Abstract
Insulin resistance is associated with accelerated atherosclerosis. Although high fructose is known to induce insulin resistance, it remains unclear as to how fructose regulates insulin receptor signaling and proliferative phenotype in vascular smooth muscle cells (VSMCs), which play a major role in atherosclerosis. Using human aortic VSMCs, we investigated the effects of high fructose treatment on insulin receptor substrate-1 (IRS-1) serine phosphorylation, insulin versus platelet-derived growth factor (PDGF)-induced phosphorylation of Akt, S6 ribosomal protein, and extracellular signal-regulated kinase (ERK), and cell cycle proteins. In comparison with PDGF (a potent mitogen), neither fructose nor insulin enhanced VSMC proliferation and cyclin D1 expression. D-[14C(U)]fructose uptake studies revealed a progressive increase in fructose uptake in a time-dependent manner. Concentration-dependent studies with high fructose (5 to 25 mM) showed marked increases in IRS-1 serine phosphorylation, a key adapter protein in insulin receptor signaling. Accordingly, high fructose treatment led to significant diminutions in insulin-induced phosphorylation of downstream signaling components including Akt and S6. In addition, high fructose significantly diminished insulin-induced ERK phosphorylation. Nevertheless, high fructose did not affect PDGF-induced key proliferative signaling events including phosphorylation of Akt, S6, and ERK and expression of cyclin D1 protein. Together, high fructose dysregulates IRS-1 phosphorylation state and proximal insulin receptor signaling in VSMCs, but does not affect PDGF-induced proliferative signaling. These findings suggest that systemic insulin resistance rather than VSMC-specific dysregulation of insulin receptor signaling by high fructose may play a major role in enhancing atherosclerosis and neointimal hyperplasia.
Keywords: Fructose, Insulin receptor substrate, Insulin, PDGF, Vascular smooth muscle cells, Proliferation
1. Introduction
Insulin resistance, characterized by metabolic abnormalities including glucose intolerance and dyslipidemia, is a risk factor of accelerated atherosclerosis (Bornfeldt and Tabas, 2011; Semenkovich, 2006; Taegtmeyer, 1996). While altered metabolic milieu is known to promote proatherogenic phenotype (Semenkovich, 2006), the contribution of vascular wall-specific insulin resistance toward atherosclerotic lesion progression remains unclear (Bornfeldt and Tabas, 2011; Semenkovich, 2006). In particular, vascular smooth muscle cells (VSMCs) that play a major role in atherosclerosis have been shown to exhibit proliferative and proapoptotic phenotypes upon target-specific deletion of insulin receptor signaling components (Lightell et al., 2011; Martinez-Hervas et al., 2014). For instance, insulin receptor gene deficiency in VSMCs leads to a decrease in insulin-induced Akt phosphorylation and an increase in extracellular signal-regulated kinase (ERK) phosphorylation with an accompanying increase in cell proliferation (Lightell et al., 2011). In a different study, siRNA-mediated downregulation of insulin receptor substrate-2 (IRS-2) in VSMCs diminishes insulin-induced phosphorylation of Akt and ERK thereby inducing a proapoptotic phenotype (Martinez-Hervas et al., 2014). Thus, dysregulation of insulin signaling in VSMCs at the level of insulin receptor and IRS-2 may result in proliferative and proapoptotic phenotypes, which would enhance and exacerbate atherosclerosis, respectively. Although high fructose consumption is known to induce insulin resistance and promote atherosclerosis (D’Angelo et al., 2005; Lu et al., 2013; Ning et al., 2015), it is unknown as to how fructose uptake in VSMCs regulates insulin receptor signaling and proliferative phenotype.
Previous studies demonstrate that high fructose diet induces insulin resistance in a rat model, as revealed by hyperinsulinemic-euglycemic clamp technique that shows a significant reduction in glucose infusion rate to maintain euglycemia (D’Angelo et al., 2005). In addition, high fructose diet-induced insulin resistance results in exaggerated atherosclerosis and neointima formation with a significant increase in smooth muscle cell accumulation (Lu et al., 2013; Ning et al., 2015). Since platelet-derived growth factor (PDGF) is a potent mitogen released at the site of arterial injury (Barrett and Benditt, 1987; Heldin and Westermark, 1999; Rubin et al., 1988), it is likely that high fructose-induced increase in neointima formation may occur through enhanced PDGF receptor signaling. Previously, we have shown that PDGF not only increases VSMC proliferation but also attenuates insulin-induced insulin receptor substrate (IRS-1/IRS-2)-associated PI 3-kinase/Akt signaling (Zhao et al., 2011). In the present study, we tested the hypothesis that high fructose-mediated dysregulation of insulin receptor signaling is associated with enhanced VSMC proliferation. Using human aortic VSMCs, we determined the effects of high fructose on: i) IRS-1 serine phosphorylation and IRS-1/IRS-2 expression; ii) insulin versus PDGF-induced changes in the phosphorylation of Akt, S6 ribosomal protein (a downstream target of mTOR/p70S6K signaling), and ERK; and iii) cell cycle proteins and proliferation.
2. Materials and methods
2.1. Materials
Recombinant human PDGF-BB was purchased from R&D Systems (Minneapolis, MN). Human insulin (Novolin R) was obtained from local pharmacy. D-[U-14C]fructose (specific activity: 240-360 mCi/mmol) was purchased from Moravek Biochemicals (Brea, CA). D-fructose was purchased from Sigma Chemical (St. Louis, MO). L-fructose was purchased from Omicron Biochemicals, Inc. (South Bend, IN). The primary antibodies for phospho-IRS1Ser636/639 (2388), IRS-1 (3407), IRS-2 (3089), phospho-44/42 MAPK (ERK1/2; 4695), 44/42 MAPK (ERK1/2; 9102), phospho-AktThr308 (2965), Akt (4691), phospho-S6 ribosomal proteinSer235/236 (4857), S6 ribosomal protein (2217), phospho-PDGFRβTyr751 (3161), PDGFRβ (3169), p27Kip1 (3686), cyclin D1 (2922), phospho-RbSer795 (9301), and β-actin (8457) were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals were from Fisher Scientific (Fair Lawn, NJ) or Sigma Chemical (St. Louis, MO).
2.2. Cell culture and treatments
Human aortic VSMCs, vascular cell basal medium and smooth muscle growth supplement (SMGS) were purchased from ATCC (Manassas, VA). SMGS constituents and their final concentrations after addition to vascular cell basal medium were as follows: 5% FBS (vol/vol), 5 ng/ml human basic fibroblast growth factor, 5 ng/ml human epidermal growth factor, 5 μg/ml insulin, 50 μg/mL ascorbic acid, 10 mM L-glutamine. VSMCs (passages 3–5) were maintained in vascular cell basal medium containing SMGS (complete medium), 5.5 mM D-glucose, and antibiotic/antimycotic solution in a humidified atmosphere of 95% air and 5% CO2 at 37°C. After the attainment of confluence (~6–7 days), VSMCs were trypsinized, centrifuged, and seeded onto petri dishes or multiwell plates. Subconfluent VSMCs were maintained under SMGS (serum)-deprived conditions for 48 h to achieve quiescence and then subjected to treatments as described in the legends to the respective figures. Equimolar concentrations of L-fructose were used as the vehicle controls for D-fructose in respective experiments.
2.3. Cell proliferation
Subconfluent VSMCs were serum-deprived for 48 h and then treated with D-fructose (5.5 or 25 mM), insulin (100 nM) or PDGF (30 ng/ml) for 96 h. Fresh serum-free media containing the respective treatments were replaced every 48 h. VSMCs were then trypsinized and the changes in cell number were determined using Countess Counter (Life Technologies, Carlsbad, CA), as described (Pyla et al., 2013).
2.4. Immunoblot analysis
Immunoblot analysis was performed as described (Osman and Segar, 2016). VSMC lysates (20 μg protein per lane) were subjected to electrophoresis using precast 4–12% NuPage mini-gels (Life Technologies). The resolved proteins were then transferred to PVDF membranes (EMD Millipore, Billerica, MA). Subsequently, the membranes were blocked in 5% nonfat milk and probed with the respective primary antibodies. The immunoreactivity was detected using HRP-conjugated horse anti-mouse secondary antibody (7076; Cell Signaling) or goat anti-rabbit secondary antibody (7074; Cell Signaling) followed by enhanced chemiluminescence (ECL; Thermo Scientific, Wilmington, DE). The protein bands were quantified by densitometric analysis using Image J.
2.5. D-[U-14C] fructose uptake studies
Subconfluent VSMCs (100,000 cells/well) were serum-deprived for 48 h and then washed twice with Krebs-Ringer-phosphate (KRP) buffer containing 130 mM NaCl, 5 mM KCl, 1.3 mM MgSO4, 10 mM Na2HPO4, 0.8 mM CaCl2 (pH 7.4). The washing buffer was removed completely and replaced with 500 μl radioactive cocktail (0.5 mM D-fructose and 0.5 μCi D-[U-14C]fructose in KRP buffer). After incubation for different time intervals (2-30 min) at 37°C, the cells were washed twice with ice-cold KRP buffer and then lysed with 500 μl lysis buffer (0.2 N NaOH and 1% SDS) with intermittent shaking for 10 min. The lysates were analyzed using liquid scintillation counter (Beckman Instruments, Inc., Fullerton, CA, Model LS-6500).
2.6. Statistical analysis
Results are expressed as the means ± S.E.M. of at least three separate experiments. Statistical analyses of the data were performed using one-way analysis of variance (ANOVA) followed by Bonferroni t-test. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. PDGF, but not fructose or insulin, enhances VSMC proliferation
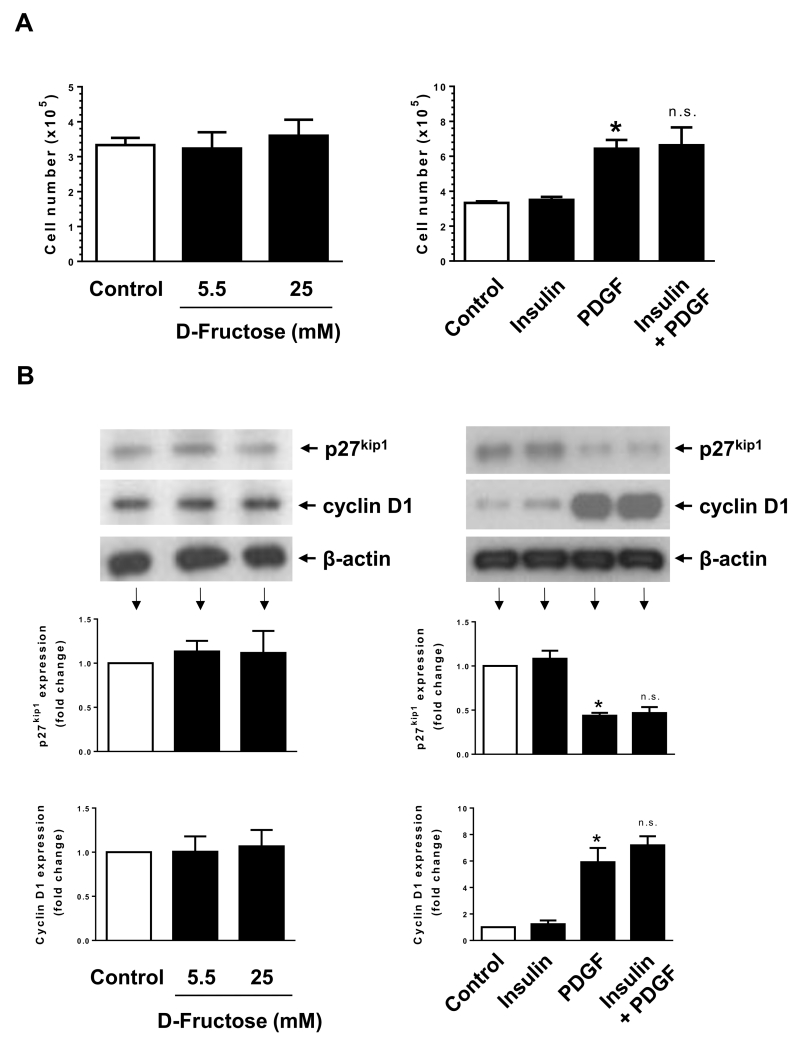
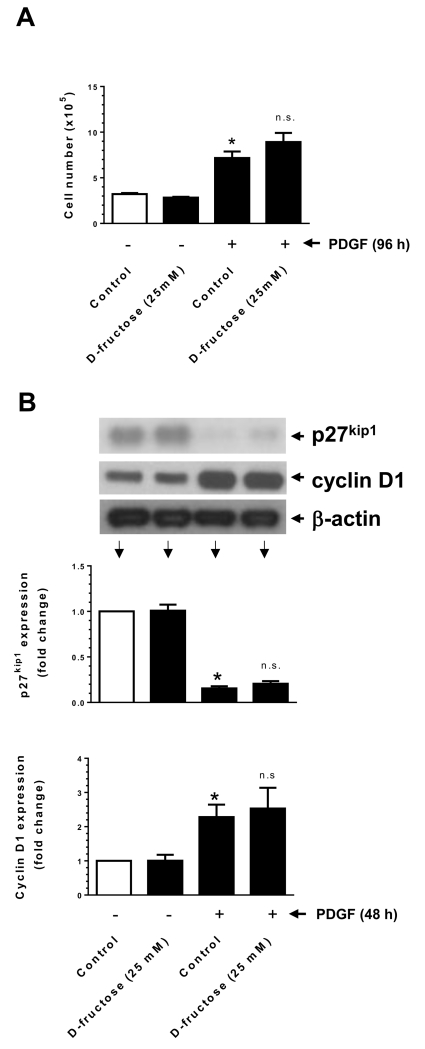
Isolated aortic VSMCs from high fructose-fed rats have been shown to exhibit enhanced proliferation in response to serum trophic factors (Miatello et al., 2001). However, a direct regulatory effect of high fructose on VSMC proliferation has not yet been examined. Insulin has been shown to enhance VSMC proliferation or maintain VSMC quiescence (Pfeifle and Ditschuneit, 1981; Wang et al., 2003). In addition, previous studies have shown that insulin treatment does not result in an increase in VSMC proliferation (Staiger et al., 2005). PDGF is a potent mitogen and is known to enhance VSMC proliferation (Owens et al., 2004). In the present study, we determined the effects of high fructose versus insulin or PDGF on VSMC proliferation and cell cycle proteins. As shown in Fig. 1A, fructose or insulin did not show significant effects on VSMC proliferation, whereas PDGF exposure led to an increase in VSMC proliferation by ~2 ± 0.5 fold. Co-incubation with insulin did not result in significant changes in PDGF-induced VSMC proliferation. In addition, fructose or insulin treatment was not associated with a decrease in the expression of p27kip1 (a cell cycle inhibitor) or an increase in the expression of cyclin D1 (a cell cycle protein) (Fig. 1B). In parallel, PDGF treatment led to a significant decrease in the expression of p27kip1 by ~56 ± 3% with an accompanying increase in cyclin D1 expression by ~6 ± 1-fold. PDGF-induced changes in p27kip1 and cyclin D1 expression were not significantly altered upon co-incubation with insulin. Furthermore, PDGF enhanced the phosphorylation of retinoblastoma (Rb) protein by ~3.8 ± 0.1 fold (data not shown). Thus, treatment of VSMCs with PDGF, but not fructose or insulin, resulted in the transition to proliferative phenotype.
Fig. 1.
Effects of fructose, insulin, versus PDGF on VSMC proliferation. (A) Serum-deprived VSMCs were exposed to D-fructose (5.5 or 25 mM), insulin (100 nM), and/or PDGF (30 ng/ml) for 96 h to determine the changes in cell number using automated counter. (B) Serum-deprived VSMCs were exposed to similar concentrations of D-fructose (for 96 h) or insulin and/or PDGF (for 48 h) to determine the changes in p27kip1 and cyclin D1 expression by immunoblot analysis. *P < 0.05 compared with control; n.s. not significant compared with PDGF alone. n = 3.
3.2. Fructose uptake occurs in a time-dependent manner in VSMCs
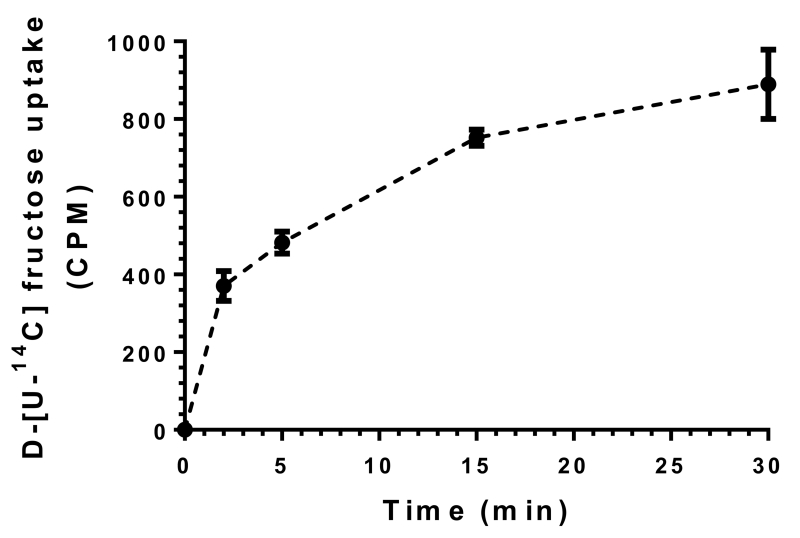
GLUT5 (fructose transporter) expression and fructose uptake have been demonstrated in several cell types including adipocytes and skeletal muscle cells (Buchs et al., 1998; Fukuzawa et al., 2013; Hajduch et al., 1998; Hajduch et al., 2003). Although we and several investigators have reported the expression of GLUT5 in VSMCs (Liu et al., 2011; Pyla et al., 2013), the ability of VSMCs to transport fructose has not been examined. As shown in Fig. 2, the uptake of D-[U-14C]fructose occurred in a time-dependent manner in VSMCs. A significant increase in fructose uptake was observed within 2 min followed by a progressive increase in fructose transport for up to 30 min.
Fig. 2.
Time course of D-fructose uptake in VSMCs. Serum-deprived VSMCs were incubated in Krebs buffer containing 0.5 μCi D-[U-14C] fructose for 2 to 30 min. Cellular uptake of radiolabeled fructose was then determined using liquid scintillation counter. n = 3.
3.3. High fructose treatment enhances IRS-1 serine phosphorylation in VSMCs
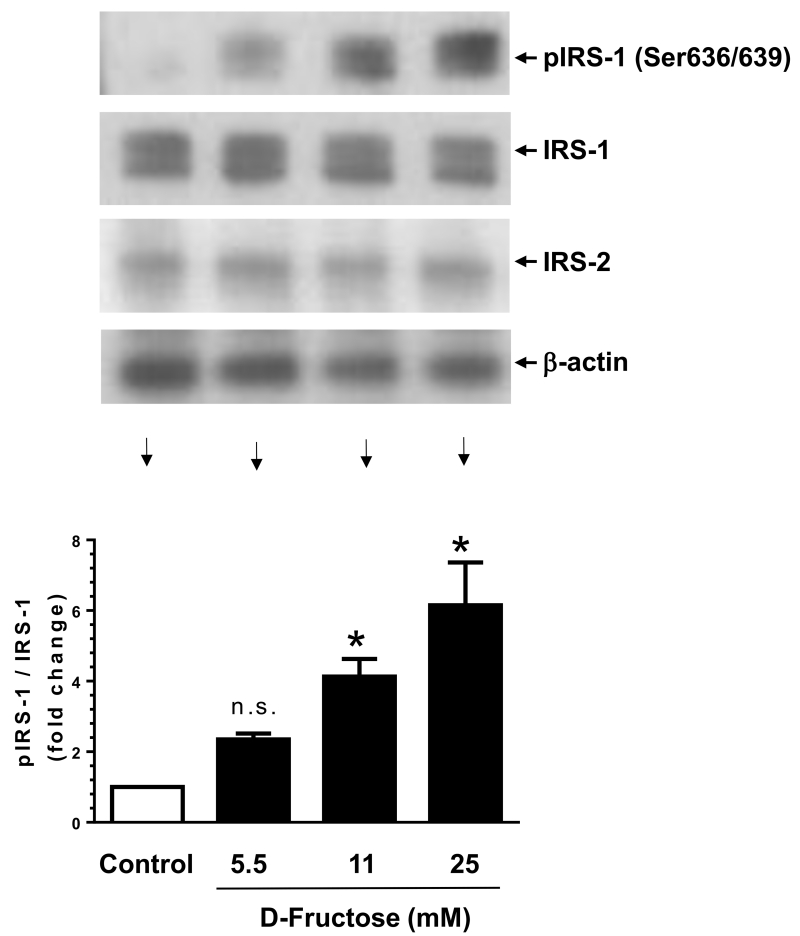
Previous studies with hepatic and skeletal muscle tissues have shown that high fructose induces IRS-1 serine phosphorylation, which is reflected by diminished insulin-induced IRS-1 tyrosine phosphorylation and PI 3-kinase activity (Bezerra et al., 2000; Wei et al., 2005). Hence, we examined the likely regulatory effects of D-fructose on IRS-1 serine phosphorylation state in VSMCs. As shown in Fig. 3, exposure of VSMCs to D-Fructose at 5.5, 11, and 25 mM concentrations led to a progressive increase in the phosphorylation of IRS-1 by ~2.3 ± 0.2-, 4.1 ± 0.5-, and 6.1 ± 1.2-fold, respectively. Under these conditions, there were no significant changes in the expression levels of IRS-1 and IRS-2 proteins. Thus, high fructose treatment in VSMCs has the potential to dysregulate the phosphorylation state of IRS-1, a key adapter protein in insulin receptor signaling.
Fig. 3.
Concentration-dependent effects of D-fructose on IRS-1 phosphorylation/expression versus IRS-2 expression in VSMCs. Serum-deprived VSMCs were exposed to increasing concentrations of D-fructose for 48 h. The cell lysates were then subjected to immunoblot analysis using primary antibodies specific for pIRS-1, IRS-1 or IRS-2. *P < 0.05 compared with control; n.s. not significant compared with control. n = 3.
3.4. High fructose attenuates insulin-induced phosphorylation of Akt, S6 ribosomal protein, and ERK in VSMCs
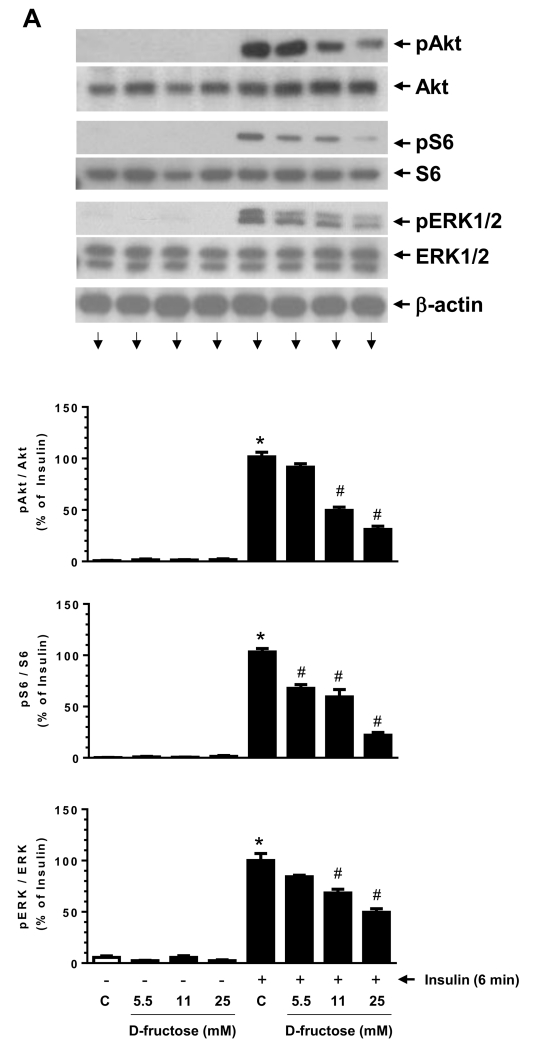
Previously, we have shown that PDGF-induced IRS-1 serine phosphorylation is associated with diminished insulin-induced PI 3-kinase/Akt signaling in VSMCs (Zhao et al., 2011). In addition, insulin resistance has been shown to suppress PI 3-kinase/Akt signaling with an accompanying activation of ERK signaling in aortic tissues (Jiang et al., 1999). To examine how fructose-induced IRS-1 serine phosphorylation impacts Akt and ERK signaling in VSMCs, we determined the effects of D-fructose pretreatment on acute insulin stimulation. As shown in Fig. 4A, D-fructose pretreatment at 5.5 mM, 11 mM, and 25 mM concentrations led to progressive decreases in insulin-induced phosphorylation of Akt, S6 ribosomal protein, and ERK1/2. In particular, D-fructose pretreatment at 25 mM concentration resulted in significant diminutions in insulin-induced phosphorylation of Akt, S6, and ERK1/2 by 69 ± 3%, 78 ± 3%, and 50 ± 4%, respectively.
Fig. 4.
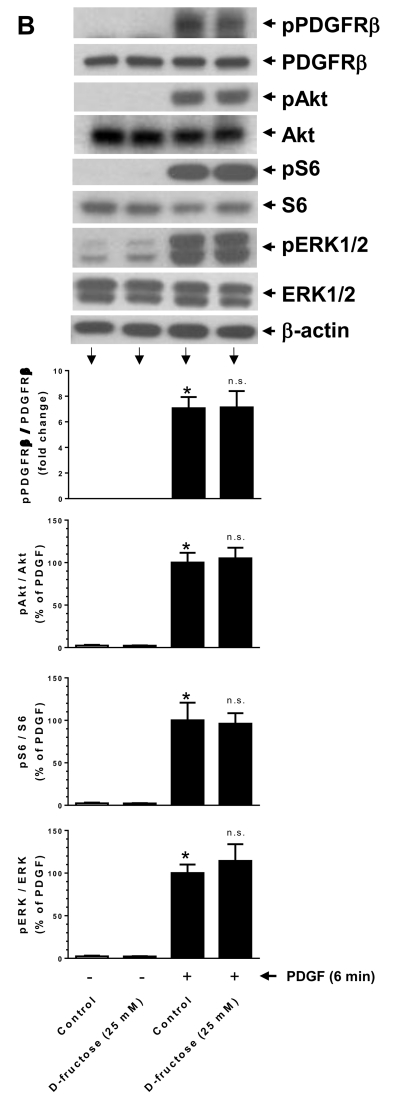
Effects of D-fructose pretreatment on insulin- versus PDGF-induced phosphorylation of Akt, S6, and ERK in VSMCs. Serum-deprived VSMCs were pretreated with the indicated concentrations of D-fructose for 48 h followed by acute stimulation with 100 nM insulin (A) or 30 ng/ml PDGF (B) for 6 min. The cell lysates were then subjected to immunoblot analysis using primary antibodies specific for pAkt, pS6, or pERK1/2. PDGF-treated cells were also probed for pPDGFRβ and PDGFRβ expression. *P < 0.05 compared with control; #P < 0.05 compared with insulin alone; n.s. not significant compared with PDGF alone. n = 3.
3.5. High fructose does not affect PDGF-induced phosphorylation of PDGF receptor-β, Akt, S6 ribosomal protein, and ERK in VSMCs
To examine whether high fructose-mediated attenuation of agonist-induced signaling events is specific for insulin, we determined the effects of high fructose on PDGF receptor signaling in parallel. As shown in Fig. 4B, D-fructose pretreatment at 25 mM concentration did not affect PDGF receptor-β expression, PDGF-induced PDGF receptor-β tyrosine phosphorylation, or PDGF-induced key proliferative signaling events including phosphorylation of Akt, S6, and ERK.
3.6. High fructose does not affect PDGF-induced VSMC proliferation or PDGF-induced changes in p27kip1 and cyclin D1 expression
To further confirm whether PDGF-induced proliferative signaling events are refractory to high fructose, we examined the changes in VSMC proliferation and expression levels of p27kip1 and cyclin D1. As shown in Fig. 5A, D-fructose pretreatment at 25 mM concentration did not result in significant changes in PDGF-induced VSMC proliferation. In addition, D-fructose pretreatment did not affect PDGF-mediated decrease in p27kip1 expression or increase in cyclin D1 expression (Fig. 5B).
Fig. 5.
Effects of D-fructose pretreatment on PDGF-induced VSMC proliferation. Serum-deprived VSMCs were pretreated with 25 mM D-fructose for 48 h followed by exposure to 30 ng/ml PDGF for: (A) 96 h to determine the changes in cell number using automated counter; or (B) 48 h to determine the changes in p27kip1 and cyclin D1 expression by immunoblot analysis, as described. *P < 0.05 compared with control; n.s. not significant compared with PDGF alone. n = 3-6.
4. Discussion
Previous studies have shown that in rodents fed a 20-40% fructose diet, circulating concentration of fructose increases at a range of 0.2 to 1 mM (Patel et al., 2015). Fructose is known to be transported passively into several tissues including skeletal muscle and adipocytes through plasma membrane-localized fructose transporter (glucose transporter-5, GLUT5) (Douard and Ferraris, 2008). The present study provides evidence for fructose uptake in VSMCs for the first time using radiolabeled 14C-fructose. Furthermore, we and several other investigators have demonstrated the expression of GLUT5 mRNA in VSMCs and aortic tissues (Liu et al., 2011; Pyla et al., 2013). In addition to the contribution from GLUT5-mediated fructose uptake, elevation of intracellular fructose concentration can occur through de novo synthesis from high glucose. This is achieved through the polyol pathway that involves the activation of aldose reductase (a rate-limiting enzyme) and the intermediary formation of sorbitol (Lanaspa et al., 2013; Yasunari et al., 1995). In this regard, exposure of VSMCs to 25 mM glucose results in the elevation of polyol pathway metabolites including sorbitol and fructose (Liu et al., 2011). Importantly, 25 mM fructose treatment has been shown to enhance intracellular fructose to a similar level with an accompanying induction of GLUT5 mRNA in VSMCs (Liu et al., 2011). The present findings reveal that at 11 to 25 mM concentrations, fructose has the potential to enhance IRS-1serine phosphorylation in VSMCs, thereby attenuating insulin-induced phosphorylation of downstream signaling components such as Akt and ribosomal protein S6. In addition, high fructose treatment inhibits insulin-induced phosphorylation of ERK. Contrary to our hypothesis, high fructose-mediated disruption of insulin receptor signaling does not affect PDGF-induced VSMC proliferation or key proliferative signaling events as evidenced by sustenance in the phosphorylation state of Akt, S6, and ERK and the expression level of cyclin D1.
Our findings on fructose dysregulation of insulin receptor signaling in VSMCs are in conformity with previous studies, which demonstrate high fructose-induced insulin resistance in hepatic tissue and skeletal muscle (Bezerra et al., 2000; Wei et al., 2005). For instance, high-fructose diet in rats leads to significant decreases in insulin-induced IRS-1 tyrosine phosphorylation and IRS-1 association with PI 3-kinase in the liver and skeletal muscle (Bezerra et al., 2000). In rat primary hepatocytes treated with high fructose, IRS-1 serine phosphorylation is increased with an accompanying decrease in insulin-induced IRS-1 tyrosine phosphorylation (Wei et al., 2005). From a mechanistic standpoint, fructose-mediated dysregulation of IRS-1 and the resultant suppression of downstream signaling events may be attributable to several factors including methylglyoxal accumulation and c-jun N-terminal kinase (JNK) activation (Dhar et al., 2008; Liu et al., 2011; Riboulet-Chavey et al., 2006; Wei et al., 2005). It is noteworthy that, in high-fructose diet-fed rats, a significant accumulation of methylglyoxal has been observed in aortic tissues (Liu et al., 2011). In addition, high fructose treatment (15 to 25 mM) under in vitro conditions has been shown to enhance methylglyoxal accumulation in aortic VSMCs (Liu et al., 2011; Wang et al., 2006). Together, these findings suggest that high fructose-mediated accumulation of methylglyoxal may promote IRS-1 serine phosphorylation, thereby disrupting insulin receptor signaling in VSMCs.
Previous studies have shown that in a rat model of obesity, there is a selective resistance to PI 3-kinase/Akt signaling but not ERK pathway in aortic tissues (Jiang et al., 1999). Furthermore, in VSMCs isolated from insulin receptor-deficient mice, a decrease in insulin-induced Akt phosphorylation is associated with an increase in ERK phosphorylation, which may decrease p27Kip1 expression to enhance proliferation (Lightell et al., 2011). Such selective insulin resistance in vascular cells would augment the atherogenic potential in insulin-resistant states (Gogg et al., 2009; Jiang et al., 1999). However, high fructose treatment diminishes insulin-induced activation of Akt, S6 (a downstream target of mTOR), and ERK in VSMCs (present study). Notably, methylglyoxal, an intermediary metabolite of fructose, has been previously shown to inhibit not only IRS-1 tyrosine phosphorylation and Akt signaling but also insulin-induced activation of ERK (Riboulet-Chavey et al., 2006). Thus, high fructose-mediated disruption of insulin receptor signaling including ERK does not result in enhanced VSMC proliferation, as evidenced in the present study.
To further understand the relationship between high fructose exposure and VSMC proliferative phenotype, the present study has also examined whether dysregulated insulin receptor signaling is associated with altered PDGF receptor signaling. Previously, high glucose has been shown to increase aldose reductase activity and fructose formation thereby upregulating PDGF receptor-β expression (Campbell et al., 2003; Kasuya et al., 1999), which in turn results in enhanced PDGF-induced VSMC proliferation. The present findings reveal that in high fructose-treated VSMCs, PDGF receptor-β expression remains unchanged. In addition, high fructose does not affect PDGF-induced PDGF receptor-β tyrosine phosphorylation. Furthermore, PDGF-induced phosphorylation of key proliferative signaling events (e.g., Akt, S6, and ERK) and the expression of cell cycle protein (e.g., cyclin D1) remain essentially the same with our without high fructose treatment. These findings suggest that, under the conditions of VSMC-specific insulin resistance resulting from high fructose-induced IRS-1 serine phosphorylation, the growth-promoting effects of PDGF will be preserved in the vessel wall.
It is noteworthy that the exaggerated neointima formation observed in previous studies with IRS-1-deficient mice has been attributed to altered metabolic milieu in insulin-resistant state (Kubota et al., 2003). High fructose is known to induce metabolic changes in the liver including enhanced de novo lipogenesis (Samuel, 2011). In addition, high fructose consumption may lead to hepatic insulin resistance and dysregulation of lipid/lipoprotein profile in the systemic circulation (Herman and Samuel, 2016; Stanhope et al., 2015). In conclusion, systemic insulin resistance rather than VSMC-specific dysregulation of insulin receptor signaling by high fructose may play a major role in enhancing atherosclerosis and neointimal hyperplasia.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute/National Institutes of Health Grant (R01-HL-097090), University of Georgia Research Foundation Fund, University of Georgia RC Wilson Pharmacy Fund, and University of Georgia College of Pharmacy Graduate Assistantship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett TB, Benditt EP. sis (platelet-derived growth factor B chain) gene transcript levels are elevated in human atherosclerotic lesions compared to normal artery. Proc Natl Acad Sci U S A. 1987;84:1099–1103. doi: 10.1073/pnas.84.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra RM, Ueno M, Silva MS, Tavares DQ, Carvalho CR, Saad MJ. A high fructose diet affects the early steps of insulin action in muscle and liver of rats. J Nutr. 2000;130:1531–1535. doi: 10.1093/jn/130.6.1531. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchs AE, Sasson S, Joost HG, Cerasi E. Characterization of GLUT5 domains responsible for fructose transport. Endocrinology. 1998;139:827–831. doi: 10.1210/endo.139.3.5780. [DOI] [PubMed] [Google Scholar]
- Campbell M, Allen WE, Silversides JA, Trimble ER. Glucose-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase-dependent upregulation of the platelet-derived growth factor-beta receptor potentiates vascular smooth muscle cell chemotaxis. Diabetes. 2003;52:519–526. doi: 10.2337/diabetes.52.2.519. [DOI] [PubMed] [Google Scholar]
- D’Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague-Dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- Dhar A, Desai K, Kazachmov M, Yu P, Wu L. Methylglyoxal production in vascular smooth muscle cells from different metabolic precursors. Metabolism. 2008;57:1211–1220. doi: 10.1016/j.metabol.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa T, Fukazawa M, Ueda O, Shimada H, Kito A, Kakefuda M, Kawase Y, Wada NA, Goto C, Fukushima N, Jishage K, Honda K, King GL, Kawabe Y. SGLT5 reabsorbs fructose in the kidney but its deficiency paradoxically exacerbates hepatic steatosis induced by fructose. PLoS One. 2013;8:e56681. doi: 10.1371/journal.pone.0056681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58:2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Darakhshan F, Hundal HS. Fructose uptake in rat adipocytes: GLUT5 expression and the effects of streptozotocin-induced diabetes. Diabetologia. 1998;41:821–828. doi: 10.1007/s001250050993. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Litherland GJ, Turban S, Brot-Laroche E, Hundal HS. Insulin regulates the expression of the GLUT5 transporter in L6 skeletal muscle cells. FEBS Lett. 2003;549:77–82. doi: 10.1016/s0014-5793(03)00773-7. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Herman MA, Samuel VT. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol Metab. 2016 doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya Y, Nakamura J, Hamada Y, Nakayama M, Sasaki H, Komori T, Chaya S, Watanabe G, Naruse K, Nakashima E, Kato K, Hotta N. An aldose reductase inhibitor prevents the glucose-induced increase in PDGF-beta receptor in cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1999;261:853–858. doi: 10.1006/bbrc.1999.1111. [DOI] [PubMed] [Google Scholar]
- Kubota T, Kubota N, Moroi M, Terauchi Y, Kobayashi T, Kamata K, Suzuki R, Tobe K, Namiki A, Aizawa S, Nagai R, Kadowaki T, Yamaguchi T. Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation. 2003;107:3073–3080. doi: 10.1161/01.CIR.0000070937.52035.25. [DOI] [PubMed] [Google Scholar]
- Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, Diggle CP, Asipu A, Petrash JM, Kosugi T, Maruyama S, Sanchez-Lozada LG, McManaman JL, Bonthron DT, Sautin YY, Johnson RJ. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nature Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightell DJ, Jr., Moss SC, Woods TC. Loss of canonical insulin signaling accelerates vascular smooth muscle cell proliferation and migration through changes in p27Kip1 regulation. Endocrinology. 2011;152:651–658. doi: 10.1210/en.2010-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang R, Desai K, Wu L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc Res. 2011;92:494–503. doi: 10.1093/cvr/cvr239. [DOI] [PubMed] [Google Scholar]
- Lu J, Ji J, Meng H, Wang D, Jiang B, Liu L, Randell E, Adeli K, Meng QH. The protective effect and underlying mechanism of metformin on neointima formation in fructose-induced insulin resistant rats. Cardiovasc Diabetol. 2013;12:58. doi: 10.1186/1475-2840-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hervas S, Vinue A, Nunez L, Andres-Blasco I, Piqueras L, Real JT, Ascaso JF, Burks DJ, Sanz MJ, Gonzalez-Navarro H. Insulin resistance aggravates atherosclerosis by reducing vascular smooth muscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovasc Res. 2014;103:324–336. doi: 10.1093/cvr/cvu115. [DOI] [PubMed] [Google Scholar]
- Miatello R, Risler N, Castro C, Gonzalez S, Ruttler M, Cruzado M. Aortic smooth muscle cell proliferation and endothelial nitric oxide synthase activity in fructose-fed rats. Am J Hypertens. 2001;14:1135–1141. doi: 10.1016/s0895-7061(01)02206-3. [DOI] [PubMed] [Google Scholar]
- Ning B, Wang X, Yu Y, Waqar AB, Yu Q, Koike T, Shiomi M, Liu E, Wang Y, Fan J. High-fructose and high-fat diet-induced insulin resistance enhances atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Nutr Metab. 2015;12:30. doi: 10.1186/s12986-015-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman I, Segar L. Pioglitazone, a PPARgamma agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol. 2016;101:54–70. doi: 10.1016/j.bcp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Patel C, Sugimoto K, Douard V, Shah A, Inui H, Yamanouchi T, Ferraris RP. Effect of dietary fructose on portal and systemic serum fructose levels in rats and in KHK−/− and GLUT5−/− mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G779–790. doi: 10.1152/ajpgi.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifle B, Ditschuneit H. Effect of insulin on growth of cultured human arterial smooth muscle cells. Diabetologia. 1981;20:155–158. doi: 10.1007/BF00262020. [DOI] [PubMed] [Google Scholar]
- Pyla R, Poulose N, Jun JY, Segar L. Expression of conventional and novel glucose transporters, GLUT1, -9, -10, and -12, in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2013;304:C574–589. doi: 10.1152/ajpcell.00275.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55:1289–1299. doi: 10.2337/db05-0857. [DOI] [PubMed] [Google Scholar]
- Rubin K, Tingstrom A, Hansson GK, Larsson E, Ronnstrand L, Klareskog L, Claesson-Welsh L, Heldin CH, Fellstrom B, Terracio L. Induction of B-type receptors for platelet-derived growth factor in vascular inflammation: possible implications for development of vascular proliferative lesions. Lancet. 1988;1:1353–1356. doi: 10.1016/s0140-6736(88)92177-0. [DOI] [PubMed] [Google Scholar]
- Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011;22:60–65. doi: 10.1016/j.tem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger K, Staiger H, Schweitzer MA, Metzinger E, Balletshofer B, Haring HU, Kellerer M. Insulin and its analogue glargine do not affect viability and proliferation of human coronary artery endothelial and smooth muscle cells. Diabetologia. 2005;48:1898–1905. doi: 10.1007/s00125-005-1874-4. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101:1144–1154. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H. Insulin resistance and atherosclerosis. Common roots for two common diseases? Circulation. 1996;93:1777–1779. doi: 10.1161/01.cir.93.10.1777. [DOI] [PubMed] [Google Scholar]
- Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52:2562–2569. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- Wang H, Meng QH, Chang T, Wu L. Fructose-induced peroxynitrite production is mediated by methylglyoxal in vascular smooth muscle cells. Life Sci. 2006;79:2448–2454. doi: 10.1016/j.lfs.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Pagliassotti MJ. Fructose selectively modulates c-jun N-terminal kinase activity and insulin signaling in rat primary hepatocytes. J Nutr. 2005;135:1642–1646. doi: 10.1093/jn/135.7.1642. [DOI] [PubMed] [Google Scholar]
- Yasunari K, Kohno M, Kano H, Yokokawa K, Horio T, Yoshikawa J. Aldose reductase inhibitor prevents hyperproliferation and hypertrophy of cultured rat vascular smooth muscle cells induced by high glucose. Arterioscler Thromb Vasc Biol. 1995;15:2207–2212. doi: 10.1161/01.atv.15.12.2207. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Biswas SK, McNulty PH, Kozak M, Jun JY, Segar L. PDGF-induced vascular smooth muscle cell proliferation is associated with dysregulation of insulin receptor substrates. Am J Physiol Cell Physiol. 2011;300:C1375–1385. doi: 10.1152/ajpcell.00670.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]