Abstract
BACKGROUND
Cardiac allograft vasculopathy (CAV) is a major cause of mortality after cardiac transplantation. High-density lipoprotein (HDL) cholesterol efflux capacity (CEC) is inversely associated with coronary artery disease. In 2 independent studies, we tested the hypothesis that reduced CEC is associated with mortality and disease progression in CAV.
METHODS
We tested the relationship between CEC and survival in a cohort of patients with CAV (n = 35). Multivariable Cox proportional hazard models demonstrated that higher levels of CEC were associated with improved survival (hazard ratio 0.26, 95% confidence interval 0.11 to 0.63) per standard deviation CEC, p = 0.002). To determine whether reduced CEC is associated with CAV progression, we utilized samples from the Clinical Trials in Organ Transplantation 05 (CTOT05) study to determine the association between CEC and CAV progression and status at 1 year (n = 81), as assessed by average change in maximal intimal thickness (MIT) on intravascular ultrasound.
RESULTS
Patients who developed CAV had reduced CEC at baseline and 1-year post-transplant. We observed a significant association between pre-transplant CEC and the average change in MIT, particularly among patients who developed CAV at 1 year (β = −0.59, p = 0.02, R2 = 0.35).
CONCLUSION
Reduced CEC is associated with disease progression and mortality in CAV patients. These findings suggest the hypothesis that interventions to increase CEC may be useful in cardiac transplant patients for prevention or treatment of CAV.
Keywords: cardiac allograft vasculopathy, cholesterol efflux capacity, high-density lipoprotein, survival, transplantation
Despite advances in therapies for end-stage heart failure, cardiac transplantation offers the best long-term survival for selected patients.1,2 Although immunosuppressive therapies have improved survival post-transplant by decreasing acute rejection, cardiac allograft vasculopathy (CAV), a form of chronic rejection, remains a major cause of post-transplant mortality.
Several murine models have shown that pharmacologically or genetically raising apolipoprotein A-I (apoA-I), the main protein constituent of high-density lipoprotein (HDL), attenuates arteriosclerosis and chronic rejection of the cardiac allograft.3,4 Adenosine triphosphate (ATP)-binding cassette transporter (ABCA1) mediates cholesterol efflux to apoA-I, forming nascent HDL particles.5 Although the causal relationship of high-density lipoprotein cholesterol (HDL-C) mass to atherosclerotic cardiovascular disease has come into question,6–9 the importance of HDL cholesterol efflux capacity (CEC) is increasingly recognized.10 Our group and others have demonstrated an inverse association between CEC and coronary disease.11,12 In the transplant population, 2 studies have demonstrated that the CEC was impaired compared with healthy controls with native hearts.13,14 Based on the available animal and human data, we hypothesized that increased CEC is protective and thus would be associated with improved survival and decreased CAV in cardiac transplant recipients.
Methods
Study population
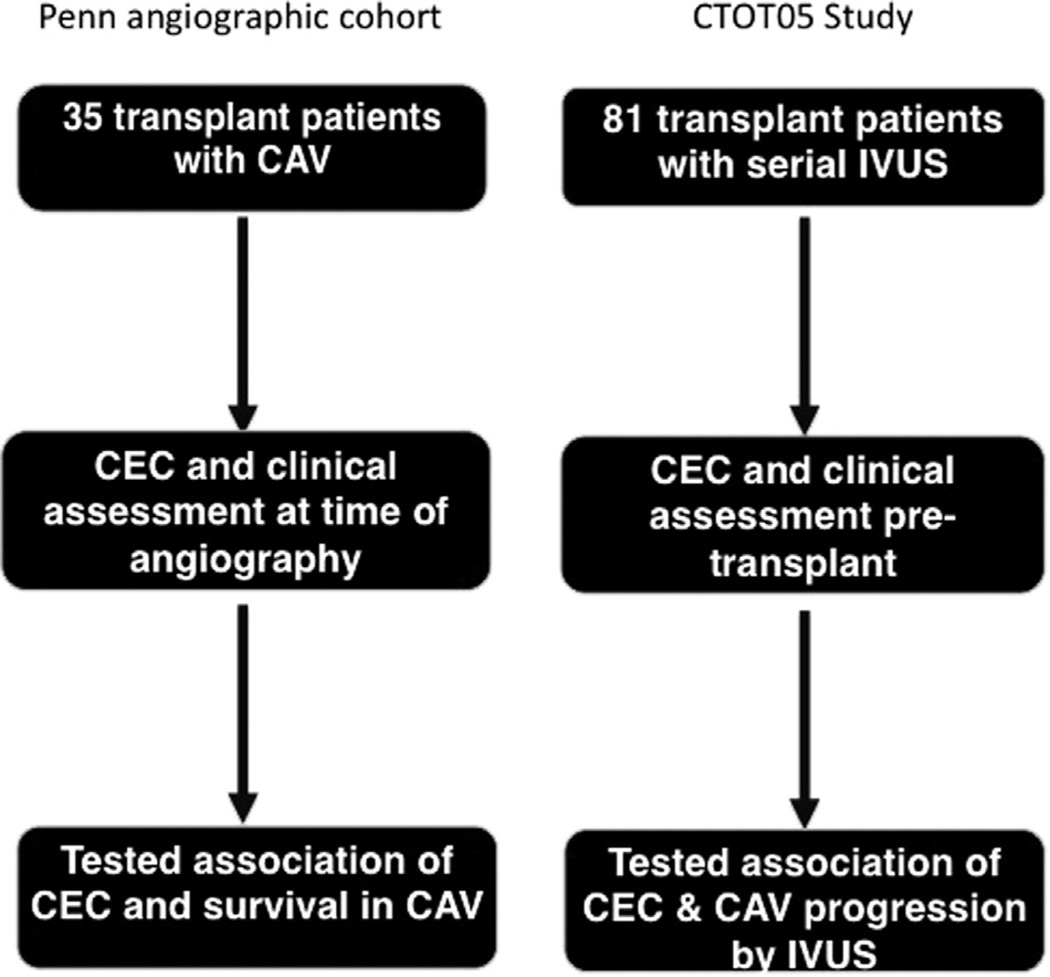
We utilized a cohort of patients recruited from our cardiac catheterization lab to test our initial hypothesis that CEC is related to survival in cardiac transplant recipients with CAV. We performed a second, independent study using samples from the Clinical Trials in Organ Transplantation 05 (CTOT05) study15 to test the hypothesis that decreased pre-transplant CEC is associated with CAV progression (see Figure 1 for flowchart detailing study designs).
Figure 1.
Study design of the Penn transplant and CTOT05 cohorts. In the Penn angiographic cohort, 35 patients with CAV had CEC measured at time of the catheterization. We tested the relationship between CEC and survival. In the CTOT05, 81 subjects had samples available for CEC assays and paired IVUS assessment of CAV. We tested the relationship between CAV status and progression, as assessed by IVUS, and CEC.
CAV cohort
At our center, we have an ongoing observational study16,17 in which patients are enrolled from the cardiac catheterization laboratory with banking of blood samples for later analysis. From this cohort we identified every transplant patient who had a banked sample between January 2009 and December 2012 and confirmed CAV status by blinded review of angiography by an interventional cardiologist (S.L.). Although patients with early CAV were not intentionally excluded, we did not identify any patients with early-stage or suspected CAV with this methodology, which is unsurprising given the low yield of angiography for capturing patients with CAV.18 CAV severity was adjudicated based on standard definitions as follows of moderate (International Society for Heart and Lung Transplantation [ISHLT] CAV2) or severe (ISHLT CAV3) vasculopathy (≥50% left main or ≥70% obstruction of at least 1 coronary vessel). Cyclosporine and tacrolimus levels were measured on the date of catheterization, and glomerular filtration rate (GFR) was calculated by the GFR EPI formula. Other clinical variables were evaluated by chart review at the time of study enrollment.
CTOT05 cohort
CTOT05 was a multicenter, observational study of 200 recipients of first cardiac allografts followed for 1 year (principal investigator: P. Heeger). The study design and results were recently reported.15 To determine the relationship between CEC and CAV progression, we measured CEC before transplant and 1 year post-transplant in subjects in whom paired intravenous ultrasound (IVUS) data and blood samples were available (n = 81). CAV was adjudicated by IVUS performed at baseline and 1 year post-transplant with measurement of the maximal intimal thickness (MIT) and total atheroma volume (TAV) for each patient. Briefly, for each coronary artery segment, the site of MIT was identified at baseline and 1 year of the study to yield a pair of measurements. The maximal change in MIT within each segment (1 year minus the baseline) was calculated for each patient. CAV was identified when at least 1 site demonstrated an increase of >0.5 mm in MIT from baseline to 1-year measurement.
Cholesterol efflux assays
Subjects were fasting at the time of cardiac catheterization. Informed consent was obtained from each patient and both studies were approved by the institutional review board of the University of Pennsylvania. Subject samples were thawed for determination of CEC, serum lipids and apolipoproteins from the same sample. Our CEC assay has been reported previously, and has a coefficient of variation of <5%.11,19 Briefly, 250,000 murine J774 macrophages were plated and allowed to attach for 6 hours before being radiolabeled for 16 hours with 2 µCi of 3H-cholesterol per milliliter. Cells were then treated with 0.3 mmol/liter 8-(4-chlorophenylthio)-cyclic AMP for 4 hours to up-regulate ABCA1. For the CTOT05, CEC assays were performed in the same manner, but additional assays were performed with probucol to inhibit ABCA1. Subsequently, efflux media containing 1.0% apolipoprotein B–depleted serum were added for 2 hours. Radioactivity in the media and remaining radioactivity in the cells after extraction were determined. CEC was calculated by dividing the counts in the media by total counts in the media and the cells. Assays were normalized by dividing the CEC of each individual patient by the CEC of a serum pool (or plasma for CTOT05 samples) run with each plate. Thus, CEC is expressed as a unitless ratio with 1.0 representing CEC equal to a pool of healthy controls, with values <1.0 representing lower-than-average CEC. The normalization of CEC to a pool of healthy controls allows comparisons to be made across assays.11
Statistical analysis
Categorical variables are presented as frequency and percent, and continuous variables as mean and standard deviation. To assess the association between CEC and survival, Cox proportional hazard models were constructed and adjusted for HDL-C,12 as well as baseline clinical variables with p ≤ 0.2 (listed in Table 1). Variables with biologic plausibility, such as medications, were also added back to the final model to test residual confounding. Survival time in Cox models was time to death or last available follow-up from study entry (the date the lab sample was obtained). Proportional hazard assumptions were verified. Among the CTOT05 sample, patient characteristics were compared between the CAV and no-CAV groups using the chi-square test or Fisher’s exact test for categorical variables and Student’s 2-sample t-test for continuous variables, and logistic regression models were constructed to examine factors associated with CAV. Linear regression analysis was used to assess the association between baseline CEC and IVUS progression of CAV as measured by the changes in MIT and TAV from baseline to 1 year. Assumptions of linearity were verified via kernel density and predicted-vs-residual plots. Baseline MIT and TAV values were log10-transformed due to violations of normality. All tests were 2-sided with p < 0.05 considered statistically significant. Analyses were performed using statistical software (STATA, version 13.1; StataCorp LP, College Station, TX).
Table 1.
Clinical Variables With HR (95% CI) From Cox Survival Analysis
| Variable | Alive (n = 20) | Dead (n = 15) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Age at transplant | 42.8 ± 2.9 | 46.5 ± 4.2 | 1.02 (0.99 to 1.07) | 0.20 |
| Male | 100% | 66.6% | 0.18 (0.05 to 0.57) | 0.004 |
| White race | 65% | 74.4% | 1.02 (0.32 to 3.30) | 0.973 |
| ICM (pre-transplant) | 25% | 46.6% | 3.2 (1.06 to 9.85) | 0.04 |
| Years from transplant | 11.5 ± 1.3 | 9.7 ± 1.2 | 0.95 (0.87 to 1.04) | 0.30 |
| Hypertension | 95% | 93.3% | 0.71 (0.09 to 5.6) | 0.75 |
| Hemoglobin A1C | 6.7 ± 0.5 | 6.8 ± 0.5 | 1.06 (0.78 to 1.47) | 0.69 |
| Diabetes | 50% | 53.3% | 0.99 (0.36 to 2.77) | 0.99 |
| BMI >30 kg/m2 | 80% | 80% | 1.23 (0.34 to 4.5) | 0.75 |
| Rejection | 75% | 60% | 0.44 (0.15 to 1.34) | 0.15 |
| GFR | 56.1 ± 5.7 | 56.8 ± 7.4 | 1.0 (0.98 to 1.02) | 0.92 |
| LDL-C (per SD) | 101.9 ± 8.6 | 80.2 ± 7.8 | 0.64 (0.31 to 1.27) | 0.20 |
| HDL-C | 48.2 ± 2.8 | 44.3 ± 3.5 | 1.03 (0.58 to 1.80) | 0.931 |
| Actively smoking | 5% | 6.6% | 2.32 (0.29 to 18.7) | 0.43 |
| Statin use | 100% | 100% | Omitted | |
| Cyclosporine | 45% | 27% | 0.52 (0.17 to 1.64) | 0.27 |
| Cyclosporine level (ng/ml) | 84.4 ± 8.4 | 79.5 ± 16.4 | 0.97 (0.93 to 1.03) | 0.38 |
| Tacrolimus | 55% | 64% | 1.92 (0.61 to 1.05) | 0.27 |
| Tacrolimus level (ng/ml) | 6.7 ± 5.7 | 5.3 ± 0.4 | 0.07 (0.52 to 1.26) | 0.35 |
| MMF | 45% | 46.6% | 1.18 (0.43 to 1.26) | 0.75 |
| Prednisone | 40% | 46.6% | 1.22 (0.44 to 3.4) | 0.70 |
| Rapamycin | 40% | 33% | 1.14 (0.37 to 3.48) | 0.82 |
| Azathioprine | 10% | 13% | 1.12 (0.25 to 5.01) | 0.89 |
BMI, body mass index; CI, confidence interval; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; ICM, ischemic cardiomyopathy; LDL-C, low-density lipoprotein cholesterol; MMF, mycophenolate mofetil; SD, standard deviation.
Results
Cholesterol efflux capacity and survival in CAV patients
We identified 35 patients at our center with CAV. Table 1 provides clinical and demographic characteristics, including hazard ratio (HR) and 95% confidence interval (CI), derived from univariate Cox survival analysis. Mean length of follow-up from study entry among CAV patients was 931 ± 97 days for survivors and 630 ± 131 days for non-survivors. Of these 35 patients, 22 were treated with tacrolimus and 13 were treated long term with cyclosporine (p = 0.27).
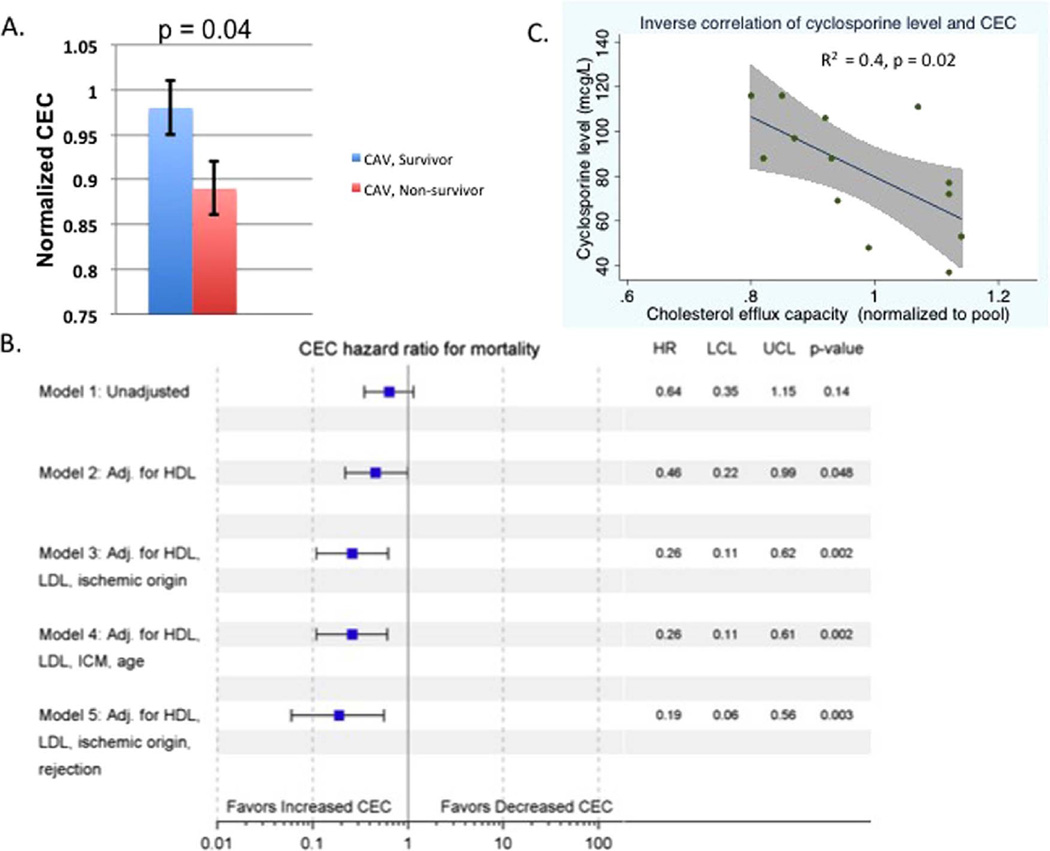
Mean CEC was significantly lower in patients with CAV who died compared with survivors (0.89 ± 0.03 vs 0.98 ± 0.03, respectively, p = 0.04; Figure 2A). Cox proportional hazard models showed that CEC was associated with survival when adjusting for HDL-C (HR 0.46 per standard deviation change in CEC, 95% CI 0.22 to 0.99, p = 0.048; Figure 2B) and after adjustment for age, history of ischemic cardiomyopathy, HDL-C and low-density lipoprotein cholesterol (LDL-C) (HR 0.26 per standard deviation CEC, 95% CI 0.11 to 0.63, p = 0.002; Figure 2B). Of the 15 patients who died, we identified 8 who died due to complications of CAV. Sensitivity analysis, excluding patients who died of other causes, reproduced the association between CEC and survival (n = 28). Because there were only 5 female patients with CAV in our study, we performed sensitivity analysis in males only and found that CEC remained significantly associated with survival (HR 0.28, 95% CI 0.08 to 0.93, p = 0.04) in our adjusted model. Furthermore, the inclusion of other clinical variables, such as race, number of rejection episodes, hemoglobin A1C, diabetes status, obesity, GFR, time elapsed since transplant and history of cytomegalovirus (CMV) viremia, did not affect the relationship between CEC and survival in our adjusted Cox proportional hazard model.
Figure 2.
CEC capacity is lower in patients with CAV who died compared to survivors with CAV. (A) CEC in patients with CAV who died compared with survivors. (B) Forest plot demonstrating hazardratio (HR) and upper and lower confidence limits (UCL and LCL, respectively) for CEC in a Cox survival analysis. (C) Scatterplot of CEC capacity versus cyclosporine dose with regression line and 95% confidence estimate around regression line.
As cyclosporine has been shown to inhibit ABCA1,20 we tested the relationship between cyclosporine levels, CEC and survival. There was a significant inverse correlation between cyclosporine levels on the day of catheterization and CEC (β = −0.02, R2 = 0.4, p = 0.02; Figure 2C). However, CEC was not significantly associated with tacrolimus dose and, in sensitivity analysis of 22 patients treated with tacrolimus, CEC remained significantly associated with survival time (HR 0.26, 95% CI 0.09 to 0.77, p = 0.02). Each immunosuppressive agent was systemically added to our Cox proportional hazard models (see Table S1 in Supplementary Material available online at www.jhltonline.org/). All models demonstrated a protective effect of CEC in relation to survival and, when both prednisone and calcineurin inhibitor (CNI) were included, there was no change in the HR between CEC and survival compared to a model with neither CNI nor prednisone.
CEC is independently associated with CAV
The aforementioned findings support the hypothesis that reduced CEC is a risk factor for mortality in transplant patients with CAV, yet previous cross-sectional data in transplant patients suggest no difference in CEC between those with and without CAV as adjudicated by angiography.14 To resolve this apparent paradox, we hypothesized that reduced CEC is associated with CAV progression and status as measured by changes in MIT on IVUS, a marker of poor prognosis in CAV and cardiac transplant. To test this hypothesis, we measured CEC both pre-transplant and at 1 year post-transplant in a cohort of CTOT05 subjects with available paired plasma samples and diagnostic IVUS exams performed shortly after transplant and at 1 year after transplant (n = 81). Change in average MIT as measured by IVUS was 0.17 ± 0.04 mm in patients who met the pre-specified IVUS end-point for CAV (>0.5 mm change in MIT by IVUS, n = 15) compared with 0.03 ± 0.01 mm in those who did not (n = 66, p < 0.001; Table 2).
Table 2.
Baseline Characteristics of Patients in the CTOT05 Study
| Variable | No CAV (n = 66) |
CAV (n = 15) |
p-value |
|---|---|---|---|
| Change in mean MIT (mm) |
0.03 ± 0.01 | 0.17 ± 0.04 | <0.001 |
| Male sex | 74% | 93% | 0.17 |
| White race | 74% | 80% | 0.75 |
| Diabetes | 32% | 40% | 0.56 |
| BMI | 27.6 ± 1.3 | 25.6 ± 0.6 | 0.18 |
| Rejection | 14% | 7% | 0.74 |
| Total cholesterol (mg/dl) |
138 ± 12.7 | 139 ± 5.2 | 0.95 |
| HDL (mg/dl) | 49 ± 2.8 | 39 ± 2.9 | 0.1 |
| LDL (mg/dl) | 76 ± 3.8 | 83 ± 9.4 | 0.48 |
| Triglyceride (mg/dl) | 77 ± 4.2 | 85 ± 11.7 | 0.51 |
BMI, body mass index; CAV, cardiac allograft vasculopathy; CTOT05, Clinical Trials in Organ Transplantation 05; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MIT, maximal intimal thickness.
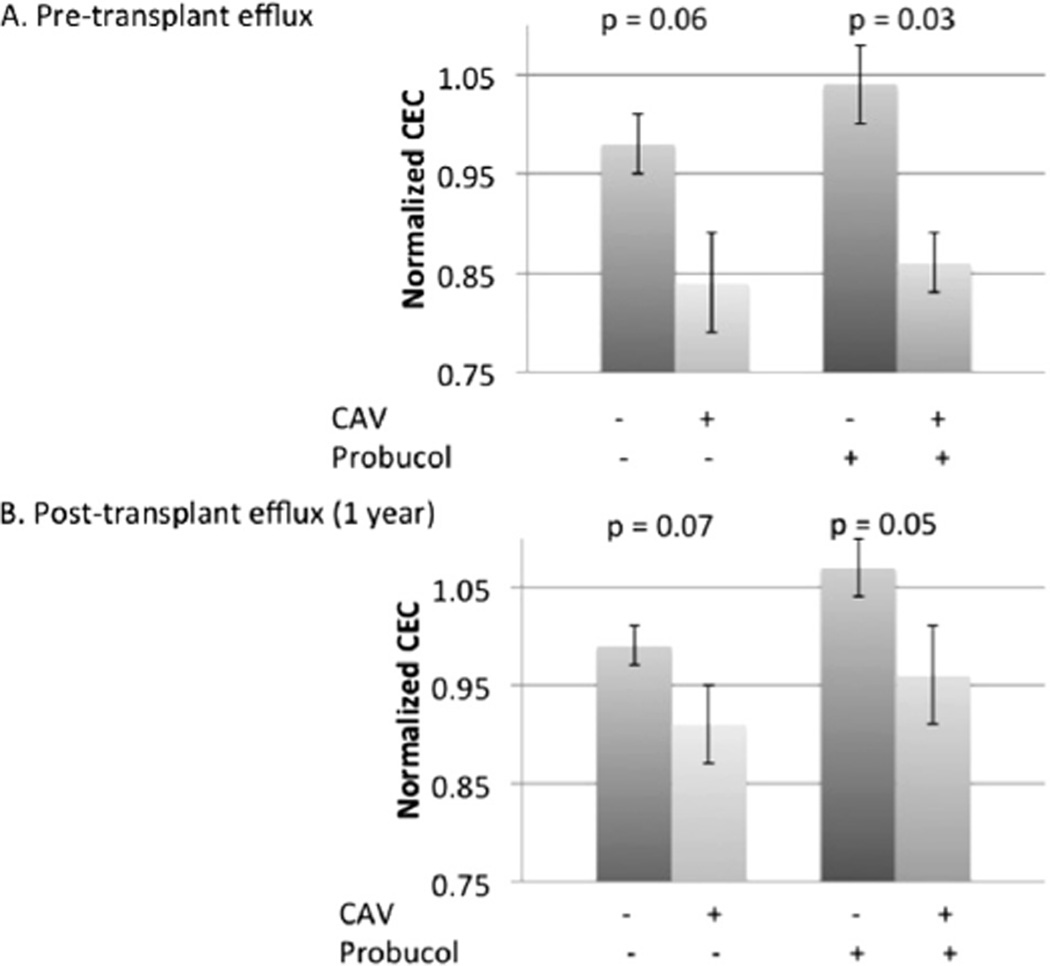
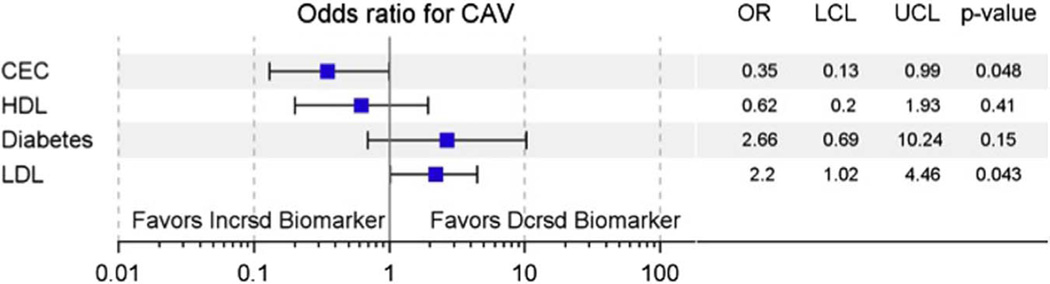
Pre-transplant basal CEC was significantly lower in subjects who met the CAV end-point compared with those who did not (0.86 ± 0.03 vs 1.04 ± 0.04, respectively, p = 0.03, in presence of probucol; 0.84 ± 0.05 vs 0.97 ± 0.03, p = 0.06, in absence of probucol; Figure 3). Both findings persisted at 1 year. Pre-transplant HDL also trended lower in CAV patients (49 ± 2.8 mg/dl vs 39 ± 2.9 mg/dl, p = 0.1; Table 2). In multivariate logistic regression evaluating metabolic factors associated with CAV, including LDL, HDL, diabetes status and CEC, CEC was independently associated with CAV, and LDL was positively associated with CAV (OR 0.35, 95% CI 0.13 to 0.99, p = 0.048 per standard deviation CEC; OR 2.14, 95% CI 1.02 to 4.46, p = 0.043 per standard deviation LDL; Figure 4). Inclusion of clinical variables, such as recipient and donor CMV antibody status, also strengthened the relationship between CEC and CAV status.
Figure 3.
CEC is significantly decreased before transplant in patients who met pre-specified intravascular ultrasound criteria for CAV versus those who did not. (A) CEC before transplant. (B) CEC at 1 year.
Figure 4.
CEC is independently associated with CAV as measured by IVUS. Forest plot depicting odds ratio (OR) with upper and lower confidence limits (UCL and LCL, respectively) for CEC, HDL-C, LDL-C and diabetes in a logistic regression model for CAV.
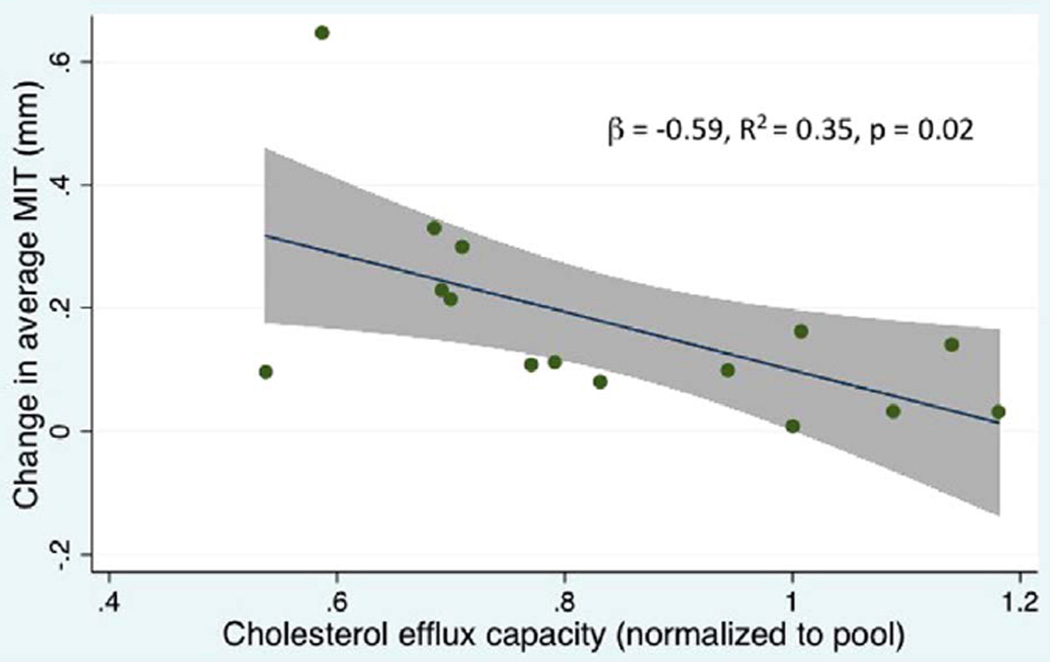
Furthermore, in the linear regression analysis, CEC showed a significant relationship with changes in MIT and total atheroma volume (TAV) in univariate analysis (data not shown) and after adjustment for baseline MIT or TAV, HDL-C or donor and recipient clinical characteristics, including HDL-C (Table 3). Among patients with CAV, there was a highly significant relationship between baseline CEC and change in average MIT (β = −0.59, R2 = 0.35, p = 0.02, n = 15; Figure 5). To our surprise, pre-transplant CEC was independently associated with baseline values of MIT and TAV early post-transplant in models adjusted for HDL-C and donor and recipient clinical characteristics (n = 81; Table 4).
Table 3.
CEC Association With Change in MIT or TAV as Measured by IVUS
| β for CEC | R2 | p-value | |
|---|---|---|---|
| MIT adjustment variables | |||
| Baseline MIT | −0.23 | 0.25 | 0.022 |
| HDL-C | −0.34 | 0.10 | 0.023 |
| Clinical characteristics | −0.29 | 0.21 | 0.047 |
| TAV adjustment variables | |||
| Baseline MIT | −0.25 | 0.18 | 0.019 |
| HDL-C | −0.39 | 0.10 | 0.009 |
| Clinical characteristics | −0.37 | 0.21 | 0.014 |
Standardized β-coefficients are presented for cholesterol efflux capacity (CEC), adjusted for baseline maximal intimal thickness (MIT) or total atheroma volume (TAV), high-density lipoprotein cholesterol (HDL-C) or additional clinical variables, including donor and recipient age, gender, race, cytomegalovirus (CMV) status and recipient diabetes. Clinical variables include donor and recipient age, gender, race, CMV status, recipient diabetes and HDL-C. IVUS, intravascular ultrasound.
Figure 5.
CEC is associated with progressive changes on intravascular ultrasound in CAV patients. Change in maximal intimal thickness over 1 year (y-axis) is plotted against normalized cholesterol efflux capacity (x-axis) with regression line and 95% confidence interval.
Table 4.
Pre-transplant CEC Associated With (Log-transformed) Baseline Post-transplant MIT (Top) or TAV (Bottom) as Measured by IVUS
| β for CEC | R2 | p-value | |
|---|---|---|---|
| MIT adjustment variables | |||
| HDL-C | −0.31 | 0.06 | 0.042 |
| Clinical characteristics | −0.29 | 0.30 | 0.044 |
| TAV adjustment variables | |||
| HDL-C | −0.32 | 0.06 | 0.03 |
| Clinical characteristics | −0.30 | 0.24 | 0.04 |
Standardized β-coefficients are presented for cholesterol efflux capacity (CEC), adjusted for high-density lipoprotein cholesterol (HDL-C) and additional clinical variables, including donor and recipient age, gender, race, cytomegalovirus (CMV) status and recipient diabetes. MIT, maximal intimal thickness; TAV, total atheroma volume.
Discussion
CAV is a major cause of morbidity and mortality in heart transplant recipients. Increased CEC reduces macrophage lipid accumulation and is associated with decreased cardiovascular events.12 The key findings of our work are that CEC was found to be associated with survival in CAV patients, independently of HDL-C and LDL-C levels, and that pre-transplant CEC was independently associated with CAV progression as adjudicated by IVUS. CEC was significantly reduced in subjects who developed rapidly progressive CAV and was associated with changes in MIT and TAV.
Although decreased CEC is associated with incident cardiovascular events in native vessel atherosclerosis,12 we observed statistically significant associations in smaller studies than what has been observed in the native coronary disease population. In contrast to studies of native coronary disease, where event rates are lower and disease progression is slower, studies in cardiac transplant recipients have demonstrated positive results with smaller sample sizes in shorter time periods.21 Using statins as a paradigm, initially small studies were reproduced by other larger studies,22,23 leading to the near universal adoption of statins in the treatment of cardiac transplant recipients. Thus, when considering our sample size in the context of the transplant literature, it is important to consider that the effects of lipid pathways may be heightened in transplant recipients compared to patients with native coronary atherosclerosis.
Furthermore, our study is consistent with a growing body of literature highlighting the importance of CEC in inflammatory vascular disease. In CAV, coronary plaque is largely non-calcified and lipid-rich, with significant macrophage accumulation24; this is similar to plaque composition in psoriasis patients, where reduced CEC was recently shown to be associated with non-calcified, lipid-rich plaque.25 The importance of increased CEC in diseases such as psoriasis and CAV may be related to decreased macrophage foam cells in plaque in the context of significant background inflammation. Furthermore, pre-transplant CEC was recently tied to graft failure in kidney transplant recipients.26 Given the similar pathophysiologic mechanisms in chronic organ rejection, CEC may be a therapeutic target after other organ transplants or in other inflammatory diseases, where cardiovascular disease remains a leading cause of mortality.25,27
Although a limitation of our study is the lack of a validation cohort of patients with advanced CAV, we observed associations between CEC with IVUS progression of CAV, a surrogate marker of poor outcomes in cardiac transplant recipients with CAV.28–30 Both the CTOT05 and our single-center experience support the concept that CEC impacts disease progression in CAV patients. The association of pre-transplant CEC with baseline measures of MIT and TAV is consistent with results from IVUS near-infrared spectroscopy studies, highlighting the role of early, rapid lipid accumulation in the pathogenesis of CAV.31,32 Furthermore, this finding suggests that studies on donor hearts performed early post-transplant may be influenced by recipient metabolic factors, and not solely reflective of donor disease.
Furthermore, the association between pre-transplant CEC and downstream CAV suggests that decreased CEC is less likely to be a consequence of immunosuppressive medications or generally poor health post-transplant. Nonetheless, we cannot rule out that medications may be modifying the relationship between CEC and survival. We have identified an inverse relationship between CEC and cyclosporine, a known inhibitor of ABCA1. The fact that CEC was reduced in CAV patients compared with controls, irrespective of the ABCA1 inhibitor probucol, suggests the potential relevance of both ABCA1-dependent and -independent pathways in CAV progression. As CEC remained significantly associated with mortality in CAV patients treated with tacrolimus, the effects of CEC on mortality in CAV patients are unlikely to be due solely to an effect of cyclosporine on CEC.
With regard to other factors that may influence the relationship between CEC and CAV outcomes, certain medications, such as mycophenolate mofetil (MMF) and rapamycin, are associated with dyslipidemia,33 but we did not observe any association between rapamycin or MMF use and CEC. Notably, all of our CAV patients were treated with statins, which may have modest efflux-lowering effects in patients with native coronary disease,34 but are known to be protective in CAV. Although our study was underpowered to detect possible effects of statin potency or patient compliance, CEC was associated with CAV independently of LDL-C levels, which should be reflective of statin use. Statins will remain the sine qua non of lipid-lowering therapy post-transplant, but our results add to the growing body of literature showing that lipid homeostasis is a critical mediator of allograft survival and suggest CEC as an alternative lipid pathway that could serve as a therapeutic target in CAV patients.
Given that an earlier cross-sectional study14 did not demonstrate an association between CEC and CAV, we tested the relationship between CEC and CAV in 2 different studies in an attempt to increase the generalizability of our findings. Although the use of a large, multicenter prospective trial with IVUS and clinical outcomes would be ideal, our study design allowed for analyses that would have been costly and time-consuming to perform prospectively. In our CAV cohort, CEC was measured at the time of clinical presentation, whereas, in the CTOT05, CEC was measured at specified time-points pre-transplant and 1 year post-transplant. The fact that 2 independent cohorts, at multiple time-points, suggested that CEC is significantly associated with disease status, progression or outcomes in CAV is remarkably consistent, particularly given the heterogeneity in care of transplant patients across many centers. Furthermore, differences in study design likely explain the discrepancies between our findings and those of Singh et al,14 who did not observe an association between CEC and CAV identified angiographically. First, our study employed IVUS progression to identify patients with CAV, which has greater sensitivity and specificity than angiography for CAV detection.32 It is likely that angiography misses individuals with milder CAV, who may be misclassified as negative.18 Second, our end-points included survival and progression of CAV, rather than solely cross-sectional association.
Although our study suggests a significant association between CEC and CAV, the mechanisms behind the increased mortality in CAV patients require further exploration. CAV progression, per se, may be one of the possible explanations, but other major targets to consider are the effects on the myocardium, including the possibility of increased arrhythmic death. In addition, certain clinical variables, such as ischemic time, donor characteristics and exact cause of death in every subject, were not available in either our single-center data or in the CTOT05, and the inability to adjust for these possible confounders is another limitation of our work. Finally, because the vast majority of patients who developed CAV were male, we were significantly underpowered to identify any differences related to recipient gender.
Most importantly, therapies that improve CEC, such as reconstituted HDL,35,36 apoA-I mimetic peptides or fibrates,37 may benefit cardiac transplant patients through both lipid and immune-modulating mechanisms. Prospective studies are needed to elucidate the specific mechanisms by which increasing CEC may protect this vulnerable group of patients.
Supplementary Material
Acknowledgments
D.J.R. is founder of Vascular Strategies. This work was supported by 5-R01-HL-11398 (Grant UL1TR000003) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and by funding from the Heart Failure Society of America. This work was also supported by the National Institute of Allergy and Infectious Diseases of the NIH (Grant U01AI063594 to P.H. and Grant AI063623 to M.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. A.J. was supported by 2T32-HL007843-16 and 5T32-HL007081-40. P. Z. received research and salary support from 5T32-HL007843-17 and UL1RR024134. We acknowledge the CTOT05 consortium for contributions to the overall success of the original CTOT05 study, which included: David A. Baran, Newark Beth Israel Medical Center, Newark, NJ; William Cotts, Northwestern University, Chicago, IL; Teresa De Marco, University of California at San Francisco, San Francisco, CA; Michael Givertz, Brigham and Women's Hospital, Boston, MA; Alain Heroux, Loyola University Medical Center, Maywood, IL; Judson Hunt, Medical City Dallas Hospital, Dallas, TX; A.G. Kfoury, Intermountain Medical Center, Murray, UT; Joren Madsen, Massachusetts General Hospital, Boston, MA; Richard N. Pierson III, University of Maryland, Baltimore, MD; Sean Pinney, Icahn School of Medicine at Mount Sinai, New York, NY; and Josef Stehlik, Craig H. Selzman, and Edward M. Gilbert, University of Utah Health Sciences Center, Salt Lake City, UT. We thank Nancy Bridges (NIH) for her contributions to overall program management; Yvonne Morrison (NIH) for her contributions to the overall project; and Ken Margulies, MD, and Abhinav Diwan, MD, for critical reading of the manuscript.
Footnotes
Disclosure statement
The other authors have no conflicts of interest to disclose.
Supplementary materials associated with this article can be found in the online version at www.jhltonline.org/.
References
- 1.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122:173–183. doi: 10.1161/CIRCULATIONAHA.109.858076. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh GR, Schnickel GT, Garcia C, et al. Inflammation/oxidation in chronic rejection: apolipoprotein a-i mimetic peptide reduces chronic rejection of transplanted hearts. Transplantation. 2007;84:238–243. doi: 10.1097/01.tp.0000268509.60200.ea. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, van Eck M, van Craeyveld E, et al. Critical role of scavenger receptor-BI-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo A-I transfer. Blood. 2009;113:755–764. doi: 10.1182/blood-2008-06-161794. [DOI] [PubMed] [Google Scholar]
- 5.Gelissen IC, Harris M, Rye KA, et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 6.HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt A, Rohatgi A. HDL cholesterol efflux capacity: cardiovascular risk factor and potential therapeutic target. Curr Atheroscler Rep. 2016;18:2. doi: 10.1007/s11883-015-0554-1. [DOI] [PubMed] [Google Scholar]
- 11.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sviridov D, Chin-Dusting J, Nestel P, et al. Elevated HDL cholesterol is functionally ineffective in cardiac transplant recipients: evidence for impaired reverse cholesterol transport. Transplantation. 2006;81:361–366. doi: 10.1097/01.tp.0000197556.83675.a6. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Jacobs F, Rader DJ, et al. Impaired cholesterol efflux capacity and vasculoprotective function of high-density lipoprotein in heart transplant recipients. J Heart Lung Transplant. 2014;33:499–506. doi: 10.1016/j.healun.2014.01.859. [DOI] [PubMed] [Google Scholar]
- 15.Starling RC, Stehlik J, Baran DA, et al. Multicenter analysis of immune biomarkers and heart transplant outcomes: results of the Clinical Trials in Organ Transplantation-05 Study. Am J Transplant. 2016;16:121–136. doi: 10.1111/ajt.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clague JR, Cox ID, Murday AJ, et al. Low clinical utility of routine angiographic surveillance in the detection and management of cardiac allograft vasculopathy in transplant recipients. Clin Cardiol. 2001;24:459–462. doi: 10.1002/clc.4960240608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagao K, Maeda M, Manucat NB, et al. Cyclosporine A and PSC833 inhibit ABCA1 function via direct binding. Biochim Biophys Acta. 2013;1831:398–406. doi: 10.1016/j.bbalip.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Ballantyne CM, Short BC, et al. Statin use and risks of death or fatal rejection in the Heart Transplant Lipid Registry. Am J Cardiol. 2005;95:367–372. doi: 10.1016/j.amjcard.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Kobashigawa JA, Moriguchi JD, Laks H, et al. Ten-year follow-up of a randomized trial of pravastatin in heart transplant patients. J Heart Lung Transplant. 2005;24:1736–1740. doi: 10.1016/j.healun.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Kotani J, Murata Y, et al. Tissue characterization of progressive cardiac allograft vasculopathy in patients with everolimus therapy compared with donor-transmitted atherosclerosis assessed using serial intravascular imaging: a case report. Transplant Proc. 2014;46:2456–2461. doi: 10.1016/j.transproceed.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Salahuddin T, Natarajan B, Playford MP, et al. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J. 2015;36:2662–2666. doi: 10.1093/eurheartj/ehv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annema W, Dikkers A, de Boer JF, et al. HDL cholesterol efflux predicts graft failure in renal transplant recipients. J Am Soc Nephrol. 2015;27:595–603. doi: 10.1681/ASN.2014090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riella LV, Gabardi S, Chandraker A. Dyslipidemia and its therapeutic challenges in renal transplantation. Am J Transplant. 2012;12:1975–1982. doi: 10.1111/j.1600-6143.2012.04084.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobashigawa JA, Tobis JM, Starling RC, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Mehra MR, Ventura HO, Stapleton DD, et al. The prognostic significance of intimal proliferation in cardiac allograft vasculopathy: a paradigm shift. J Heart Lung Transplant. 1995;14(suppl):S207–S211. [PubMed] [Google Scholar]
- 30.Mehra MR, Ventura HO, Stapleton DD, et al. Presence of severe intimal thickening by intravascular ultrasonography predicts cardiac events in cardiac allograft vasculopathy. J Heart Lung Transplant. 1995;14:632–639. [PubMed] [Google Scholar]
- 31.Zheng B, Maehara A, Mintz GS, et al. In vivo comparison between cardiac allograft vasculopathy and native atherosclerosis using near-infrared spectroscopy and intravascular ultrasound. Eur Heart J Cardiovasc Imaging. 2015;16:985–991. doi: 10.1093/ehjci/jev017. [DOI] [PubMed] [Google Scholar]
- 32.Javaheri A, Saha N, Lilly SM. How to approach the assessment of cardiac allograft vasculopathy in the modern era: review of invasive imaging modalities. Curr Heart Fail Rep. 2016;13:86–91. doi: 10.1007/s11897-016-0283-y. [DOI] [PubMed] [Google Scholar]
- 33.Spinelli GA, Felipe CR, Park SI, et al. Lipid profile changes during the first year after kidney transplantation: risk factors and influence of the immunosuppressive drug regimen. Transplant Proc. 2011;43:3730–3737. doi: 10.1016/j.transproceed.2011.08.074. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls SJ, Ruotolo G, Brewer HB, et al. Cholesterol efflux capacity and pre-beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. J Am Coll Cardiol. 2015;66:2201–2210. doi: 10.1016/j.jacc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Javaheri A, Kolansky DM, Cuchel M. Reconstituted high-density lipoprotein therapies: a cause for optimism. Arterioscler Thromb Vasc Biol. 2014;34:1800–1802. doi: 10.1161/ATVBAHA.114.304156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gille A, Easton R, D'Andrea D, et al. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34:2106–2114. doi: 10.1161/ATVBAHA.114.303720. [DOI] [PubMed] [Google Scholar]
- 37.Khera AV, Millar JS, Ruotolo G, et al. Potent peroxisome proliferator-activated receptor-alpha agonist treatment increases cholesterol efflux capacity in humans with the metabolic syndrome. Eur Heart J. 2015;36:3020–3022. doi: 10.1093/eurheartj/ehv291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.