive function measured by the Wechsler Intelligence Tests for Children-III and performed much worse than comparative arms of HIV exposed uninfected (moderate performing) and HIV unexposed uninfected (best performing) controls[2] (see Table 1). In this study, HIV remained the most significant predictor of poor cognitive function after adjustment for family income and relationship with primary caregiver (OR = 6.2). Among HIV infected children in Kinshasa, there was greater improvement in motor function compared to cognitive scores following HAART, and children who initiated HAART at younger age and in early disease stage (WHO Clinical stage 1–2) experienced the greatest gains [3].
Table 1.
Effect of HAART on neurocognitive deficits in HIV infected children.
| Author Setting | HAART regimens duration | Spectrum and measures of Neurocognitive deficit after HAART | Associations |
|---|---|---|---|
| Jeremy R, Pediatrics 2005 489 HIV +VE (4–17 yrs) US | Protease inhibitor based 48 weeks | No change in neurocognitive scores except vocabulary | Viral suppression not linked with improvement in NP scores |
| Puthanakit, AIDS Care 2010 121 (39 HIV +VE, 40 exposed, 40 unexposed. Thailand | NNRTI based median 35 weeks | No change in Verbal scales of WISC after ART | HIV infected had lower verbal scale of WISC than controls. |
| Shanbagh. Arch Pediatr. Adolesc. Med 2005 146 HIV +VE US | Compared pre–HAART 1996 and post-HAART | Progressive encephalopathy dropped from 27% to 12% | |
| Wolters, Martin Dev. Neuropsychology 30(2) 41 HIV +VE (6–11 years) US National Cancer Institute. | Protease-inhibitor based. At least 1 year | WISC-III. Overall function in the average range | Minimal to Moderate CT scan abnormalities and low CD4 count correlated with lower neurocognitive scores. |
These data indicate that among HIV infected children receiving HAART, neurocognitive impairment remains a significant problem. Table 1 summarizes selected studies on the effect of HAART on neurocognitive function in pediatric HIV. Given the persistence of neurocognitive deficits in important domains, especially those that determine learning capability in HAART-treated children and adolescents, it is imperative to develop and evaluate potential interventions to remediate persistent neurocognitive deficits, even in clinically stable pediatric HIV patient populations.
Medical care and ARV treatment in preventing neurodevelopmental disabilities
Research from high-income countries suggests that children treated with a protease inhibitor (PI) based antiretroviral (ARV) regimen have normal global cognition scores [4] and that ARV treatment duration is associated with a reduction in neurological impairment [5]. However, evidence is largely limited to subtype-B, which is responsible for only 11% of global HIV infections [6]. The effect of ARVs on a child’s cognition and behavior in low and middle income countries (LMICs) is still not well defined [4]. Different studies suggest that HIV-1 mutations differ by subtype [7, 8], which might lead to increased resistance to ARVs [6]. Also, another potential cause for persisting neurodevelopmental deterioration in children in LMIC is treatment with ARV regimes with poor CNS penetration, resulting in sub-therapeutic levels that generate HIV resistance [9]. Research suggests that providing ARV with better CNS penetration can result in improved neurocognitive outcomes among adults [10]. Additional research is needed to define ARV effectiveness by specific HIV-1 subtype and the role that time of ARV initiation has on preventing or reversing neurodevelopmental deficits, and psychiatric problems in adolescents [11, 12]. Priority needs to be given to research that includes children and adolescents, who are likely to be on life-long treatment in a context of limited therapeutic options, long-term toxicity, challenging treatment adherence and risk of developing ARV resistance [13].
Virology and immunological biomarkers have been identified as independent factors of disease progression in adults living with HIV [14]. Few and inconsistent results have been published in pediatric samples. Higher CD4+ and CD8+ T-cell counts [15, 16], as well as higher CD4 percentages in children >1 year of age, have been associated with better neurobehavioral functioning [12]. Significant associations between T-cell activation and HIV associated CNS disease have been reported [17]. Different CD4+ and CD8+ T-cell subsets in blood have been used to assess the association of immune cell activation and behavioral and cognitive outcomes[17–19], leading to contradictory results. Higher CD4+ CD38+ HLADR+ T-cells have been associated with a neuroprotective effect in young HIV+ children (<1 year) [18]. In contrast, low immune activation defined as ≤ 5% CD8+HLADR+ among HIV+ infants (<2 months) was consistently associated with better psychomotor development [19]. Further examination of the role of immune activation in HIV disease among children is warranted so that clinical management can be modified accordingly and potential neuropsychological and psychiatric problems perhaps prevented.
For HIV-infected African children, enhanced access to ARV medications and their increased effectiveness has changed the prognosis from a uniformly deadly disease early in childhood, to one in which survival well into adolescence is not uncommon [11]. As a result, pediatric HIV illness is increasingly becoming a sub-acute, chronic disease marked by developmental lag and progressive encephalopathy (PE) [12]. Even so, Koekkoek and others have concluded that antiretroviral therapy alone is not sufficient to reverse the neurodevelopmental consequences of pediatric HIV infection [13–15]. HAART may even contribute to neuromotor decline over time [13,16]. In American children, Jeremy and colleagues studied HIV-infected children enrolled in several of the NIH-sponsored PACTG HAART studies and found that only 1 of 13 neuropsychological measures significantly improved. They concluded that “treatment strategies for children with HIV disease need to be re-evaluated so that they consider restoration of neuropsychological functioning in addition to lowering the viral load” [17].
Another important consideration is whether HAART initiated earlier in the child’s development, irrespective of clinical status, can result in better developmental outcomes for the child over the long-term. Van Rie and colleagues in the DR Congo concluded that earlier treatment onset can increase the likelihood of a more normal trajectory in motor and perhaps cognitive development [19]. The Van Rie et al and Boivin et al studies with Congolese children included three comparison groups: those infected with HIV, those exposed to HIV in utero but not infected, and non-infected children not exposed to HIV. In both studies, children not infected but exposed to HIV were still at greater developmental and neuropsychological risk than children not infected and not exposed [7,8]. In order to better understand the developmental and neurocognitive benefits of HAART, it is important to disentangle the effects of compromised caregiving due to maternal HIV status and illness (distal neurodevelopmental effects) from the direct effects of HIV infection on the child (proximal effects of the disease on the CNS). The best way to do this is to recruit a comparison group of HIV-exposed (in utero) but uninfected children who live in a similar developmental milieu in terms of quality of caregiving as affected by the impact of HIV on the household. Using this approach, Laughton and colleagues documented in a randomized controlled trial that very early initiation of treatment with HAART among South African children with HIV resulted in better neurodevelopmental outcomes [11]. The same seems to be true in an observational study of very young Ugandan children [5].
Early childhood and brain development
Early childhood is a period of rapid and dramatic change in the cognitive, emotional, social, and behavioral domains. Emotions have been shown to play a very central role in infant and child development, with early emotional experiences being shown to leave a permanent effect on brain structure and not only on behavior [20, 21]. For example, positive experiences may result in an increase in beta-endorphin and dopamine (“positive brain” chemicals) levels, while negative experiences may trigger the production of stress hormones, such as cortisol. This chemical activity may have long lasting effects. If a child has many unhappy early experiences the development of dopamine and opiate receptors may be reduced, which may result in diminished capacity to experience pleasure and enjoy reward later in life [22].
Cerebral plasticity provides the means by which early experience sculpts a child’s genetic blueprint into her ultimate profile of neurocognitive and affective function. This product of neurocognitive sculpting produces a dynamic brain/behavior product, emerging along a continuum from highly adaptive and functional, to disorganized and pathological. An important theory on how early childhood intervention achieves brain/behavior rehabilitative benefit is based on work by Mahncke and colleagues [23]. Central to their theory is the notion of negative and positive neuroplasticity. They define brain plasticity as the capacity for physical and functional brain change that can either be strengthened or degraded in a bi-directional manner, depending on the circumstances [23].
Bonnier included child-caregiver care enhancement as a way to achieve neuroprotection when it provides optimal environmental conditions during a critical period of neurodevelopment. She concluded in her review that the most effective programs involved both the parents and the child in achieving improved cognitive outcomes through improved child–parent interactions [24]. Cognition improved more than motor skills; and gains were greater for more at-risk families with lower maternal education. Pnina Klein developed the mediational interaction for sensitizing caregivers (MISC) approach to improve cognitive and social development among young children by training their caregivers in practical strategies for enhancing their children’s development through daily interactions in the home. MISC principles can be readily translated into actions within the cultural and contextual constraints of everyday living in each of their families [25–27].
Distal Effects of the HIV epidemic as children survive into adolescence
Enhanced access to HAART medications for children in the developing world has changed the prognosis for infected children from a uniformly deadly disease early in childhood, to one in which survival well into adolescence is not uncommon [28]. Programs such as the UN Global Fund and PEPFAR have dramatically enhanced access to HAART for HIV infected children in participating African countries such as Uganda [29]. As a result, pediatric HIV illness is increasingly becoming a sub-acute, chronic disease [30, 31]. HIV African children are now able to survive longer, but they remain at significant risk developmentally because of psychosocial distress from HIV-related orphanhood [32]. Therefore, it is important to consider strategies for enhancing their cognitive and psychosocial development in the face of HIV disease developmental encephalopathy, psychosocial distress, and seriously compromised caregiving [33].
Over 90% of pediatric HIV infections and AIDS deaths occur in Africa and more than 11 million children have lost at least one parent to AIDS [34]. In Uganda, about 1 million children are orphans with one or both parents dead (UNICEF definition) and a new child is orphaned every 14 seconds [35]. When considering how to best address the global public health burden of the developmental effects of HIV on children, the African context is clearly paramount. In rural areas of sub-Sahara Africa, the family must rely upon labor-intensive subsistence agriculture to provide for the nutritional needs of the family. Because of this, maternal HIV disease and illness can severely disrupt not only the nurturing capacity of the mother for her children, but also food security for the entire family [36–38]. Chronic nutritional hardship can severely undermine early childhood development [39, 40]. Because of this, the AIDS epidemic can also have devastating consequences for non-infected children of HIV parent(s).
Research conducted among children and adolescents in Uganda [41], Kenya [42], the Democratic Republic of Congo [43], Rwanda [44], Thailand and Cambodia [45] has consistently showed the detrimental effects of HIV infection on cognition, intelligence, behavior, memory and psychomotor outcomes. These neurocognitive deficits can result in negative school performance and other social interactions. A small study (N=84) among Ugandan adolescents showed that 51% had significant psychological distress within the last 12 months, and 46% met ICD-10 criteria for anxiety disorders [46]. However, the cognitive, behavioral and psychiatric symptoms associated with specific HIV-1 subtypes on adolescents are not known.
Children with perinatally acquired HIV are at risk for neurocognitive delays and psychiatric symptoms as they progress through middle-childhood into adolescence. Youth infected with HIV are at increased risk of developmental and neuropsychological disturbances due to both, the direct effects of the HIV virus on brain structures involved in the regulation of emotion, behavior, and cognition and the indirect effects of social stressors, poverty, illness and trauma [47–49]. These disadvantages can seriously undermine academic and social achievement and therefore require urgent attention [50–52]. Following the scale-up of antiretroviral therapy supported by PEPFAR, survival of perinatally-infected children in sub-Saharan Africa has dramatically improved [53, 54], increasing the population of HIV+ adolescents. UNAIDS reports that 2 million adolescents between the ages of 10–19 years are living with HIV globally, and over 90% live in sub-Saharan Africa [34]. While initial focus was rightly placed on improved survival, there is increasing need to focus on the emerging challenges of adolescents living with HIV, specifically in identifying those at higher risk of developing neurobehavioral problems. As growing numbers of children progress into adolescence, clinical emotional and behavioral disorders have been identified as major concerns among perinatally-infected adolescents [55].
For example, in Uganda where our pediatric HIV research group has evaluated the neurodevelopmental effects of perinatal pediatric HIV, an estimated 177,000 children below 15 years of age were living with HIV in 2013, with 9,600 new infections projected to happen in the same year[56]. Children in Uganda are exposed to multiple potential developmental insults including malaria, malnutrition, parasitic infection, HIV/AIDS, trauma and abuse, unstable living conditions, and limited access to education. With an estimated 5 million children, Uganda ranks in the top ten countries for number of disadvantaged children at risk of failing to meet their developmental milestones [57]. It is likely that adolescents living in LMICs face recurrent and cumulative psychosocial stressors associated with their HIV status (i.e., medication adherence, disclosure) in addition to being confronted with psychosocial issues common to their developmental stage (i.e., initiation of sexual activity), and their social milieu (i.e., death of parents and siblings, responsibility for the welfare of other siblings, stigma, discrimination). The few studies on Uganda HIV+ adolescents show considerable rates of depression and anxiety symptoms [46].
An Intervention Model: Mediational Intervention for Sensitizing Caregivers (MISC) in early childhood development
One to 5 years of age is a critical developmental period for children, during which time they develop the dynamic capacity to benefit from new learning experiences. There is a general consensus from developmental research that adult-child interactions are of central importance in this process [24]. Farah and colleagues (2008) observed a relationship between parental nurturance and memory development. This relationship was consistent with the animal literature on maternal buffering of stress hormone effects on hippocampal development [58]. Rao et al. (2009) observed that parental nurturance at age 4 predicts the volume of the left hippocampus in adolescence, with warmer and more loving nurturance associated with smaller hippocampal volume. Also, the association between parental nurturance and hippocampal volume disappears at 8 years of age. They concluded that this supports the existence of a sensitive developmental period for brain maturation, especially before 4 years of age [59]. The caregiver provides for secure emotional attachments in a nurturing environment, creating learning experiences that allows a child’s neurocognitive ability to blossom [60, 61]. Effective mediational behaviors by caregivers were found to be significantly related to children’s social-emotional stability and the willingness to explore and learn about the world around them [62, 63].
While the role of effective caregiving in fostering optimal neurocognitive development during sensitive periods in early childhood has been studied in a wide range of cultural settings and across various populations of children with special needs, evaluation of interventions to improve caregiving have not been done in low-resource non-Western settings. Nor have they been done with African children affected by HIV. Caregiver training can help the caregiver interact with their child in a way that promotes development and growth even in the face of adversity. Boivin and colleagues have shown that caregiver training to improve the home-based developmental milieu of very young Ugandan children with HIV can significantly enhance their cognitive development, and that such interventions are both feasible and well-accepted by the local Ugandan population [64].
Theoretical foundation of MISC training
The MISC approach has a clear and well-developed theoretical foundation. Unlike models based on simple direct learning through stimulating the senses with an enriched environment [27, 57, 65–68], MISC is a mediational approach based on Feuerstein’s theory of cognitive modifiability [60, 69]. The fundamental premise of this approach is that mediated learning best occurs interactively, when the caregiver interprets the environment for the child. To do so, the caregiver must be sensitive to the child’s cognitive and emotional needs, interests and capacities. As such, MISC has a strong emphasis on the importance of the social/interactive/emotional domains as integrally linked to intellectual and cognitive development.
MISC learning is accomplished by training caregivers in mediational processes as focusing (gaining the child’s attention and directing them to the learning experience in an engaging manner); exciting (communicating emotional excitement, appreciation, and affection with the learning experience); expanding (making the child aware of how that learning experience transcends the present situation and can include past and future needs and issues, therefore extending beyond the immediate need of the moment); encouraging (emotional support of the child to foster a sense of security and competence); and regulating (helping direct and shape the child’s behavior in constructive ways with a goal towards self-regulation).
Most of the MISC training of caregivers is devoted to helping parents become aware and develop practical strategies for focusing, exciting, expanding, encouraging, and regulating the child as learning opportunities arise in the course of natural everyday caregiver/child interactions [27, 68, 70, 71]. It begins by trying to understand and highlight the caregivers’ objectives for childrearing and their goals for the ideal child and ideal parent. It asks parents/caregivers what outcomes they hope to achieve. This process raises parental awareness regarding their own attitudes about childrearing, perception of the child, perception of themselves as caregivers, awareness of the child’s emotional and cognitive needs, and awareness of the impact of parental/caregiver interactive behavior. Because of the facilitative nature of the program, it does not rely on outside resources or materials, and can be implemented with most children in a variety of contexts where caregiver/child interactions naturally take place.
The families in the district where our preliminary study took place are primarily from the Baganda tribe in Uganda, a tribe which traditionally highly values children and emphasizes the importance of the effective and loving nurture of children for the future betterment of families and communities [72]. The cultural emphasis on the nurturing of children is a good fit for the MISC, which is a method for sensitizing mothers to the positive aspects of their current childrearing interactions. Similarly to what Klein found in Ethiopia, the initial results from Kayunga and Tororo indicated that the principles of MISC are simple and can be easily understood by caregivers and associated with their own childrearing goals [25]. As a result, caregivers noted how MISC principles can be readily translated into actions within the cultural and contextual constraints of everyday living in each of their families [27, 68]. When culturally appropriate and effectively implemented, caregiver training interventions to support the care and nurture of household children can improve functionality, psychosocial support, and emotional wellbeing of the mothers. These benefits are strongly related to the neurodevelopmental wellbeing of young African children [73].
HAART and the neuropsychological effects of HIV in adolescence
Following the scale-up of HAART supported by United States President’s Emergency Plan for AIDS Relief (PEPFAR), survival of perinatally HIV infected children in sub-Saharan Africa has dramatically improved [53, 54]. While initial focus was rightly placed on improved survival, there is increasing need to focus on the quality of life for African children living with HIV. Cognitive, psychiatric, and behavioral (neuropsychological) disorders are emerging as a major concern in HAART-treated perinatally infected children as they progress into adolescence. Such problems can seriously undermine academic and social achievement and therefore require urgent attention [50–52]. There is emerging data on poor academic performance by HIV infected children, with a significant number of children experiencing delay in achieving academic targets.
A large study among HIV infected adolescents in New York found one-third to be attending special education mainly attributable to problems in reading or math. Several other studies have explored school performance among HIV infected children and adolescents and identified poorer outcomes compared with children without HIV [74–76]. Therefore, interventions targeting HIV children who remain at risk for poor neurocognitive and behavioral function are urgently needed. In a systematic review of cognitive development and pediatric HIV, 81% of 54 studies found HIV to have a detrimental effect on neurocognitive development [77, 78]. While most neurocognitive domains can be affected, pediatric HIV has the most substantial effect on attention, memory, and visual spatial processing speed[78–82]. These are the very domains of neurocognitive development in African children that have been most effectively improved through computer cognitive rehabilitation training (CCRT), discussed further below [83].
Computerized Cognitive Rehabilitation Training (CCRT) with children with HIV
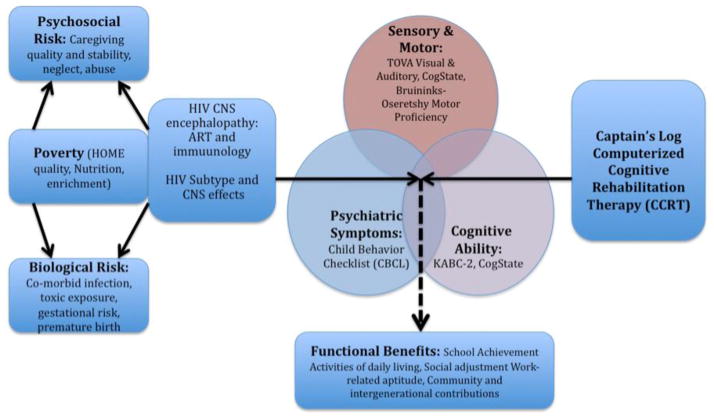
We can identify factors that can serve as early predictors to identify those who are potentially at higher risk of having negative neurocognitive and behavioral outcomes. Such neurobehavioral biomarkers can allow for early intervention using primary and secondary behavioral prevention and treatment strategies [84–86]. This is so that psychological support and treatment services can be developed in low-resource settings with a high prevalence of adolescents with HIV. Figure 1 is based on a conceptual model proposed by Walker and colleagues[87, 88] and illustrates how our assessment plan was used to evaluate the neuropsychological benefits of CCRT in Ugandan school-age children with HIV [83, 89–91]. This work could eventually be the basis for a similar intervention plan extending into adolescence for children especially at risk.
Figure 1. Model of the major risk factors and developmental domains for our study children with HIV.
This figure depicts the computerized cognitive rehabilitation training (CCRT) program used (Captain’s Log) (far right), neuropsychological outcome assessment domains from Table 2 (Sensory/Motor, Cognitive, Psychiatric), principal proximal severity of illness factors serving as moderatoring and modifying variables (HIV subtype, extent of progressive encephalopathy), and more distal risk control variables (far left) affecting the three principal neurodevelopmental domains (Sensory/Motor, Cognitive, Psychiatric). This type of clinical study design allows for the more accurate assessment of the direct impact of severity of HIV on brain/behaviour benefits derived from such interventions as CCRT.
In our most recent study findings with school-age Ugandan children with HIV, both Captain’s Log CCRT and the locked (limited) version of Captain’s Log (active control arm) significantly enhanced global cognitive ability and executive functioning as measured by the Kaufman Assessment Battery for Children (2nd ed.) (KABC-II) (see Table 2 and Figure 1) [92]. In a separate test, we evaluated the neuropsychological benefits of Brain Powered Games (BPG), a set of games within an African village motif developed by Michigan State University telecommunications professor Brian Winn and his programming team [93]. Our analyses documented significant improvements on measures of attention and processing speed from the Tests of Variables of Attention (TOVA; see www.tovatest.com). These disparate findings (i.e., improvements in attention and processing speed versus global ability and executive functioning) between BPG and Captain’s Log CCRT suggest that combining BPG and CCRT training successively may provide for a more comprehensive and complementary neuropsychological benefit from CCRT intervention.
Table 2.
The principal outcomes from a neuropsychological assessment battery used to evaluate all of the principal motor and neurocognitive domains known to be affected by pediatric HIV disease. This battery can be used to evaluate the benefits of computerized cognitive rehabilitation training (CCRT) or other behavioral interventions for African children affected by HIV [91]
| Developmental Domain | Muscle Tonus | Cognition | Intellect / Achievement | Affect Adjustment | |||
|---|---|---|---|---|---|---|---|
| Motor Function | Visual Spatial Memory | Auditory Verbal Memory | Central Executive Function | Executive Reasoning | Language/Numeric | Social / Emotional | |
| Kaufman Assessment Battery for Children (KABC-II) | Sequential & Simultaneous Processing & Learning | Sequential Processing & Learning | Simultaneous Processing & Learning | Planning | Learning Knowledge (verbal and quantitative) | ||
| Tests of Variables of Attention (TOVA) | Reaction time speed | D prime, ADHD, Omission Errors | Impulsivity, Commission Errors | ||||
| Bruininks-Oseretsky Test of Motor Proficiency | Total Motor Proficiency | ||||||
| Behavior Rating Inventory for Executive Functions School Age (BRIEF) | Working Memory | Monitoring | Plan/Organize & Shift & Global Executive Function | Emotional Control & Inhibit | |||
| SRQ-20 | Attention Problems | Internalizing (emotional) and Externalizing (behavioral) symptoms | |||||
Summary and Conclusions
An estimated 200 million children under age 5 in low- and middle-income countries (LMIC) fail to reach their developmental potential. Advances in neuroscience show that exposure to poverty-related cumulative risk during childhood affects brain structure and compromises the development potential of these children by limiting opportunities for cognitive stimulation. This is especially the case in African children affected by both the proximal (infection) and distal (compromised caregiving and learning environments) in resource-constrained settings.
Although there are evidence-based programs for promoting cognitive development among children living in poverty in low- and middle-income countries, because they have generally only been implemented by highly-skilled providers, who are costly and limited in supply, the possibility of scaling up and disseminating such programs is limited. One such program is the MISC, a program that uses specialized providers to train caregivers to be more attuned to their child’s needs. This program has been shown to achieve significant developmental and long-term academic gains in children within impoverished communities when implemented by educated providers [25]. As children survive into middle childhood and adolescence, CCRT can provide an effective way to enhance the neurocognitive skills foundational to academic and community-based learning and achievement.
However, the financial costs and human resources needed to expand the reach of such program may be prohibitive in its current format, which includes individual treatment over 12-months by technical or university-educated providers. The next logical step to evaluate dissemination and implementation strategies of a group-based MISC and CCRT programs in the most cost-effective and sustainable manner possible, appropriate to the cultural context.
This review provides evidence for the efficacy of such interventions in the African context in addressing the developmental needs of children affected by HIV. We conclude that MISC- and CCRT-type interventions can be packaged with the needed ARV and medical support treatment services so as to be efficiently and successfully implemented in a group-based peer-delivered model. Such bundling of medical and behavioral services considerably enhances the potential for scaling up and sustaining of such evidence-based behavioral interventions.
The implications for such a proposal are significant in that they address a serious global health problem in a vulnerable population for whom few medical/behavioral evidence-based programs exist. Dissemination and implementation science research for such medical/behavioral treatment packages will have considerable public health impact for African children affected by HIV in that 1) they provide for the systematic evaluation of strategies that could lead to a sustainable translation of an evidence-based program into practice. 2) They can lead to a better understanding of the critical factors pertaining to sustainability and intervention package program fidelity; and 3) they can provide for cost data that will be accessible by policy makers to inform their decision on further supporting the program’s dissemination. Beyond the needs of HIV-affected children in Africa, such an initiative has far-reaching implications for promoting improved child development and reducing the burden of emotional and behavioral problems globally. That is, where-ever at-risk impoverished children face significant adversity with few highly trained providers (medical or otherwise) to meet their needs.
Footnotes
Conflict of Interest
Michael J. Boivin, Horacio Ruisenor-Escudero, and Itziar Familiar-Lopez declare that they have no conflict of interest
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Michael J. Boivin, Email: boivin@msu.edu, Department of Psychiatry and the Department of Neurology & Ophthalmology, Michigan State University Department of Psychiatry, University of Michigan, 909 Fee Road, Rm 321 West Fee Hall, East Lansing Michigan 48894 USA, Phone: 765 506-2163, FAX: 517 432-2893.
Horacio Ruisenor-Escudero, Email: horaciore@gmail.com, Department of Psychiatry, Michigan State University, 909 Fee Road, Rm 321 West Fee Hall, East Lansing, Michigan 48894 USA, Phone: 517 432-4204, FAX: 517 432-2893.
Itziar Familiar-Lopez, Email: ifamiliar@gmail.com, Department of Psychiatry, Michigan State University, 909 Fee Road, Rm 321 West Fee Hall, East Lansing, Michigan 48894 USA, Phone: 517 432-4204, FAX: 517 432-2893.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Jeremy RJ, Kim S, Nozyce M, Nachman S, McIntosh K, Pelton SI, et al. Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005;115:380–387. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- 2.Puthanakit T, Aurpibul L, Louthrenoo O, Tapanya P, Nadsasarn R, Insee-ard S, et al. Poor cognitive functioning of school-aged children in thailand with perinatally acquired HIV infection taking antiretroviral therapy. AIDS Patient Care STDS. 2010;24:141–146. doi: 10.1089/apc.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2009;52:636–642. doi: 10.1097/QAI.0b013e3181b32646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326–1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- 5.Brahmbhatt H, Boivin M, Ssempijja V, Kigozi G, Kagaayi J, Serwadda D, et al. Neurodevelopmental benefits of antiretroviral therapy in Ugandan children aged 0–6 years with HIV. J Acquir Immune Defic Syndr. 2014;67:316–322. doi: 10.1097/QAI.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor BS, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;359:1965–1966. doi: 10.1056/NEJMc086373. [DOI] [PubMed] [Google Scholar]
- 7.Brenner BG, Oliveira M, Doualla-Bell F, Moisi DD, Ntemgwa M, Frankel F, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. Aids. 2006;20:F9–13. doi: 10.1097/01.aids.0000232228.88511.0b. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Cajas JL, Pant-Pai N, Klein MB, Wainberg MA. Role of genetic diversity amongst HIV-1 non-B subtypes in drug resistance: a systematic review of virologic and biochemical evidence. AIDS Rev. 2008;10:212–223. [PubMed] [Google Scholar]
- 9.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. Aids. 25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26:1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. This review article concludes that even with HAART treatment from an early age and with good medical care and support, there is still a high prevalence of developmental and psychosocial need among children with HIV, especially as they age through middle childhood into adolescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. The Pediatric Infectious Disease Journal. 2013 doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18555. doi: 10.7448/IAS.16.1.18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev. 2013;254:143–169. doi: 10.1111/imr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcotte TD, Deutsch R, McCutchan JA, Moore DJ, Letendre S, Ellis RJ, et al. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch Neurol. 2003;60:1406–1412. doi: 10.1001/archneur.60.10.1406. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Ramon S, Bellon JM, Resino S, Canto-Nogues C, Gurbindo D, Ramos JT, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics. 2003;111:E168–175. doi: 10.1542/peds.111.2.e168. [DOI] [PubMed] [Google Scholar]
- 17.Jennings C, Rich K, Siegel JN, Landay A. A phenotypic study of CD8+ lymphocyte subsets in infants using three-color flow cytometry. Clin Immunol Immunopathol. 1994;71:8–13. doi: 10.1006/clin.1994.1044. [DOI] [PubMed] [Google Scholar]
- 18.Kapetanovic S, Aaron L, Montepiedra G, Burchett SK, Kovacs A. T-cell activation and neurodevelopmental outcomes in perinatally HIV-infected children. AIDS. 2012;26:959–969. doi: 10.1097/QAD.0b013e328352cee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mekmullica J, Brouwers P, Charurat M, Paul M, Shearer W, Mendez H, et al. Early immunological predictors of neurodevelopmental outcomes in HIV-infected children. Clinical Infectious Diseases. 2009;48:338–346. doi: 10.1086/595885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson RJ, Slagter HA. Probing emotion in the developing brain: functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Ment Retard Dev Disabil Res Rev. 2000;6:166–170. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Galderisi S, Mucci A. Emotions, brain development, and psychopathologic vulnerability. CNS Spectr. 2000;5:44–48. doi: 10.1017/s1092852900007537. [DOI] [PubMed] [Google Scholar]
- 22.Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- 23.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses inthe aged: scientific bases for a novel intervention. In: Moller AR, editor. Progress in Brain Research. Amsterdam: Elsevier B.V; 2006. pp. 81–109. [DOI] [PubMed] [Google Scholar]
- 24.Bonnier C. Evaluation of early stimulation programs for enhancing brain development. Acta Paediatr. 2008;97:853–858. doi: 10.1111/j.1651-2227.2008.00834.x. [DOI] [PubMed] [Google Scholar]
- 25.Klein P, Rye H. Interaction-Oriented Early Intervention in Ethiopia: the MISC Approach. Infants and Young Children. 2004:17. [Google Scholar]
- 26.Klein PS. Garland Reference Library of Social Science. New York, NY: Garland Press; 1996. Early Intervention: cross-cultural experiences with a mediational approach. [Google Scholar]
- 27.Klein PS. Seeds of hope: twelve years of early intervention in Africa. Oslo, Norway: unipub forlag; 2001. [Google Scholar]
- 28.Armstrong F, Willen E, Surgen K. Handbook of Pediatric Psychology. 3. 2003. HIV/AIDS in Children and Adolescents; pp. 359–374. [Google Scholar]
- 29.O’Hare BA, Venables J, Southall D. Child health in Africa: 2005 a year of hope? Arch Dis Child. 2005;90:776–781. doi: 10.1136/adc.2004.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bass E. The two sides of PEPFAR in Uganda. Lancet. 2005;365:2077–2078. doi: 10.1016/S0140-6736(05)66717-7. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. ABC in Uganda: success or subterfuge. HIV AIDS Policy and Law Review. 2005;10:23–24. [PubMed] [Google Scholar]
- 32.Nyamukapa CA, Gregson S, Lopman B, Saito S, Watts HJ, Monasch R, et al. HIV-Associated Orphanhood and Children’s Psychosocial Distress: Theoretical Framework Tested With Data From Zimbabwe. American Journal of Public Health. 2008;98:133–141. doi: 10.2105/AJPH.2007.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose S. ProQuest Information & Learning, Jul 1997. AAM9719709. 1997. An examination of adaptive functioning in HIV infected children: Exploring the relationships with HIV disease, neurocognitive functioning, and psychosocial characteristics; p. 409. Dissertation Abstracts International: Section B: The Sciences and Engineering. [Google Scholar]
- 34.UNAIDS. UNAIDS Report on the global AIDS epidemic/2010. Joint United Nations Programmes on HIV/AIDS; 2010. [Google Scholar]
- 35.Ronald AR, Sande MA. HIV/AIDS care in Africa today. Clin Infect Dis. 2005;40:1045–1048. doi: 10.1086/428360. [DOI] [PubMed] [Google Scholar]
- 36.Caruso N. Refuge from the Lord’s Resistance Army in Uganda: a report from a Medecins Sans Frontieres team leader. Emerg Med Australas. 2006;18:295–298. doi: 10.1111/j.1742-6723.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 37.Fabiani M, Nattabi B, Opio AA, Musinguzi J, Biryahwaho B, Ayella EO, et al. A high prevalence of HIV-1 infection among pregnant women living in a rural district of north Uganda severely affected by civil strife. Trans R Soc Trop Med Hyg. 2006;100:586–593. doi: 10.1016/j.trstmh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Orach CG, De Brouwere V. Integrating refugee and host health services in West Nile districts, Uganda. Health Policy Plan. 2006;21:53–64. doi: 10.1093/heapol/czj007. [DOI] [PubMed] [Google Scholar]
- 39.Foster G, Williamson J. A review of current literature on the impact of HIV/AIDS on children in sub-Saharan Africa. Aids. 2000;14(Suppl 3):S275–284. [PubMed] [Google Scholar]
- 40.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54:1001–1009. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamau JW, Kuria W, Mathai M, Atwoli L, Kangethe R. Psychiatric morbidity among HIV-infected children and adolescents in a resource-poor Kenyan urban community. AIDS Care. 2012;24:836–842. doi: 10.1080/09540121.2011.644234. [DOI] [PubMed] [Google Scholar]
- 43.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Msellati P, Lepage P, Hitimana DG, Van Goethem C, Van de Perre P, Dabis F. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics. 1993;92:843–848. [PubMed] [Google Scholar]
- 45.Bunupuradah T, Kosalaraksa P, Vibol U, Hansudewechakul R, Sophonphan J, Kanjanavanit S, et al. Impact of antiretroviral therapy on quality of life in HIV-infected Southeast Asian children in the PREDICT study. AIDS Patient Care STDS. 2013;27:596–603. doi: 10.1089/apc.2013.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musisi S, Kinyanda E. Emotional and behavioural disorders in HIV seropositive adolescents in urban Uganda. East Afr Med J. 2009;86:16–24. doi: 10.4314/eamj.v86i1.46923. [DOI] [PubMed] [Google Scholar]
- 47.Scharko AM. DSM psychiatric disorders in the context of pediatric HIV/AIDS. AIDS Care. 2006;18:441–445. doi: 10.1080/09540120500213487. [DOI] [PubMed] [Google Scholar]
- 48.Benton TD. Psychiatric considerations in children and adolescents with HIV/AIDS. Child Adolesc Psychiatr Clin N Am. 2010;19:387–400. x. doi: 10.1016/j.chc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Benton TD. Treatment of psychiatric disorders in children and adolescents with HIV/AIDS. Curr Psychiatry Rep. 2010;12:104–110. doi: 10.1007/s11920-010-0092-z. [DOI] [PubMed] [Google Scholar]
- 50.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 52.Foster C, Fidler S. Optimizing antiretroviral therapy in adolescents with perinatally acquired HIV-1 infection. Expert Rev Anti Infect Ther. 2010;8:1403–1416. doi: 10.1586/eri.10.129. [DOI] [PubMed] [Google Scholar]
- 53.Ciaranello AL, Chang Y, Margulis AV, Bernstein A, Bassett IV, Losina E, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 55.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc. 2013;16:18593. doi: 10.7448/IAS.16.1.18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.UNAIDS. UNAIDS Report on the global AIDS epidemic/2010. Joint United Nations Report on HIV/AIDS 2013; 2013. [Google Scholar]
- 57.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 59.Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feuerstein R. Instrumental enrichment: Redevelopment of cognitive functions of retarded performers. New York: University Park Press; 1980. [Google Scholar]
- 61.Vygotsky LS. Mind in Society: The Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 62.Feurerstein R. Then Dynamic Assessment of Retarded Performers. 1979. [Google Scholar]
- 63.Feurerstein R. Instrumental Enrichment: Redevelopment of Cognitive Functions of Retarded Performers. 1980. [Google Scholar]
- 64**.Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, et al. A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr. 2013;163:1409–1416. doi: 10.1016/j.jpeds.2013.06.055. This publication is the first to document that a caregiver training program can significantly enhance neurodevelopmental outcomes in African children with HIV. This study also documents that such a program can improve mental health outcomes in the caregivers and survival rates among younger children with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grantham-McGregor S, Schofield W, Harris L. Effect of psychosocial stimulation on mental development of severely malnourished children: an interim report. Pediatrics. 1983;72:239–243. [PubMed] [Google Scholar]
- 66.Grantham-McGregor S, Schofield W, Powell C. Development of severely malnourished children who received psychosocial stimulation: six-year follow-up. Pediatrics. 1987;79:247–254. [PubMed] [Google Scholar]
- 67.Grantham-McGregor S, Stewart ME, Schofield WN. Effect of long-term psychosocial stimulation on mental development of severely malnourished children. Lancet. 1980;2:785–789. doi: 10.1016/s0140-6736(80)90395-5. [DOI] [PubMed] [Google Scholar]
- 68.Klein PS, Rye H. Interaction-oriented early intervention in Ethiopia: the MISC approach. Infants and Young Children. 2004;17:340–354. [Google Scholar]
- 69.Feuerstein R. The dynamic assessment of retarded performers. New York: University Park Press; 1979. [Google Scholar]
- 70.Klein P. Early Intervention: Cross-cultural experiences with a mediational approach. New York, NY: Garland Press; 1996. [Google Scholar]
- 71.Klein PS. More Intelligent and Sensitive Child. Ramat-Gan, Israel: Bar-Ilan University; 1985. [Google Scholar]
- 72.Minde KK. Psychological problems in Ugandan school children: a controlled evaluation. Journal of Child Psychology and Psychiatry. 1975;16:49–59. doi: 10.1111/j.1469-7610.1975.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 73.Bass JK, Nakasujja N, Familiar-Lopez I, Sikorskii A, Murray SM, Opoka R, et al. Association of caregiver quality of care with neurocognitive outcomes in HIV-affected children aged 2–5 years in Uganda. AIDS Care. 2016 doi: 10.1080/09540121.2016.1146215. http://dx.doi.org/10.1080/09540121.2016.1146215:1-8. [DOI] [PMC free article] [PubMed]
- 74.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 76.Rabiner DL, Murray DW, Skinner AT, Malone PS. A Randomized Trial of Two Promising Computer-Based Interventions for Students with Attention Difficulties. Journal of Abnormal Child Psychology. 2010:131–142. doi: 10.1007/s10802-009-9353-x. [DOI] [PubMed] [Google Scholar]
- 77.Koekkoek S, de Sonneville LMJ, Wolfs RFW, Licht R, Greelen SPM. Neurocognitive function profile in HIV-infected school-age children. European Journal of Paediatric Neurology. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Dev Neuropsychol. 2006;30:633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- 79.Bisiacchi PS, Suppiej A, Laverda A. Neuropsychological evaluation of neurologically asymptomatic HIV-infected children. Brain Cogn. 2000;43:49–52. [PubMed] [Google Scholar]
- 80.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Tardieu M, Mayaux MJ, Seibel N, Funck-Brentano I, Straub E, Teglas JP, et al. Cognitive assessment of school-age children infected with maternally transmitted human immunodeficiency virus type 1. J Pediatr. 1995;126:375–379. doi: 10.1016/s0022-3476(95)70451-5. [DOI] [PubMed] [Google Scholar]
- 82.Boivin MJ, Ruel TD, Boal HE, Bangirana P, Cao H, Eller LA, et al. HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS. 2010;24:1163–1170. doi: 10.1097/qad.0b013e3283389dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83**.Bangirana P, Boivin MJ, Giordani B. Computerized Cognitive Rehabilitation Therapy (CCRT) for African Children: Evidence for Neuropsychological Benefit and Future Directions. In: Boivin MJ, Giordani B, editors. Neuropsychology of Children in Africa: Perspectives on Risk and Resilience. New York: Springer Science+Business Media; 2013. pp. 277–298. This review chapter is the first to document the evidence supporting the neuropsychological benefit of computerized cognitive rehabilitation training (CCRT) with African children with HIV and those surviving severe malaria. This review also considers strategies for scaling such interventions to the community level. [Google Scholar]
- 84.Lowenthal ED, Marukutira TC, Chapman J, Mokete K, Riva K, Tshume O, et al. Psychosocial assessments for HIV+ African adolescents: establishing construct validity and exploring under-appreciated correlates of adherence. PLoS ONE. 9:e109302. doi: 10.1371/journal.pone.0109302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 14:627–639. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cowan F, Pettifor A. HIV in adolescents in sub-Saharan Africa. Curr Opin HIV AIDS. 2009;4:288–293. doi: 10.1097/COH.0b013e32832c7d10. [DOI] [PubMed] [Google Scholar]
- 87.Engle PL, Black MM, Behrman JR, Cabral de Mello M, Gertler PJ, Kapiriri L, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 88.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 89.Boivin MJ, Bangirana P, Tomac R, Parikh S, Opoka RO, Nakasujja N, et al. Neuropsychological benefits of computerized cognitive rehabilitation training in Ugandan children surviving cerebral malaria and children with HIV. BMC Proceedings. 2008;2:P7. [Google Scholar]
- 90.Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, et al. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24:667–673. doi: 10.1037/a0019312. [DOI] [PubMed] [Google Scholar]
- 91.Boivin MJ, Giordani B. Neuropsychological assessment of African children: evidence for a universal basis to cognitive ability. In: Chiao JY, editor. Cultural Neuroscience: Cultural Influences on Brain Function. New York, NY: Elsevier Publications; 2009. pp. 113–135. [DOI] [PubMed] [Google Scholar]
- 92.Boivin MJ, Nakasujja N, Sikorskii A, Opoka RO, Giordani B. A Randomized Controlled Trial to Evaluate if Computerized Cognitive Rehabilitation Improves Neurocognition in Ugandan Children with HIV. AIDS Res Hum Retroviruses. 2016;32:743–55. doi: 10.1089/aid.2016.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giordani B, Novak B, Sikorskii A, Bangirana P, Nakasujja N, Winn BM, et al. Designing and evaluating Brain Powered Games for cognitive training and rehabilitation in at-risk African children. Global Mental Health. 2015;2:1–14. doi: 10.1017/gmh.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]