Abstract
We have synthesized and characterized MP-III-022 ((R)-8-ethynyl-6-(2-fluorophenyl)-N,4-dimethyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxamide) in vitro and in vivo as a binding- and efficacy-selective positive allosteric modulator of GABAA receptors containing the α5 subunit (α5GABAARs). By approximation of the electrophysiological responses which the estimated free rat brain concentrations can induce, we demonstrated that convenient systemic administration of MP-III-022 in the dose range 1-10 mg/kg may result in a selective potentiation, over a wide range from mild to moderate to strong, of α5βγ2 GABAA receptors. For eliciting a comparable range of potentiation, the widely studied parent ligand SH-053-2′F-R-CH3 containing an ester moiety needs to be administered over a much wider dose range (10-200 mg/kg), but at the price of activating non-α5 GABAARs as well as the desired α5GABAARs at the highest dose. At the dose of 10 mg/kg, which elicits a strong positive modulation of α5GABAARs, MP-III-022 caused mild, but significant muscle relaxation, while at doses 1-10 mg/kg was devoid of ataxia, sedation or an influence on the anxiety level, characteristic for non-selective benzodiazepines. As an amide compound with improved stability and kinetic properties, MP-III-022 may represent an optimized tool to study the influence of α5GABAARs on the neuronal pathways related to CNS disorders such as schizophrenia, Alzheimer's disease, Down syndrome or autism.
Keywords: amide compound, receptor efficacy, binding assay, free brain concentration, rat kinetics, basic behavior
1. Introduction
Gamma-aminobutyric acid (GABA)A-receptors mediate the majority of inhibitory neurotransmission in adult brain and represent a prominent therapeutic target. Most notably, benzodiazepines are GABAergic drugs widely used in treatment of anxiety, insomnia, epilepsy and muscular spasms, and elucidation of their mechanism of action has helped the understanding of the role of various GABAA receptor populations in normal and pathological conditions. Specifically, the vast majority of pentameric GABAA receptors contain an α1, α2, α3 or α5 subunit together with a neighboring γ2 subunit, and benzodiazepines such as diazepam act as positive allosteric modulators at all these receptors (Rudolph and Möhler, 2014).
Among the diazepam-sensitive target populations, α5GABAARs are the least abundant (<5% of total) and with the most restricted distribution (Rudolph and Möhler, 2014), both of which are excellent characteristics for development of subtype selective ligands. Most of the patents in the field of benzodiazepines claim the therapeutic usefulness of both, positive and negative selective modulators of α5GABAARs (Guerrini and Ciciani, 2012). As studies in α5GABAAR knock-out mice revealed pro-cognitive effects (Collinson et al., 2002; Crestani et al., 2002), development of negative allosteric modulators selective for these receptors has been initiated firstly (Maubach, 2003), and resulted in claims of their effectiveness in cognitive symptoms of schizophrenia, Down syndrome or mood disorders (Guerrini and Ciciani, 2012; Soh and Lynch, 2015). Development of positive allosteric modulators of α5GABAARs has been recognized more recently as a potentially fruitful approach in treatment of aging-related dementia (Koh et al., 2013) and cognitive impairment in schizophrenia (Gill et al., 2011) and autism (Mendez et al., 2013). Moreover, the results obtained in mice with the central nucleus of the amygdala–specific deletion of α5-GABAARs (Botta et al., 2015) as well as in mice bearing three point-mutated subunits (Behlke et al., 2016), suggest that positive allosteric modulation of α5-GABAARs could be expected to interfere with maladaptive fear generalization and/or induce anxiolytic activity, which is an additional impetus for development of the appropriate selective ligands.
Although discovery of ligands selective for distinct GABAA receptor subtypes has become a research priority (Skolnick, 2012), many encouraging preclinical results failed to translate to the clinical setting (e.g. Atack et al., 2011). Suboptimal selectivity or kinetics, lack of the needed magnitude of receptor modulation and poor tolerability or toxicity are among possible explanations for such failure (Atack, 2010; 2011). We have recently published an updated model of the benzodiazepine binding site pharmacophore of α5GABAARs (Clayton et al., 2015). The present study aimed to obtain an α5GABAAR positive modulator with improved selectivity, and suitable to elicit a wide range of in vivo modulatory actions on the α5GABAA receptor population within attainable doses. In this vein, we have synthesized and pharmacokinetically and pharmacologically characterized MP-III-022, an amide ligand prepared from the ester precursor SH-053-2′F-R-CH3, which has been characterized and widely studied as one of the most selective positive modulators at α5GABAARs (Gill et al., 2011; Fischer et al, 2010; Savić et al., 2010; Drexler et al., 2013; Soto et al., 2013; Gallos et al., 2015).
2. Materials and methods
2.1. Chemistry
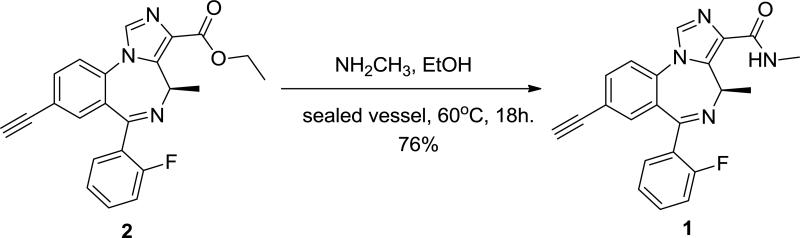
(R)-8-Ethynyl-6-(2-fluorophenyl)-N,4-dimethyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxamide (MP-III-022) (1) was synthesized as follows.
Ester 2 (1.0 g, 2.58 mmol) [35] was added to a sealed vessel fitted with a septum at 0°C and treated with methyl amine (15 ml; 33% wt solution in ethanol). The vessel was sealed with a cap and stirred at 60°C for 18 h. The solution was then cooled to rt and the methyl amine and ethanol were removed under reduced pressure. The residue was purified by a wash column (silica column, 4:1 EtOAc:hexanes) to afford amide 1 as a white powder (731 mg, 1.96 mmol, 76%). Characterization of compound 1: 1H NMR (300 MHz, CDCl3) δ 7.88 (s, 1H), 7.67 (dd, J = 16.3, 7.8 Hz, 2H), 7.55 (d, J = 8.3 Hz, 1H), 7.50 – 7.41 (m, 2H), 7.29 – 7.18 (m, 2H), 7.04 (t, J = 9.3 Hz, 1H), 6.93 (q, J = 7.4 Hz, 1H), 3.16 (s, 1H), 2.97 (d, J = 5.0 Hz, 3H), 1.29 (d, J = 6.4 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 162.24, 161.81, 158.48, 138.25, 137.12, 135.73, 134.41, 133.57, 132.53, 131.57, 129.03, 125.15, 124.92, 124.58, 122.50, 116.33, 166.05, 81.25, 79.99, 49.42, 25.73, 25.27. HRMS (LCMS-IT-TOF) Calc. for C22H18FN4O (M + H)+ 373.1459, found: 373.1462. Detailed spectra are provided in the Supporting Information (Fig. S1 – S2).
2.2. Competition binding assays
2.2.1. Materials
3H-Flunitrazepam (specific activity 83 Ci/mmol) was purchased from Perkin Elmer NEN (New England Nuclear )(Waltham, Massachusetts, USA). Diazepam (7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4, benzodiazepine-2-one) from Nycomed (Opfikon, Switzerland). Standard chemicals came from Sigma-Aldrich (St. Louis, Missouri, USA)
2.2.2. Culturing of human embryonic kidney 293 cells
Human embryonic kidney (HEK) 293 cells (American Type Culture Collection ATCC® CRL-1574™) were maintained in Dulbecco's modified Eagle medium (DMEM, high glucose, GlutaMAX™ supplement, Gibco 61965-059, ThermoFisher, Waltham, Massachusetts, USA) supplemented with 10% fetal calf serum (Sigma-Aldrich F7524, St. Louis, Missouri, USA), 100 U/ml Penicillin-Streptomycin (Gibco 15140-122, ThermoFisher, Waltham, Massachusetts, USA) and MEM (Non-Essential Amino Acids Gibco 11140-035, ThermoFisher, Waltham, Massachusetts, USA) on 10cm Cell culture dishes (Cell+, Sarstedt, Nürnbrecht, Germany) at 37°C and 5% CO2.
HEK293 cells were transfected with cDNAs encoding rat GABAA-receptor subunits subcloned into pCI expression vectors. The ratio of plasmids used for transfection with the calcium phosphate precipitation method [36] were 3μg α(1,2,3 or 5): 3μg β3 and 15 μg γ2 per 10 cm dish. Medium was changed 4-6 h after transfection.
Cells were harvested 72 days after transfection by scraping into phosphate buffered saline. After centrifugation (10 min, 12000 g, 4°C) cells were resuspended in TC50 (50 mM Tris-Citrate pH=7.1), homogenized with an Ultra-Turrax® (IKA, Staufen, Germany) and centrifuged (20 min, 50 000g). Membranes were washed three times in TC50 as described above and frozen at −20°C until use.
2.2.3. Radioligand binding assay
Frozen membranes were thawed, resuspended in TC50 and incubated for 90 min at 4°C in a total of 500 μl of a solution containing 50 mM Tris/citrate buffer, pH=7.1, 150 mM NaCl and 2 nM [3H]-Flunitrazepam in the absence of presence of either 5 μM diazepam (to determine unspecific binding) or various concentrations of receptor ligands (dissolved in DMSO, final DMSO-concentration 0.5%). Membranes were filtered through Whatman GF/B filters and the filters were rinsed twice with 4 ml of ice-cold 50mM Tris/citrate buffer. Filters were transferred to scintillation vials and subjected to scintillation counting after the addition of 3 ml Rotiszint Eco plus liquid scintillation cocktail. Nonspecific binding determined in the presence of 5 μM Diazepam was subtracted from total [3H]-Flunitrazepam binding to result in specific binding.
In order to determine the equilibrium binding constant KD of 3H-Flunitrazepam for the various receptor-subtypes, membranes were incubated with various concentrations of 3H-Flunitrazepam in the absence or presence of 5 μM Diazepam.
2.2.4. Data calculation
Saturation binding experiments were analyzed using the equation Y=Bmax*X/(KD+X). Nonlinear regression analysis of the displacement curves used the equation: log(inhibitor) vs. response - variable slope with Top=100% and Bottom=0% Y=100/(1+10^((logIC50-x)*Hillslope)). Both analyses were performed using GraphPad Prism version 5.0a for Mac OS X, GraphPad Software, La Jolla California USA, www.graphpad.com.
Drug concentrations resulting in half maximal inhibition of specific 3H-Flunitrazepam binding (IC50) were converted to Ki values by using the Cheng-Prusoff relationship [37] Ki= IC50/(1+(S/KD)) with S being the concentration of the radioligand (2 nM) and the KD values listed in Table S1.
2.3. Electrophysiological experiments
cDNAs of rat GABAA receptor subunits were used for generating the respective mRNAs that were then injected into Xenopus laevis oocytes (Nasco, WI) as described previously [38]. For electrophysiological recordings, oocytes were placed on a nylon-grid in a bath of Xenopus Ringer solution (XR, containing 90 mM NaCl, 5 mM HEPES-NaOH (pH 7.4), 1 mM MgCl2, 1 mM KCl and 1 mM CaCl2). The oocytes were constantly washed by a flow of 6 ml/min XR which could be switched to XR containing GABA or MP-III-022 and GABA. Test compounds were diluted into XR from DMSO-solutions resulting in a final concentration of 0.1% DMSO perfusing the oocytes. For current measurements the oocytes were impaled with two microelectrodes (2–3 mΩ) which were filled with 2 mM KCl. To test for modulation of GABA-induced currents by MP-III-022, a concentration of GABA was used that was titrated to trigger 3-5% of the respective maximum GABA-elicited current of the individual oocyte (EC3-5). At this low GABA concentration, within-day and between-day currents are reproducible and effects of drugs (% of modulation) are much higher than that at higher GABA concentrations. GABA at EC3-5 was then coapplied with varying concentrations of MP-III-022 until a peak response was observed. Between two applications, oocytes were washed in XR for up to 15 min to ensure full recovery from desensitization. All recordings were performed at room temperature at a holding potential of –60 mV using Dagan TEV-200 two-electrode voltage clamp amplifiers (Warner Instruments, Hamden, CT). Data were digitized, recorded and measured using Digidata 1322A and 1550 data acquisition systems (Axon Instruments, Union City, CA). Results of concentration response experiments were graphed using GraphPad Prism 4.00 (GraphPad Software, San Diego, CA).
2.4. Studies in rats
Male outbred Wistar rats were supplied by Military Farm, Belgrade, Serbia. Rats were housed in groups of five and were maintained under standard laboratory conditions (21 ± 2°C, relative humidity 40-45%) with free access to pellet food and tap water. They were kept on 12:12 h light/dark cycle with lights on at 07.00 h. All handling and testing took place during the light phase of the diurnal cycle.
Behavioral experiments were carried out on nine weeks old Wistar male rats weighing 200–250 g. The animals received one or two intraperitoneal (i.p.) injections, one of which was the solvent if not otherwise stated. The behavior was recorded by a ceiling–mounted camera and analyzed by ANY–maze Video Tracking System software (Stoelting Co., USA). Throughout the study, experimentally naïve animals were used. Experiments were approved by the Ethical Commission on Animal Experimentation of the Faculty of Pharmacy in Belgrade and were carried out in accordance with the EEC Directive 86/609.
The ligands MP-III-022, SH-053-2′F-R-CH3 and XLi-093 were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin–Milwaukee, USA. XLi-093 is an α5GABAA receptor selective antagonist (Savić et al., 2009). D-Amphetamine-sulfate was purchased from Sigma–Aldrich, and flumazenil from Feicheng BoYuan Fine Chemicals Co., Ltd, China. The ligands MP-III-022, SH-053-2′F-R-CH3, XLi-093 and flumazenil were suspended/dissolved with the aid of sonication in the solvent (85% distilled water, 14% propylene glycol, and 1% Tween 80), while d-amphetamine was dissolved in saline. All compounds were administered i.p. in a volume of 2 ml/kg, 20 min before the behavioral testing, with the exception of the experiment with d-amphetamine, in which the animal's behavior was recorded immediately after the respective treatments.
For determination of free fractions in plasma and brain tissue, stock solutions of MP-III-022 and SH-053-2′F-R-CH3 were prepared in dimethyl sulfoxide (DMSO).
2.4.1. Pharmacokinetic study
Rats were divided in two cohorts of six groups which corresponded to predetermined time intervals (5, 10, 20, 40, 60 and 180 min), each containing three animals. MP-III-022 and SH-053-2′F-R-CH3, dosed at 2.5 mg/kg and 10 mg/kg, respectively, were administered by i.p. injection in a volume of 2 ml/kg. Additional experiments were performed in order to determine brain and plasma concentration 20 min after i.p. injection, as well as to calculate free brain and plasma levels of MP-III-022 dosed at 1, 2.5, 10, 15 and 20 mg/kg, and SH-053-2′F-R-CH3 dosed at 10, 20, 30, 150 and 200 mg/kg. The blood samples were collected in heparinized syringes via cardiac puncture of rats anesthetized with ketamine solution (10% Ketamidor, Richter Pharma Ag, Wels, Austria, dosed i.p. at 100 mg/kg), and centrifuged at 800 rcf for 10 min to obtain plasma. Thereafter, rats were decapitated and brains were weighed, homogenized in 5 ml of methanol and centrifuged at 3340 rcf for 20 min. To determine the concentration of MP-III-022 and SH-053-2′F-R-CH3 in plasma and supernatants of brain tissue homogenates, MP-III-022 and SH-053-2′F-R-CH3 were extracted from these samples by solid phase extraction, using Oasis HLB cartridges (Waters Corporation, Milford, Massachusetts). The procedure of sample preparation and determination of MP-III-022 and SH-053-2′F-R-CH3 by ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) with Thermo Scientific Accela 600 UPLC system connected to a Thermo Scientific TSQ Quantum Access MAX triple quadrupole mass spectrometer (Thermo Fisher Scientific, San Jose, California), equipped with electrospray ionization (ESI) source, has been already described in detail (Obradović et al., 2014).
2.4.2. In vitro hydrolytic stability study in rat plasma
MP-III-022 and SH-053-2′F-R-CH3 were tested for their hydrolytic stability, utilizing blank rat plasma spiked with the respective ligand (0.2 μg/ml) and internal standard (SH-I-048A; synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin–Milwaukee, USA), and incubated in vitro at 37 °C. Aliquots (10 μl) of this solution were then collected at various time intervals, diluted with acetonitrile (90 μl) to precipitate any protein and analyzed using UPLC–MS/MS as above.
2.4.3. Plasma protein and brain tissue binding studies
The rapid equilibrium dialysis assay used to determine free fraction of MP-III-022 and SH-053-2′F-R-CH3 in rat plasma and brain tissue was the same as in Obradović et al., 2014.
2.4.4. Grip strength
Muscle strength was assessed by the grip strength meter (Ugo Basile, Milan, Italy, model 47105). When pulled by the tail, the rat grasps the trapeze connected to a force transducer, and the apparatus measures the peak force of experimenter's pull (in grams) necessary to overcome the strength of the animal's forelimbs grip. Six groups received one of the following treatments 20 minutes before testing: solvent, MP-III-022 (1, 2.5, 10, 15 and 20 mg/kg). Each animal was given three consecutive trials, and the median value of three readings, normalized against body weight, was used for further statistics.
2.4.5. Rotarod test
Motor performance was assessed using an automated rotarod (Ugo Basile, Italy). Before testing, rats were trained for three days until they could remain for 180 s on the rod revolving at 15 rpm. On the fourth day, selection was made and rats fulfilling the given criteria were included in the experiment. Seven groups received one of the following treatments 20 minutes before testing: solvent, MP-III-022 (10, 15 and 20 mg/kg) , SH-053-2′F-R-CH3 (200 mg/kg) and 15 mg/kg MP-III-022 in combination with XLi-093 (20 mg/kg) or flumazenil (15 mg/kg). Latency to fall from the rotarod was recorded automatically.
2.4.6. Intravenous pentylenetetrazole infusion test
A butterfly cannula (needle size 25 G, ¾ in.) attached to a 20 ml syringe prefilled with pentylenetetrazole (PTZ) solution was used. The syringe was held in adjustable motor driven infusion pump (Stoelting Co., Wood Dale Illinois, USA). Before infusing PTZ, the rat's tail vein was dilated with warm water. Single rat was then placed in the restrainer. A butterfly needle was inserted into the lateral tail vein and PTZ was infused at a constant rate of 0.5 ml/min. The animal was observed throughout the infusion by two trained and blind observers. The threshold doses of PTZ (mg/kg) required to elicit clonic and tonic seizures were calculated using the following formula: volume of PTZ (ml) × concentration of PTZ (mg/ml)/body weight (kg).
2.4.7. Spontaneous and amphetamine induced locomotor activity
Activity of single rats in a clear Plexiglas chamber (40×25×35 cm) under dim red light (20 lux) was recorded for a total of 45 min, without any habituation period but with 20 min acclimatization time. One of two doses of MP-III-022 (10 and 15 mg/kg) or solvent were applied 20 min before testing. The same apparatus was used for assessing the amphetamine-induced locomotor activity, with an adapted protocol. Here, rats received the appropriate treatment (solvent, 1, 2.5 or 10 mg/kg MP-III-022) and were immediately placed in the tracking chamber. Behavior was recorded for 20 min and then rats were injected with saline or 0.5 mg/kg amphetamine and locomotor activity was recorded for an additional 60 min.
2.4.8. Behavior in the elevated plus maze
The apparatus was constructed of black Plexiglas and consisted of a maze elevated to a height of 50 cm with two open (50 × 10 cm) and two enclosed arms (50 × 10 × 40 cm), connected by a junction area (central platform) measured 10 × 10 cm. A ledge 0.3 cm high surrounding the open arms was added. The illumination in the experimental room consisted of one red neon tube fixed on the ceiling, giving light intensity of 10 lux on the surface of the closed arms. At the beginning of the experiment, single rats were placed in the center of the maze, facing one of the enclosed arms, and their behavior was recorded for 5 min. An entry into an open or closed arm was scored when 90% of the animal crossed the virtual line separating the central square of the maze from the arm, whereas an exit occurred when more than 90% of the animal left the respective arm. After each trial, the maze was cleaned with dry and wet towels. The rats received the appropriate treatment (solvent, 1, 2.5 or 10 mg/kg MP-III-022) and 20 min later were placed in the maze.
2.5. Statistics
All numerical data presented in figures are given as the mean ± S.E.M. Pharmacokinetic parameters were calculated using PK Functions for Microsoft Excel software (by Joel Usansky, Atul Desai, and Diane Tang-Liuwere). For the binding study, as well as in the PTZ, grip strength, rotarod test and the elevated plus maze, one-way ANOVA were applied. Post hoc comparisons, where applicable, were performed using SNK test, with exception of the binding study, where Tukey test was applied. Statistical analysis was performed using SigmaPlot 11 (Systat Software Inc., Richmond, USA) software. Differences were considered significant when P < 0.05.
3. Results
3.1. Chemistry
As depicted in Scheme 1, (R)-8-Ethynyl-6-(2-fluorophenyl)-N,4-dimethyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxamide (1) was synthesized in a 76% yield by stirring ethyl ester (2) with methyl amine in a sealed vessel.
Scheme 1.
Synthesis of MP-III-022 (1)
3.2. Binding studies
Membranes from HEK-293 cells transiently expressing different GABAA receptor subunit combinations were characterized using 3H-Flunitrazepam binding. In order to determine radioligand affinities to the four tested receptor subtypes (which are needed to convert the IC50 values of a test compound into Ki values), saturation binding experiments were performed. Cells were incubated with various concentrations of 3H-flunitrazepam and binding data were subjected to Scatchard analysis, as described in the methods section. The equilibrium dissociation constants of the ligand-receptor complex obtained in saturation binding experiments for four GABAA receptor subtypes are presented in Table S1.
In other experiments the potency of MP-III-022 to inhibit 2 nM 3H-flunitrazepam binding to membranes from HEK-293 cells expressing four different receptor subtypes was studied. Membranes from HEK cells transfected with the four subunit combinations were incubated with 2 nM 3H-flunitrazepam in the absence or presence of either 5 μM diazepam (to determine nonspecific binding) or increasing concentrations of MP-III-022. IC50 values (i.e. drug concentrations resulting in half maximal inhibition of specific 3H-flunitrazepam binding) were converted to Ki values by using the Cheng-Prusoff relationship (for details see methods-section).
The determined Ki values (Table 1) of α5GABAARs differ statistically significantly from α1-(P=0.0011) and from α3-containing receptors (P=0.0041) (one-way ANOVA analysis followed by Tukey's multiple comparison test).
Ligand MP-III-022 exerted negligible activity in 50 other receptor and enzyme assays (B. Roth et al., NIMH Psychoactive Drug Screening Program, UNC, available at http://pdsp.med.unc.edu). The only exception was the κ opioid receptor (KOR), at which the secondary binding study gave the Ki value of 381 nM.
3.3. Pharmacokinetic, plasma stability and free fraction studies
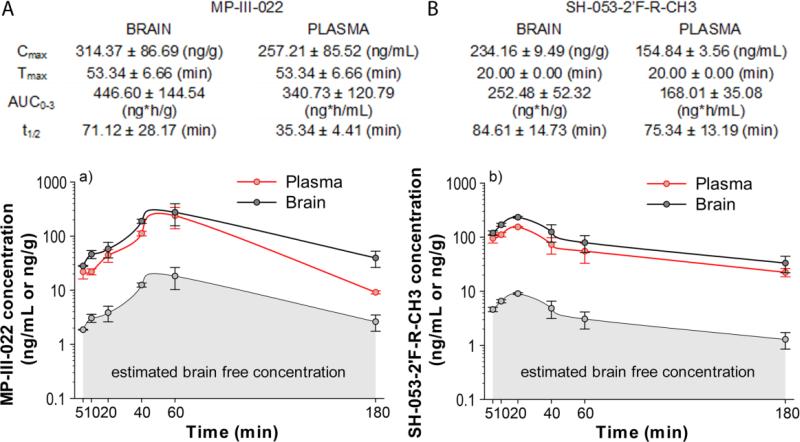
The concentration–time profiles of MP-III-022 and SH-053-2′F-R-CH3 in rat plasma and brain after intraperitoneal administration of the 2.5 mg/kg and 10 mg/kg dose, respectively, with the calculated pharmacokinetic parameters, are given in Fig. 1.
Fig. 1.
Plasma and brain concentration–time profiles of MP-III-022 (A) and SH-053-2′F-R-CH3 (B) after intraperitoneal administration of the 2.5 mg/kg and 10 mg/kg dose, respectively (n = 3 per time point). Cmax = maximum concentration in brain or plasma; Tmax = time of maximum concentration in brain or plasma; t1/2 = elimination half-life from brain or plasma; AUC0-3 = area under the brain or plasma concentration–time curve from 0 to 3 h
The doses are chosen to elicit a moderate degree of selective potentiation at α5GABAARs (see vertical markers in Fig. 2 and explanation below). AUC (area under the curve from time 0 to 180 min post-dosing) values for plasma and brain were 2-fold and 1.75-fold, respectively, greater for MP-III-022 than for SH-053-2′F-R-CH3, despite the fact that MP-III-022 was administered at a 4-fold lower dose. In line with in vivo data of superior systemic exposure, as assessed by AUC, the amide compound MP-III-022 displayed an excellent in vitro metabolic stability during incubation in rat plasma; this is in clear contrast with the values obtained with the less stable ester compound SH-053-2′F-R-CH3 (Table 2).
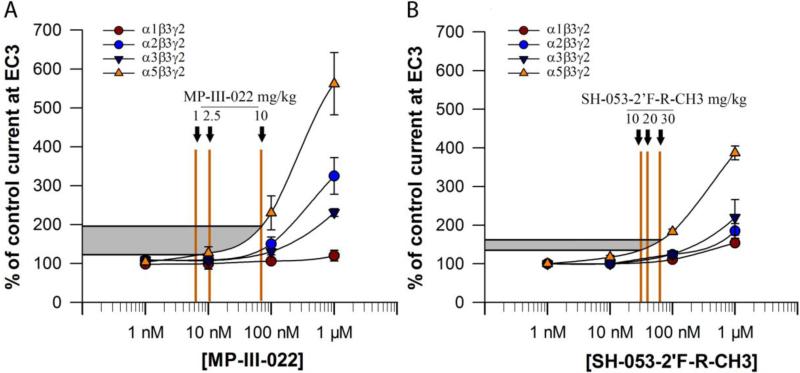
Fig. 2.
The approximated electrophysiological responses elicited by the estimated free brain concentrations of MP-III-022 (given in Table 2) and SH-053-2′F-R-CH3 (given in Table 3), presented on the concentration-response curves of MP-III-022 (A) and SH-053-2′F-R-CH3 (B) at rat recombinant α1-, α2-, α3,- and α5β3γ2 GABAA receptors measured at GABA EC3 (eliciting 3% of the maximal GABA current in the respective subtype). Brain tissue density of 1.04 g/ml (Weaver et al., 2001) was used to convert brain concentrations from mg/kg (Tables 3 and 4) into mg/ml. The shaded range delineates the interpolated lower and upper limit of potentiation at α5GABAARs in the dose range used; the vertical lines mark the approximated free brain concentration of the given dose of each of two ligands.
The concentrations of MP-III-022 in rat plasma (nmol/l) and brain tissue (nmol/kg), both total and free (estimated), measured 20 min after i.p. injection of MP-III-022 dosed at 1, 2.5, 10, 15 and 20 mg/kg, are shown in Table 3. Free concentrations of MP-III-022 were calculated by multiplying the obtained total plasma and brain concentrations with the appropriate free fractions (13.51% for plasma and 6.61% for brain tissue) determined by rapid equilibrium dialysis. The analogous results for SH-053-2′F-R-CH3 over the same 20-fold dose schedule, but in the range from 10 mg/kg to 200 mg/kg, are presented in Table 4. Free fractions for this ester compound were 68.55% for plasma and 3.87% for brain.
3.4. Electrophysiological effects
Dose response curves for MP-III-022 from two-electrode voltage clamp experiments are shown in Fig. 2A, while the exact data are available in the Supplementary table S2. At the α5β3γ2 GABAA receptors, MP-III-022 elicited a significant allosteric modulatory efficacy at concentrations starting at 10 nM, and the potentiation values obtained at concentrations approaching 100 nM can be described as strong efficacy (above 300%). On the other hand, its activity tended to be weakly modulatory at α2- and α3-containing receptors only at concentrations of about 100 nM and above. MP-III-022 lacked modulatory actions at α1-containing GABAA receptors in the whole range of in vivo free brain concentrations which were obtained (Table S3). For comparison, a concentration of 100 nM of SH-053-2′F-R-CH3 resulted in 111 ± 2%, 124 ± 9%, 125 ± 8%, and 183 ± 7% of control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively (Savić et al., 2010). Analogous to the data with MP-III-022, in Fig. 2B dose response curves for SH-053-2′F-R-CH3 (Savić et al., 2010) are presented with approximated actions of in vivo obtainable free brain concentrations. The exact values of potentiation read from the curves in Fig. 2 (Table S3), suggest that efficacy selectivity of MP-III-022 is obtainable in the range from mild (below 140%) via moderate (about 160%) to strong (above 300%) potentiation at α5GABAARs. Importantly, MP-III-022 reached strong potentiation at α5GABAARs already at 100 nM effective concentration, at which the effects on non-α5GABAARs were still at and below 150% (see Fig. 2A and Table S3). In contrast, the concentration of the parent compound needed to elicit 300% potentiation at α5GABAARs was > 300nM, requiring exceptionally high doses of SH-053-2′F-R-CH3, and concurrently generating in non-α5 receptors the interpolated potentiation in the range of 150 to 200%.
3.5. Grip strength, rotarod test and intravenous PTZ infusion test
MP-III-022 induced a dose-dependent decrease of muscle tension as measured by grip strength (Table 5). The first muscle relaxant effect of MP-III-022 was observed at the 10 mg/kg dose.
The rats treated with 10 mg/kg MP-III-022 displayed normal motor coordination and balance as assessed in the rotarod test, far different from the animals treated with the 15 mg/kg, and especially 20 mg/kg dose (Table 5). The ataxic effect of 15 mg/kg MP-III-022 was preventable by flumazenil, but not XLi-093. Namely, one-way ANOVA (F(2,18) = 5.05, P = 0.018) and Student–Newman–Keuls (SNK) post hoc revealed significant differences in performance between the rats treated with 15 mg/kg MP-III-022 alone or in combination with 20 mg/kg of XLi-093, and the combination of 15 mg/kg MP-III-022 and 15 mg/kg flumazenil (P = 0.021 and P = 0.019, respectively, Table 5). When testing SH-053-2′F-R-CH3 on the rotarod, the first, mild and insignificant deterioration (154±26 s vs. 180 s with control animals; n = 4) was noticed at 200 mg/kg.
One-way ANOVA applied to the PTZ test (Table 5) did not show a statistically significant effect of treatment with either of two tested doses of MP-III-022 selective for α5GABAARs (F(2,30) = 1.50, P = 0.239).
3.6. Spontaneous and amphetamine induced locomotor activity
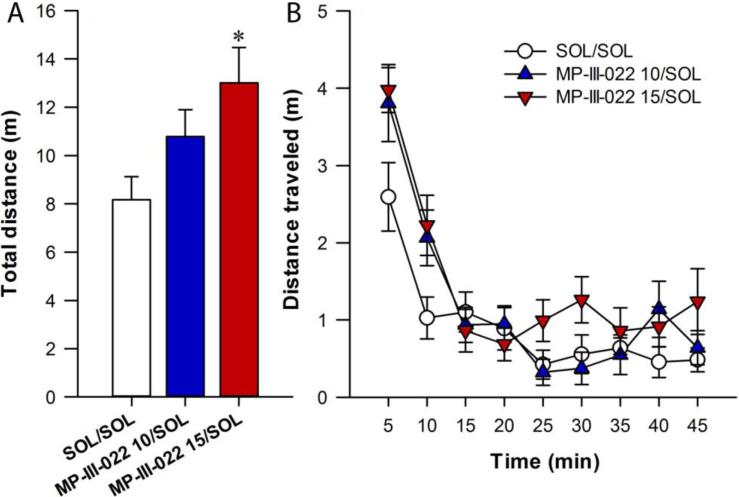
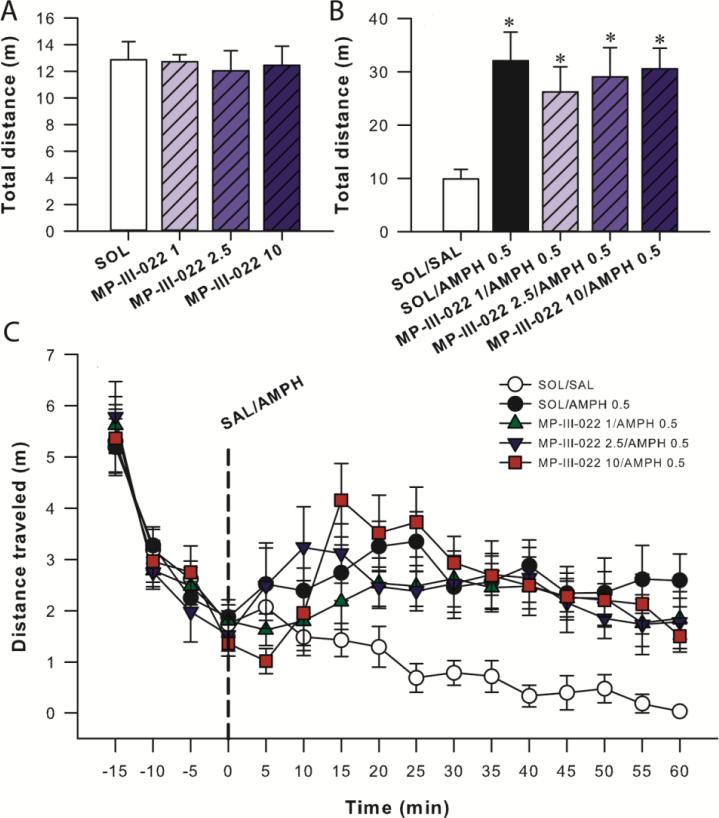
One-way ANOVA for total distance traveled revealed a significant effect of treatment (F(2,25) = 3.64, P = 0.041) and analysis of the SNK test showed differences between control and 15 mg/kg MP-III-022 (P = 0.029, Fig 3A). Similarly, when the two-way repeated measures ANOVA was applied on distance traveled in the 5-min bins, a significant effect of treatment and time was revealed (F(2,25) = 3.64, P = 0.041, F(8,200) = 32.90, P < 0.001, respectively), without a significant interaction of factors (F(16,200) = 1.63, P = 0.065, Fig. 3B). The post hoc SNK test showed the differences between control and 15 mg/kg MP-III-022 (P = 0.032).
Fig. 3.
The effects of 10 and 15 mg/kg MP-III-022 intraperitoneally on the total distance traveled (A) and on distance traveled in 5-min bins (B) during 45 min in SLA. *P < 0.05 compared to control (SOL/SOL) group. All data are presented as the mean +/± S.E.M. Number of animals per treatment group was 8-10. SOL = solvent, MP-III-022 10 = 10 mg/kg MP-III-022, MP-III-022 15 = 15 mg/kg MP-III-022.
One-way ANOVA applied on data with three selective doses of MP-III-022 (1, 2.5 and 10 mg/kg) from the first part of the amphetamine-induced locomotor activity test did not show any effect of treatment (F(3,28) = 0.08, P = 0.970, Fig. 4A). The same lack of effect was observed with two-way repeated measures ANOVA on results of 5-min intervals: Treatment: F(3,28) = 0.08, P = 0.970; Time: F(3,84) = 78.36, P < 0.001: Interaction: F(9,84) = 0.49, P = 0.879.
Fig. 4.
The effect of 1, 2.5, and 10 mg/kg MP-III-022 intraperitoneally on locomotor activity during 20 minutes before amphetamine treatment (A), the influence of 1, 2.5, and 10 mg/kg MP-III-022 on amphetamine induced hyperlocomotion during 60 min (B) and effect on distance traveled in 5-min bins during all testing (C) in locomotor activity test. *P < 0.05 compared to control (SAL/SAL) group. All data are presented as the mean + or ± S.E.M. Number of animals per treatment group was 8. SOL = solvent, SAL = vehicle, AMPH 0.5 = 0.5 mg/kg amphetamine, MP-III-022 1 = 1 mg/kg MP-III-022, MP-III-022 2.5 = 2.5 mg/kg MP-III-022, MP-III-022 10 = 10 mg/kg MP-III-022
Analysis of data from 60-min recordings after administration of amphetamine with one-way ANOVA revealed a significant effect on total distance traveled (F(3,35) = 4.16, P = 0.007); post hoc SNK results were given in Fig. 4B. When two-way ANOVA with repeated measures was applied on distance traveled in 5-min bins, the same significant effect was observed (Treatment: F(4,35) = 4.16, P = 0.007, Time: F(11,385) = 5.47, P < 0.001, Interaction: F(44,385) = 2.02, P < 0.001). The SNK test confirmed differences between control and all amphetamine-treated groups.
3.7. Elevated plus maze
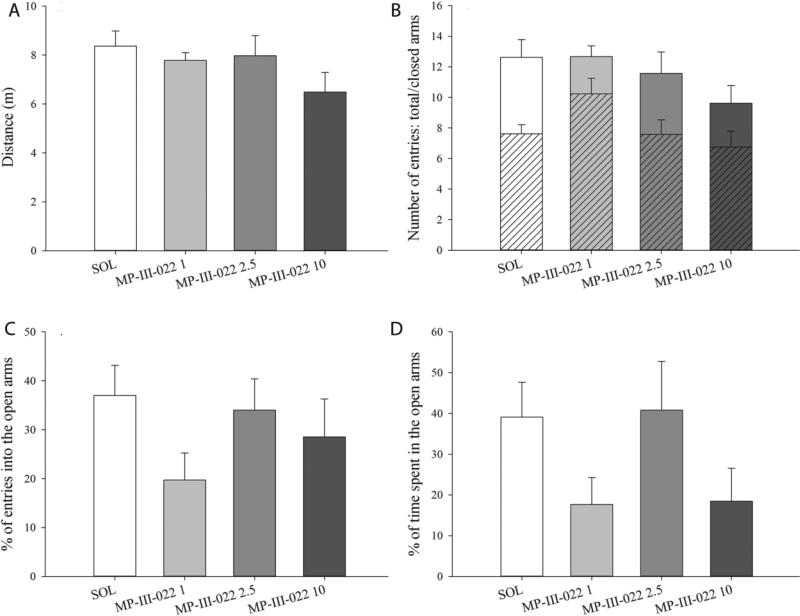
In the elevated plus maze, treatment with MP-III-022 in the dose range 1 mg/kg to 10 mg/kg did not affect total distance traveled (F(3,28) = 1.593, P=0.213; Fig 5A), closed and total arm entries (F(3,28) = 2.822, P=0.057 and F(3,28) = 1.749, P=0.180, respectively; Fig 5B), percent of open arm entries (F(3,28) = 1.455, P=0.248; Fig 5C) or percent of open arm time (F(3,28) = 2.106, P=0.122; Fig 5D).
Fig. 5.
The effect of 1, 2.5, and 10 mg/kg MP-III-022 administered i.p. on: distance traveled (A), total and closed arm entries (B), % of entries into the open arms (C), and % of time spent in the open arms (D) in the elevated plus maze during 5 minutes of recording. All data are presented as the mean ± S.E.M. Number of animals per treatment group was 8 for SOL = solvent, 9 for MP-III-022 1 = 1 mg/kg MP-III-022, 7 for MP-III-022 2.5 = 2.5 mg/kg MP-III-022 and 8 for MP-III-022 10 = 10 mg/kg MP-III-022.
4. Discussion
The Ki values demonstrate that MP-III-022 possesses a 15, 6 and 12-fold selectivity in binding to α5GABAARs when compared to binding to α1, α2 and α3-containing GABAA receptors, respectively. For comparison, the Ki values for the parent ligand SH-053-2′F-R-CH3 at α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors were 759.1, 948.2, 768.8 and 95.2 nM, respectively (Savić et al., 2010), which means that relative selectivity of the two ligands is of the same order of magnitude. It is noteworthy that MP-III-022 is the least selective for those GABAA receptors, containing the α2 subunit, for which SH-053-2′F-R-CH3 is the most selective (6-fold vs. 10-fold ratio). However, selectivity for α5- against α1-containing GABAA receptors is 15-fold for MP-III-022, and 8-fold for SH-053-2′F-R-CH3; this approximately two-fold greater selectivity ratio may be relevant because α1-containing GABAA receptors are the subtype predominantly connected with adverse effects of potentiation at GABAA receptors (Rudolph and Möhler, 2014).
The value of 381 nM for affinity at KOR is of the same order of magnitude as that of SH-053-2′F-R-CH3 (240 nM; B. Roth et al., NIMH Psychoactive Drug Screening Program, UNC, available at http://pdsp.med.unc.edu). Although these are at least 100-fold weaker affinities than that of the declared KOR selective ligands (Frankowski et al., 2012), the KOR Ki for MP-III-022 is on par with that determined for α2GABAARs (360 nM), and KOR-mediated effects might be seen after the doses (15 mg/kg and above) connected with high free brain concentrations of this ligand, at which selectivity for α5-containing receptors was lost, as discussed below.
It is well known that many enzymes (carboxylesterase, butyrylcholinesterase, paraoxonase, acetylcholinesterase, albumin esterase) are involved in both, drug metabolism and bioconversion of the ester-based prodrugs; particularly carboxylesterases have been found virtually throughout the body (Takai et al., 1997; Liederer and Borchardt, 2006). Moreover, the ethyl function of an ethyl ester, as present in SH-053-2′F-R-CH3, may be especially labile and subject to beta-oxidation (Lijinsky et al., 1982; Bodor and Buchwald, 2004). For these reasons, a series of amides were synthesized (J. Cook, M. Poe, Guanguan Li, et al., unpublished results, provisional patent filed in 2016), based on the pharmacophore/receptor model (Clayton et al., 2015). The N-methyl analog MP-III-022 fits this model well, and analysis of the rodent data demonstrated that MP-III-022, as a highly brain-bioavailable benzodiazepine, provides an incrementally more favorable pharmacokinetic profile for in vivo investigation of the role of α5GABAARs than SH-053-2′F-R-CH3. Especially, a comparison of the unbound plasma fractions demonstrates that for each molecule of MP-III-022 present free in plasma there were five such molecules of SH-053-2′F-R-CH3, suggesting that the latter ligand was much more susceptible to enzymes, as the performed in vitro metabolic stability study proved.
The combined in vitro and pharmacokinetic results suggest that MP-III-022 in the dose range 1-10 mg/kg can selectively and gradually potentiate the α5GABAARs in a wide span from 138% to 314%. At higher doses (15 mg/kg and above), the selectivity of receptor activation disappears, and other, mostly α2/α3-containing GABAA receptors, would begin to substantially contribute to the overall pharmacological activity of MP-III-022. In contrast, the estimated potentiation of GABA-mediated currents at α5GABAARs obtained with SH-053-2′F-R-CH3 reached the value of 280% after a huge, experimentally inconvenient dose of 200 mg/kg. In this vein, SH-053-2′F-R-CH3 is not as applicable as MP-III-022 for in vivo studies of the role of strong potentiation of α5GABAARs. When compared with other published ligands besides SH-053-2′F-R-CH3, MP-III-022 also demonstrated an improved selectivity for α5βγ2 GABAA receptors, both in binding and functional assays (cf. Soh and Lynch, 2015). Studies assessing the behavioral activity of diazepam in knock-in mice, which harbor single point-mutated receptors, have attributed the muscle relaxant actions of benzodiazepines to the α2/α3/α5-subtypes (Rudolph and Möhler, 2014). We have previously demonstrated that the α5-selective antagonist XLi-093 can significantly diminish the myorelaxant actions of diazepam in rats (Milić et al., 2012). Consistently, the first muscle relaxant effect of MP-III-022 was observed at a dose (10 mg/kg) postulated to elicit a selective strong potentiation of α5-GABAA receptors.
The rotarod studies in knock-in mice have suggested that the α1-, but not the α2- or α3-containing GABAA receptors contribute to the ataxic action of diazepam (Rudolph and Möhler, 2014). Moreover, the administration of L-838,417, a ligand with significant, but comparably small efficacy at α2-, α3- and α5-GABAARs, did not cause deficits in rotarod performance in rats (Knabl et al., 2008) or mice (McKernan et al., 2000). Nonetheless, the experiments using the nonselective, α1- and α5-subunit-selective antagonists flumazenil, βCCt and XLi-093, respectively, have suggested that an effect of diazepam mediated by α2- or α3-, but not α5-containing GABAA receptors may have contributed to the impairing influence of diazepam on the rotarod performance of rats (Milić et al., 2012). Accordingly, the present results imply that a pronounced modulatory action of MP-III-022 at α2/3GABAARs (the estimated respective potentiations at α2- and α3GABAARs were 225% and 175% after the 15 mg/kg dose, and 280% and 200% after the 20 mg/kg dose; for comparison, the estimated potentiation at α2GABAARs after the dose of 10 mg/kg was 138% and the estimated free concentration was only 19% of Ki value for this receptor subtype) still can induce ataxia. The differences in dose-dependency of behavioral response in two tests demonstrate that the rotarod-ataxic effects of MP-III-022 may encompass, but do not solely rely on muscle relaxation. SH-053-2′F-R-CH3 induced a mild and insignificant rotarod incapacitation at the dose as high as 200 mg/kg, at which the estimated potentiations at α2- and α3GABAARs were 171% and 155%, respectively, i.e. were lower than those after the 15 mg/kg dose of MP-III-022. Higher doses of SH-053-2′F-R-CH3 most probably required for marked incapacitation (for comparison, the S-isomer of this ligand, SH-053-2′F-S-CH3, displayed a median incapacitating dose of 219 mg/kg (Savić et al., 2008)) were not tested due to ethical constraints and irrelevancy, as they need to be accompanied by a distinct lack of selectivity of receptor subtype action. Keeping in mind the efficacy and kinetic properties of MP-III-022, the whole set of grip strength and rotarod results unambiguously demonstrated that the 15 mg/kg dose of MP-III-022 is behaviorally non-selective. Thus, the PTZ test was performed with selective doses only and the results were consistent with the proposed contribution of the non-α5 containing receptors to the anticonvulsant action of GABAergic drugs (Crestani et al., 2002).
The spontaneous locomotor activity test was performed with the highest selective (10 mg/kg) and lowest non-selective (15 mg/kg) dose of MP-III-022. The slight hyperlocomotion observed after treatment with the 15 mg/kg dose could be ascribed to a kind of behavioral disinhibition induced by potentiation of α2/3-containing GABAA receptors unopposed by activity at α1-containing receptors (Savić et al., 2009). As an important corollary, the assumption that α5GABAARs may contribute to the sedative properties of benzodiazepine site agonists (Savić et al., 2008; 2010) would most probably not apply to a substantial level of potentiation, such as that elicited by 10 mg/kg MP-III-022. Recent studies with allegedly selective positive as well as negative modulators of α5GABAA receptors reported a slight, but still significant suppression of the amphetamine-induced locomotor stimulation in the sensitized, behaviorally altered, animals (Gill et al., 2011; Redrobe et al., 2012); the present data demonstrate the lack of influence of α5 receptor-selective doses of MP-III-022 on amphetamine's effects in normal wild-type animals.
The lack of significant effects of MP-III-022 in the elevated plus maze is in agreement with a number of previous studies examining the relation between α5GABAARs and anxiety (Crestani et al., 2002; Dawson et al., 2006; Savić et al., 2008; 2010). It is less clear how these results can be linked with the recent suggestions that generalization of fear and anxiety is mediated by α5-subunit containing neurons in the central nucleus of the amygdala, where reduced expression of α5-GABAARs was proved to be anxiogenic (Botta et al., 2015), or that the anxiolytic effect of diazepam is preserved in mice rendered sensitive to its modulatory action solely via α5-GABAARs (Behlke et al., 2016). The latter studies were performed in genetically modified mice, while the present study dealt with native rats, so that both, species and genetic diversity may have been the source of variability. Moreover, observation of the relative time spent in open arms in the elevated plus maze (Fig. 5D) may suggest a potential of MP-III-022 to induce, under certain conditions, an anxiogenic-like action, at a low and high, but not middle dose. This may be apparently at odds with the finding, based on non-parametric statistical analysis, that an affinity-selective negative modulator at α5-GABAARs exerted both a U-shaped anxiogenic (decreased open-arm activity) and stimulatory effect (increased closed-arm activity) in the murine elevated plus maze (Navarro et al., 2002). As we previously discussed for locomotor influences (Savić et al., 2008), the behavioral output in general, and anxiety in particular, may be modulated by certain, experimentally hardly accessible, ‘discontinuous effective windows’ generated by neurons expressing α5-GABAARs.
5. Conclusions
By approximation of the electrophysiological responses which estimated free rat brain concentrations can induce, we demonstrated that systemic administration of MP-III-022 at doses between 1 and 10 mg/kg should result in a wide range of selective potentiation at α5GABAARs, from mild to moderate to strong, the latter of which is not conveniently attainable after dosing with the parent compound SH-053-2′F-R-CH3. This was not accompanied by overt behavioral impairments which would be suggestive of safety issues. Namely, in the rotarod, pentylenetetrazole as well as spontaneous and amphetamine-induced locomotor activity tests, MP-III-022 at doses up to 10 mg/kg proved to be behaviorally silent in rats, and the only motor effect of that dose was a mild, though significant muscle relaxation expectedly observed in the grip strength test. Moreover, MP-III-022 proved to be devoid of influence on the anxiety level. Thus, it would be possible to selectively assess the behavioral consequences of eliciting a wide range of potentiation of α5GABAARs in a set of rodent behavioral tests modeling human disorders, especially those with cognitive impairment.
Supplementary Material
Table 1.
Binding affinities of MP-III-022 (Ki) for the different receptor subtypes
| α1β3γ2S | α2β3γ2S | α3β3γ2S | α5β3γ2S |
|---|---|---|---|
| 850 ± 110 nM | 360 ± 56 nM | 660 ± 170 nM | 55 ± 17 nM |
The concentrations resulting in half maximal inhibition of radioligand binding were converted into Ki values by using the Cheng-Prusoff relationship and the respective KD values given in Table S1. Ki values are presented as mean values ± S.E.M. from 3 independent experiments performed in duplicates.
Table 2.
Estimated in vitro metabolic stability (total plasma concentration of MP-III-022 or SH-053-2’F-R-CH3 in 30, 60, 120 and 240 minutes). Mean ± S.E.M., n = 3
| MP-III-022 | μM | % | SH-053-2’F-R-CH3 | μM | % |
|---|---|---|---|---|---|
| 0 min | 0.5556 | 100 | 0 min | 0.5556 | 100 |
| 30 min | 0.5402 ± 0.0146 | 97.23 | 30 min | 0.4258 ± 0.0042 | 76.64 |
| 60 min | 0.5403 ± 0.0193 | 97.25 | 60 min | 0.3487 ± 0.0040 | 62.77 |
| 120 min | 0.5472 ± 0.0007 | 98.50 | 120 min | 0.2126 ± 0.0036 | 38.27 |
| 240 min | 0.5374 ± 0.0063 | 96.74 | 240 min | 0.0291 ± 0.0023 | 5.24 |
Table 3.
Total and estimated free concentration of MP-III-022 (1, 2.5, 10, 15 and 20 mg/kg) in plasma and brain 20 minutes after intraperitoneal treatment in rats. Mean ± S.E.M., n = 3-6
| Dose (mg/kg) | 1 | 2.5 | 10 | 15 | 20 | |
|---|---|---|---|---|---|---|
| Plasma (nmol/l) | Total | 62.43 ± 4.97 | 120.01 ± 31.74 | 645.07 ± 166.29 | 3709.24 ± 2012.04 | 5378.01 ± 1936.91 |
| Free | 8.47 ± 0.67 | 16.29 ± 4.31 | 87.57 ± 22.57 | 503.51 ± 273.13 | 730.04 ± 262.93 | |
| Brain (nmol/kg) | Total | 96.47 ± 12,04 | 156.46 ± 50.33 | 1030.52 ± 278.03 | 4804.44 ± 2821.77 | 8465.45 ± 886.18 |
| Free | 6.38 ± 0.80 | 10.34 ± 3.33 | 68.12 ± 18.38 | 317.57 ± 186.52 | 559.57 ± 58.58 | |
Table 4.
Total and estimated free concentration of SH-053-2’F-R-CH3 (10, 20, 30, 150 and 200 mg/kg) in plasma and brain 20 minutes after intraperitoneal treatment in rats. Mean ± S.E.M., n = 2-4
| Dose (mg/kg) | 10 | 20 | 30 | 150 | 200 | |
|---|---|---|---|---|---|---|
| Plasma (nmol/l) | Total | 406.43 ± 76.45 | 420.63 ± 70.01 | 555.51 ± 68.18 | 1604.96 ± 271.88 | 2737.87 ± 286.86 |
| Free | 278.60 ± 52.39 | 288.32 ± 47.99 | 380.78 ± 46.73 | 1100.14 ± 186.36 | 1876.71 ± 196.63 | |
| Brain (nmol/kg) | Total | 752.00 ± 132.84 | 1003.57 ± 87.55 | 1663.84 ± 378.29 | 3816.79 ± 965.21 | 8374.25 ± 2322.03 |
| Free | 29.10 ± 5.14 | 38.85 ± 3.39 | 64.40 ± 14.64 | 147.74 ± 37.36 | 324.16 ± 89.88 | |
Table 5.
Effect of different intraperitoneal doses of MP-III-022 in the grip strength, rotarod, and pentylenetetrazole (PTZ) test; mean ± S.E.M.
| grip strength the peak force of experimenter's pull necessary to overcome the strength of the animal's grip | ||
|---|---|---|
| Treatment | Mean ± S.E.M. (kg/kg) | n |
| SOL | 2.87 ± 0.17 | 10 |
| 1 mg/kg MP-III-022 | 2.57 ± 0.08 | 10 |
| 2.5 mg/kg MP-III-022 | 2.41 ± 0.28 | 8 |
| 10 mg/kg MP-III-022 | 1.94 ± 0.22b,d | 8 |
| 15 mg/kg MP-III-022 | 1.86 ± 0.16a | 5 |
| 20 mg/kg MP-III-022 | 1.78 ± 0.16c,d | 11 |
| rotarod test the time remaining on the rod revolving at 15 rpm | ||
|---|---|---|
| Treatment | Mean ± S.E.M. (s) | n |
| 10 mg/kg MP-III-022 | 180 | 3 |
| 15 mg/kg MP-III-022 | 69.09 ± 25.58 | 9 |
| 15 mg/kg MP-III-022/20 mg/kg XLi-093 | 74.61 ± 34.03 | 6 |
| 15 mg/kg MP-III-022/15 mg/kg FLU | 175.17 ± 4.83a,d | 6 |
| 20 mg/kg MP-III-022 | 6.67 ± 4.81 | 3 |
| PTZ test the threshold doses of PTZ required to elicit seizures | ||
|---|---|---|
| Treatment | Mean ± S.E.M. (mg/kg PTZ) | n |
| SOL | 53.38 ± 1.84 | 11 |
| 1 mg/kg MP-III-022 | 60.99 ± 6.11 | 11 |
| 10 mg/kg MP-III-022 | 50.99 ± 3.72 | 11 |
Grip strength: a P<0.05, b P<0.01, c P<0.001 compared to SOL, d P<0.05 compared to combination of 1 mg/kg MP-III-022. Rotarod: a P<0.05 compared to 15 mg/kg MP-III-022, d P<0.05 compared to combination of 15 mg/kg MP-III-022 and 20 mg/kg XLi-093; SOL = solvent, FLU = flumazenil
Acknowledgements
Financial support was provided by the Ministry of Education, Science and Technological Development, R. Serbia – Grant No. 175076 (MMS) and by the Austrian Science Fund (FWF) grant P 27746 (ME). We also wish to thank the NIH (MH096463, NS076517) for generous financial support (JMC). The authors acknowledge support from the Milwaukee Institute for Drug Discovery. Analytical instrumentation support was provided by University of Wisconsin - Milwaukee's Shimadzu Laboratory for Advanced and Applied Analytical Chemistry.
We appreciate the skilled work of Dr. Bojan Marković, who performed the mass spectrometry measurements. We also thank Ms Karin Schwarz and Ms Friederike Steudle for excellent technical assistance in radioligand binding experiments and Dr. Roshan Puthenkalam for some oocyte measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
TTS, ME, PS, JMC and MMS conceived and designed the experiments. MMP synthesized and characterized the compound. TTS, AS, BD, SR, PS and ME performed the biological assays and analyzed the data. MMS, TTS, MP, PS, ME and JMC wrote the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
References
- Atack JR. GABAA receptor alpha2/alpha3 subtype-selective modulators as potential nonsedating anxiolytics. Curr. Top. Behav. Neurosci. 2010;2:331–360. doi: 10.1007/7854_2009_30. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABAA receptor subtype-selective modulators. II. α5-selective inverse agonists for cognition enhancement. Curr. Top. Med. Chem. 2011;11:1203–1214. doi: 10.2174/156802611795371314. [DOI] [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Street LJ, Dawson GR, Tye S, Van Laere K, Bormans G, Sanabria-Bohórquez SM, De Lepeleire I, de Hoon JN, Van Hecken A, Burns HD, McKernan R-M, Murphy MG, Hargreaves RJ. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J. Psychopharmacol. 2011;25:314–328. doi: 10.1177/0269881109354927. [DOI] [PubMed] [Google Scholar]
- Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson AJ, Yee BK, Zeilhofer HU, Engin E, Rudolph U. A Pharmacogenetic ‘Restriction-of-Function’ Approach Reveals Evidence for Anxiolytic-Like Actions Mediated by α5-Containing GABA(A) Receptors in Mice. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.49. doi: 10.1038/npp.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor N, Buchwald P. Designing safer (soft) drugs by avoiding the formation of toxic and oxidative metabolites. Mol Biotechnol. 2004;26:123–132. doi: 10.1385/MB:26:2:123. [DOI] [PubMed] [Google Scholar]
- Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, Lu T, Poe MM, Xu L, Cook JM, Rudolph U, Sah P, Ferraguti F, Lüthim A. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clayton T, Poe MM, Rallapalli S, Biawat P, Savić MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA, Cook J-M. A review of the updated pharmacophore for the alpha 5 GABA(A) benzodiazepine receptor model. Int J Med Chem. 2015;2015:430248. doi: 10.1155/2015/430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J. Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Zhou H, Huang S, Sarma PVVS, Zhang C. Stereospecific anxiolytic and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. 2009 PCT WO2006/004945A1, US Patent 7,618,958.
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc. Natl. Acad. Sci. USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J. Pharmacol. Exp. Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Drexler B, Zinser S, Huang S, Poe MM, Rudolph U, Cook JM, Antkowiak B. Enhancing the function of alpha5-subunit-containing GABAA receptors promotes action potential firing of neocortical neurons during up-states. Eur. J. Pharmacol. 2013;703:18–24. doi: 10.1016/j.ejphar.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr, Cook JM, Furtmüller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankowski KJ, Hedrick MP, Gosalia P, Li K, Shi S, Whipple D, Ghosh P, Prisinzano TE, Schoenen FJ, Su Y, Vasile S, Sergienko E, Gray W, Hariharan S, Milan L, Heynen-Genel S, Mangravita-Novo A, Vicchiarelli M, Smith LH, Streicher JM, Caron MG, Barak LS, Bohn LM, Chung TD, Aubé J. Discovery of small molecule kappa opioid receptor agonist and antagonist chemotypes through a HTS and Hit refinement strategy. ACS Chem. Neurosci. 2012;3:221–236. doi: 10.1021/cn200128x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW., Sr. Selective targeting of the α5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2015;308:L931–L942. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini G, Ciciani G. Benzodiazepine receptor ligands: a patent review. Expert. Opin. Ther. Pat. 2012;23:843–866. doi: 10.1517/13543776.2013.782005. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer U-B, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABAA α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–152. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- Lijinsky W, Saavedra JE, Reuber MD, Singer SS. Esophageal carcinogenesis in F344 rats by nitrosomethylethylamines substituted in the ethyl group. J Natl Cancer Inst. 1982;68:681–684. [PubMed] [Google Scholar]
- Maubach K. GABA(A) receptor subtype selective cognition enhancers. Curr. Drug. Targets. CNS. Neurol. Disord. 2003;2:233–239. doi: 10.2174/1568007033482779. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Horder J, Myers J, Coghlan S, Stokes P, Erritzoe D, Howes O, Lingford-Hughes A, Murphy D, Nutt D. The brain GABA-benzodiazepine receptor alpha-5 subtype in autism spectrum disorder: a pilot [(11)C]Ro15-4513 positron emission tomography study. Neuropharmacology. 2013;68:195–201. doi: 10.1016/j.neuropharm.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milić M, Divljaković J, Rallapalli S, van Linn ML, Timić T, Cook JM, Savić MM. The role of α1 and α5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav. Pharmacol. 2012;23:191–197. doi: 10.1097/FBP.0b013e3283512c85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JF, Burón E, Martín-López M. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABA(A) receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1389–1392. doi: 10.1016/s0278-5846(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Obradović A.Lj., Joksimović S, Poe MM, Ramerstorfer J, Varagic Z, Namjoshi O, Batinić B, Radulović T, Marković B, Roth BL, Sieghart W, Cook JM, Savić MM. SH-I-048A, an in vitro non-selective super-agonist at the benzodiazepine site of GABAA receptors: the approximated activation of receptor subtypes may explain behavioral effects. Brain. Res. 2014;1554:36–48. doi: 10.1016/j.brainres.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramerstorfer J, Furtmüller R, Vogel E, Huck S, Sieghart W. The point mutation gamma 2F77I changes the potency and efficacy of benzodiazepine site ligands in different GABAA receptor subtypes. Eur. J. Pharmacol. 2010;636:18–27. doi: 10.1016/j.ejphar.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe JP, Elster L, Frederiksen K, Bundgaard C, de Jong IE, Smith GP, Bruun AT, Larsen PH, Didriksen M. Negative modulation of GABAA α5 receptors by RO4938581 attenuates discrete sub-chronic and early postnatal phencyclidine (PCP)-induced cognitive deficits in rats. Psychopharmacology. 2012;221:451–468. doi: 10.1007/s00213-011-2593-9. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Huang S, Furtmüller R, Clayton T, Huck S, Obradović DI, Ugresić ND, Sieghart W, Bokonjić DR, Cook JM. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008;33:332–339. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- Savić MM, Milinković MM, Rallapalli S, Clayton T, Sr, Joksimović S, Van Linn M, Cook JM. The differential role of alpha1- and alpha5-containing GABA(A) receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. Int. J. Neuropsychopharmacol. 2009;12:1179–1193. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Majumder S, Huang S, Edwankar RV, Furtmüller R, Joksimović S, Clayton T, Sr, Ramerstorfer J, Milinković MM, Roth BL, Sieghart W, Cook JM. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends. Pharmacol. Sci. 2012;33:611–620. doi: 10.1016/j.tips.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh MS, Lynch JW. Selective modulators of α5-Containing GABAA receptors and their therapeutic significance. Curr. Drug. Targets. 2015;16:735–746. doi: 10.2174/1389450116666150309120235. [DOI] [PubMed] [Google Scholar]
- Soto PL, Ator NA, Rallapalli SK, Biawat P, Clayton T, Cook JM, Weed MR. Allosteric modulation of GABA(A) receptor subtypes:effects on visual recognition and visuospatial working memory in rhesus monkeys. Neuropsychopharmacology. 2013;38:2315–2325. doi: 10.1038/npp.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Matsuda A, Usami Y, Adachi T, Sugiyama T, Katagiri Y, Tatematsu M, Hirano K. Hydrolytic profile for ester- or amide-linkage by carboxylesterases pI 5.3 and 4.5 from human liver. Biol Pharm Bull. 1997;20:869–873. doi: 10.1248/bpb.20.869. [DOI] [PubMed] [Google Scholar]
- Weaver BM, Staddon GE, Mapleson WW. Tissue/blood and tissue/water partition coefficients for propofol in sheep. Br J Anaesth. 2001;86:693–703. doi: 10.1093/bja/86.5.693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.