Abstract
Background
This study aimed to identify how the activity of large-scale brain networks differs between mood states in bipolar disorder. The authors measured spontaneous brain activity in subjects with bipolar disorder in mania and euthymia and compared these states to a healthy comparison population.
Methods
23 subjects with bipolar disorder type I in a manic episode, 24 euthymic bipolar I subjects, and 23 matched healthy comparison (HC) subjects underwent resting state fMRI scans. Using an existing parcellation of the whole brain, we measured functional connectivity between brain regions and identified significant differences between groups.
Results
In unbiased whole-brain analyses, functional connectivity between parietal, occipital, and frontal nodes within the dorsal attention network (DAN) were significantly greater in mania than euthymia or HC subjects. In the default mode network (DMN), connectivity between dorsal frontal nodes and the rest of the DMN differentiated both mood state and diagnosis.
Limitations
The bipolar groups were separate cohorts rather than subjects imaged longitudinally across mood states.
Conclusions
Bipolar mood states are associated with highly significant alterations in connectivity in two large-scale brain networks. These same networks also differentiate bipolar mania and euthymia from a HC population. State related changes in DAN and DMN connectivity suggest a circuit based pathology underlying cognitive dysfunction as well as activity / reactivity in bipolar mania. Altered activities in neural networks may be biomarkers of bipolar disorder diagnosis and mood state that are accessible to neuromodulation are promising novel targets for scientific investigation and possible clinical intervention.
Keywords: Bipolar, Mania, fMRI, Network, Imaging, Euthymia
Introduction
Bipolar disorder is a debilitating psychiatric disorder estimated to affect between 2 and 5% of the population (Merikangas et al., 2007). It may be instructive to examine the instability of neural activity in the varying mood states in bipolar disorder for clues into the mechanisms of specific mood states and the underlying physiology of bipolar disorder itself. A growing body of scientific inquiry has examined changes in neural activity associated with specific cognitive tasks such as emotion and reward processing (reviewed in (Phillips and Swartz, 2014; Strakowski et al., 2012)). Drawing upon these findings from the primarily task-based fMRI literature, we recently examined the “resting state” (rsfMRI) functional connectivity of bipolar mania when compared to bipolar euthymia (Brady et al., 2016). That analysis examined functional connectivity to brain regions selected from a consensus model of the neurobiology of bipolar disorder (Strakowski et al., 2012). We observed mood state specific aberrant connectivity between the amygdala and brain regions implicated in emotion regulation even under rest (non-task) conditions.
We sought to complement our prior study of mood related connectivity of select cortical and subcortical regions with a more data-driven analysis of functional connectivity across the entire brain. The analysis of rsfMRI has demonstrated the presence of large-scale brain networks whose function is altered in psychiatric and neurologic diseases e.g. (Baker et al., 2014; Yeo et al., 2011; Zhou and Seeley, 2014). In bipolar disorder comparatively few studies have sought to examine whole brain measures of network activity and connectivity and there is a growing call to incorporate these studies into a bipolar imaging literature that has most often examined local networks(Chase and Phillips, 2016). Several recent studies have examined large scale brain networks to differentiate bipolar disorder from other diseases (reviewed in (Chase and Phillips, 2016; Lois et al., 2014).
We examined spontaneous neural activity in bipolar disorder to understand how whole brain connectivity is altered in subjects with bipolar disorder in different mood states. We compared rsfMRI data from subjects diagnosed with bipolar disorder type I in a manic state to euthymic bipolar I subjects and a healthy comparison group. We then used a data driven approach to examine changes in brain network activity across and between these groups. We measured the low-frequency oscillations of the blood-oxygen-level dependent (BOLD) signal and conducted a whole-brain analysis of the functional connectivity (FC) between all grey matter regions. We then examined FC values for significant group differences across and between groups. We chose to use an existing atlas that parcellates grey matter into regions defined by functional connectivity (Craddock et al., 2012). In doing this we hoped to capture differences in large scale network activity that does not adhere to anatomical boundaries. While this can be accomplished by independent component analysis (ICA) (see (Meda et al., 2012) for example), the method utilized here would also allow us to isolate differences in individual nodes of networks that may otherwise be broadly similar between groups.
Primarily, we hypothesized that FC measures would differentiate mood states in bipolar disorder as well as differentiating subjects diagnosed with bipolar disorder, whatever their mood state, from matched healthy comparison subjects.
Methods
Participants
The McLean Hospital Institutional Review Board approved the study, and all participants gave written informed consent before participating. Bipolar subjects were recruited as in our previous studies (Brady et al., 2012; Brady et al., 2016; Ongur et al., 2008). Almost all (21/23) of the manic bipolar subjects were recruited from McLean Hospital inpatient units while hospitalized for a manic episode. Euthymic bipolar patients were primarily (17/24) recruited by contacting bipolar subjects who had previously been hospitalized on McLean Hospital inpatient units. Recruiting of both patient groups occurred contemporaneously and there was no change in clinical assessment, imaging procedure or scanner software during the time that all of the subjects were recruited.
Diagnosis was determined using the Structured Clinical Interview for the DSM-IV (SCID)(First and New York State Psychiatric Institute. Biometrics Research, 2007). Patients were assessed by trained research staff, who carried out monthly reliability exercises where a study subject was interviewed in the presence of the research team. All subjects in the patient group met DSM-IVTR criteria for the diagnosis of bipolar disorder type I. Healthy comparison (HC) subjects did not meet criteria for any Axis I psychiatric disorder currently or historically.
Exclusion criteria included age outside the range of 18–65, any neurological illness, positive pregnancy test or lactation, electroconvulsive therapy in the last three months, history of head trauma with a loss of consciousness lasting more than a few minutes, and contraindications to magnetic resonance imaging.
47 subjects with bipolar disorder type I, and 23 HC subjects participated in the study. Of the bipolar subjects, 23 subjects met DSMIVTR criteria for a manic episode and almost all (21/23) were currently hospitalized. 24 subjects with bipolar disorder were euthymic and in outpatient treatment. Almost all of the euthymic bipolar subjects (23/24) had a previous history of hospitalization and the median time since last hospitalization was 6.5 months. All euthymic subjects did not meet DSM criteria for any mood episode in the month prior to scanning. Subjects diagnosed with bipolar disorder were assessed using the SCID, Young Mania Rating Scale (YMRS), Montgomery–Asberg Depression Rating Scale (MADRS), Positive And Negative Syndrome Scale (PANSS), and North American Adult Reading Test (NAART) on the day of the study. HC subjects were assessed using the SCID. Demographic, clinical, and medication regimen information are summarized in Table 1. Chlorpromazine equivalents (CPZE) were calculated as in (Woods, 2003).
Table 1.
Detailed demographics, clinical, and medication information of the study population.
| Bipolar Subjects (n=47) | Bipolar-Manic Subjects (n=23) | Bipolar-Euthymic Subjects (n=24) | Healthy Control Subjects (n=23) | Statistic | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Demographics | Bipolar vs Control | Control vs Mania | Mania vs Euthymia | Euthymia vs Control | ||||
| Age in years, mean (SD) | 29.3 (11.5) | 27.7 (11.1) | 30.9 (11.9) | 29.7 (10.9) | MW p=.527 |
MW p=.186 |
MW p=.076 |
MW p=.840 |
| Sex | df=1 χ2=.003 p=.956 |
df=1 χ2=.107 p=.743 |
df=1 χ2=.295 p=.587 |
df=1 χ2=.045 p=.831 |
||||
| male | 33 | 17 | 16 | 16 | ||||
| female | 14 | 6 | 8 | 7 | ||||
| Clinical Characteristics | ||||||||
| YMRS, mean (SD) | 14.4 (13.3) | 26.9 (5.70) | 2.33 (3.40) | n/a | MW p<.001 |
|||
| MADRS, mean (SD) | 8.89 (7.02) | 12.6 (6.60) | 5.33 (5.46) | n/a | MW p<.001 |
|||
| PANSS, mean (SD) | 49.3 (14.3) | 60.4 (10.3) | 38.6 (8.11) | n/a | MW p<.001 |
|||
| PANSS positive subscale, mean (SD) | 15.9 (8.11) | 22.7 (4.85) | 9.33 (4.24) | n/a | MW p<.001 |
|||
| Anticonvulsants | 17/47 | 8/23 | 9/24 | n/a | df=1 χ2= .038 p=.846 |
|||
| Antipsychotics | 37/47 | 22/23 | 15/24 | n/a | df=1 χ2=7.07 p=.006 |
|||
| CPZ equivalents, mean (SD) | 254 (253) | 370 (254) | 143 (200) | n/a | MW p=0.001 |
|||
| Benzodiazepines | 10/47 | 6/23 | 4/24 | n/a | df=1 χ2=.622 p=.430 |
|||
| Lithium | 34/47 | 18/23 | 16/24 | n/a | df=1 χ2= .789 p=.374 |
|||
| Antidepressants | 1/47 | 0/23 | 1/24 | n/a | df=1 χ2=.979 p=.322 |
|||
| framewise displacement in mm, mean (SD) | .136 (.058) | .140 (.066) | .133 (.051) | .121 (.071) | t(119) = 1.265 p=.208 |
t(73) = 1.218 p=.227 |
t(62)= .536 p=.594 |
t(84) = .918 p=.361 |
Abbreviations: MW: Mann-Whitney U Test, PANSS: Positive And Negative Symptom Scale, SD: Standard Deviation, YMRS: Young Mania Rating Scale.
MRI data acquisition
Data were acquired on a 3T Siemens scanner using a standard head coil. The echoplanar imaging parameters were as follows: repetition time, 3000 milliseconds; echo time, 30 milliseconds; flip angle, 85°; 3 × 3 × 3-mm voxels; field of view, 216; and 47 axial sections collected with interleaved acquisition and no gap. Structural data included a high-resolution, multiecho, T1-weighted, magnetization-prepared, gradient-echo image. In addition, all participants underwent two resting fMRI runs with the instructions ‘remain still, stay awake, and keep your eyes open’. Video recording of the eyes was used while in the scanner to confirm the awake, eye-open state. Each functional run lasted 6.2 minutes (124 time points).
MRI data processing
The imaging data were preprocessed using the Data Processing Assistant for Resting-State fMRI (DPARSF(Chao-Gan and Yu-Feng, 2010),). To minimize effects of scanner signal stabilization, the first four images were omitted from all analysis. Scans with head motion exceeding 2mm or 20 of maximum rotation through the resting-state run were discarded. This step resulted in a total of 35 analyzed scans from 23 bipolar mania subjects, 46 analyzed scans from 24 bipolar euthymia subjects, and 40 analyzed scans from 23 HC subjects. Functional and structural images were co-registered. Structural images were then normalized and segmented into gray, white and CSF partitions.. A Friston 24-parameter model(Friston et al., 1996) was used to regress out head motion effects from the realigned data. The CSF and white matter signals as well as the linear trend were also regressed as nuisance covariates. After realigning, slice timing correction, and co-registration, framewise displacement (FD) was calculated for all resting state volumes (Power et al., 2012). All volumes with a FD greater than .2mm were regressed out as nuisance covariates. After nuisance covariate regression the resultant data were band pass filtered to select low frequency (.01-.08Hz) signals. Filtered data were normalized by into MNI space and then smoothed by a Gaussian kernel of 8mm3 full-width at half maximum (FWHM). Voxels within a group derived gray matter mask were used for further analyses. While the combination of GSR with volume editing effectively removes motion effects, there remain concerns about the effect of GSR to distort group differences (Gotts et al., 2013; Power et al., 2014; Yan et al., 2013). We therefore processed the resting state data twice, once as above and a second time with global signal regression.
Functional Connectivity Analysis
We measured whole-brain changes in FC by utilizing an existing brain atlas that parcellates brain grey matter into 200 functionally distinct subunits (Craddock et al., 2012). We then calculated correlation coefficients for the BOLD timecourses between each ROI and all 199 other ROIs for each subject using DPARSF(Chao-Gan and Yu-Feng, 2010), generating a 200×200 matrix of correlation coefficients for each subject. We used DPARSF to Fisher’s Z transform these R values generating a 200×200 matrix of z-transformed functional connectivity (zFC) values for each subject.
Statistical Analyses
Group identity and functional connectivity
We performed a one-way ANOVA comparing zFC values for all ROI pairs in the 200×200 matrix for the three groups (bipolar mania, bipolar euthymia, and HC). We then corrected the resulting p-values for multiple comparisons using a false discovery rate (FDR) of q <.05. Post-Hoc testing for differences between individual groups was performed using Tukeys HSD test. We then repeated this analysis using resting state data that had been preprocessed using the additional step of global signal regression. Only results that were statistically significant under both preprocessing methods are presented here.
Medication dosage and functional connectivity
Given differences in antipsychotic medication prescription between bipolar manic and euthymic groups, we examined the effect of antipsychotic dosage on FC values by computing the Pearson’s correlation coefficient between prescribed antipsychotic dosage (in chlorpromazine equivalents, CPZE) and each of the zFC values in the 200×200 matrix for the 47 subjects with bipolar disorder.
Voxel-wise differences in functional connectivity
To complement the ROI to ROI based changes in functional connectivity and better integrate these results with extant FC literature; we performed an exploratory analysis of how mania symptomatology correlates with FC at the individual voxel level (i.e. a ROI to voxel analysis). We did this by utilizing the DPARSF toolkit to generate whole brain maps of zFC values for all subjects using ROIs identified in the ROI to ROI analysis. These maps were then entered into SPM8 (Statistical and Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm) to generate maps of how whole brain FC to selected ROIs varies with group membership (mania versus euthymia and euthymia vs HC).
Table and figure generation
Graphs of relationships between group and zFC values were generated using SPSS Version 23. Projections of ROIs and T contrast maps onto cortical surfaces was accomplished using Caret (Van Essen et al., 2001)
Results
Subject Characteristics
Table 1 lists demographic and clinical characteristics of the study population. When grouped according to DSM criteria, bipolar subjects in a DSMIVTR defined manic state were significantly more symptomatic than subjects in a euthymic state on all symptoms scales. Increased MADRS scores in mania typically came from scores on the reduced sleep, concentration difficulties, and inner tension items. All bipolar subjects were prescribed medication. All antipsychotic medications prescribed in both groups were second generation. Subjects in the manic group were more likely to be prescribed anti-psychotics and at higher doses than the euthymic group. The two bipolar mood state sub-groups did not differ in prevalence of lithium, anti-epileptic drugs, anti-depressants, or benzodiazepines in their medication regimens. Bipolar subjects had a higher mean framewise displacement (FD) than HC subjects. The manic sub-group had a higher mean FD than the euthymic sub-group or HC subjects but there were no significant differences in FD between any of the groups. NAART testing, an estimate of pre-morbid intellectual ability, was available for all 23 manic bipolar subjects and 21 of the euthymic bipolar subjects. Scores on the NAART for the manic (115 +/− 9.23) and euthymic (120 +/− 7.37) bipolar subject groups did not show significant differences (p=.245). In comparing illness history, information about duration of illness was available for 46/47 bipolar subjects and information about the number of prior hospitalizations was available for 45/47 bipolar subjects. Duration of illness in the manic (6.81 +/− 7.06 years) and euthymic (9.04 +/− 11.7 years) groups was not significantly different (p=.438). The number of prior hospitalizations in the manic (2.05 +/− 3.32) and euthymic (3.0 +/− 2.77) groups was not significantly different (p=.307).
ROI to ROI Functional connectivity changes related to bipolar diagnosis and mood state
Dividing all grey matter regions into 200 individual ROIs and measuring zFC values between ROIs reveal ROI-pair relationships that demonstrate significant differences across the three groups. Out of 1.99 × 104 unique ROI-ROI pairs, twenty-two ROI pairs demonstrated significant (p-FDR corrected <.05) across group differences regardless of the preprocessing strategy used (GSR vs no GSR). These ROI pairs (including hemisphere, lobe and Brodmann area locations) and zFC values (after preprocessing without GSR) are reported in Table 2. ANOVA F-statistic and Post-hoc testing using Tukey’s HSD tests for between group differences are also reported in Table 2. A graphic representation of all significant ROI pairs projected onto the cortical surface is presented with bar graphs for zFC scores for each group in Supplementary Figure 1. ROI numbering corresponds to Craddock et al.(Craddock et al., 2012).
Table 2. Brain regions whose functional connectivity differs with bipolar diagnosis and mood state.
After dividing grey matter regions into 200 individual ROIs, twenty-two pairs of regions demonstrated significant across group differences in functional connectivity after FDR correction. Columns 1 & 2 identify ROIs (with lobar and Brodmann area location). ROI numbering corresponds to the ROIs generated by Craddock et al.(Craddock et al., 2012) Columns 3–5 are mean and SD for Fishers Z transformed correlation values for each ROI pair for each subject group. Column 6 is the across group F-statistic and uncorrected p-value. Columns 7–9 are between group p-values for Tukeys HSD post-hoc testing. Significant between group results are highlighted in green.
| ROI 1 | ROI 2 | (mean +/− SD) | One-way ANOVA | post-hoc Tukey HSD p-value | ||||
|---|---|---|---|---|---|---|---|---|
| BP-euthy | BP-mania | HC | mania v. euthymia | euthymi a v. HC | mania v HC | |||
| 1: L occipital, BA19 | 131: L occipital, BA18/19 | 1.18 +/− .308 | 1.31 +/− .268 | 1.45 +/− .214 | F(2,118) = 11.182, p = 3.57 × 10-5 | 0.0766 | 1.88E-05 | 0.0609 |
| 34: L frontal, BA6/44 | 115: R frontal, BA4/6 | .535 +/− .275 | .711 +/− .270 | .398 +/− .333 | F(2,118) = 10.587, p = 5.90 × 10-5 | 0.0243 | 0.0809 | 3.18E-05 |
| 38: R frontal, BA9 / 46 | 49: R temporal, BA21/22 | .376 +/− .281 | .557 +/− .204 | .649 +/− .316 | F(2,118) = 11.098, p = 3.83 × 10-5 | 0.0107 | 3.09E-05 | 0.32 |
| 38: R frontal, BA9 / 46 | 116: L parietal, BA2/40 | .300 +/− .228 | .243 +/− .294 | .055 +/− .217 | F(2,118) = 11.188, p = 3.55 × 10-5 | 0.565 | 3.35E-05 | 0.00369 |
| 38: R frontal, BA9 / 46 | 140: R temporal, BA21/22 | .169 +/− .290 | .371 +/− .201 | .448 +/− .255 | F(2,118) = 13.671, p = 4.57 × 10-6 | 0.0018 | 5.13E-06 | 0.399 |
| 38: R frontal, BA9 / 46 | 186: R frontal, BA8/9 | .473 +/− .322 | .685 +/− .262 | .767 +/− .320 | F(2,118) = 10.642, p = 5.64 × 10-5 | 0.00711 | 5.81E-05 | 0.476 |
| 38: R frontal, BA9 / 46 | 187: R frontal, BA6/8 | .549 +/− .263 | .687 +/− .181 | .817 +/− .231 | F(2,118) = 14.423, p = 2.49 × 10-6 | 0.0229 | 1.24E-06 | 0.0452 |
| 49: R temporal, BA21/22 | 61: L frontal, BA9/46 | .391 +/− .230 | .621 +/− .235 | .613 +/− .288 | F(2,118) = 11.454, p = 2.84 × 10-5 | 0.000255 | 0.00024 | 0.991 |
| 61: L frontal, BA9/46 | 140: R temporal, BA21 | .303 +/− .205 | .468 +/− .203 | .558 +/− .273 | F(2,118) = 13.79, p = 4.15 × 10-6 | 0.00473 | 3.11E-06 | 0.211 |
| 61: L frontal, BA9/46 | 174: R parietal, BA7/31 | .415 +/− .196 | .581 +/− .213 | .666 +/− .314 | F(2,118) = 11.638, p = 2.44 × 10-5 | 0.00888 | 1.95E-05 | 0.299 |
| 70: L Occipital, BA17/18/19 | 89: R Occipital, BA17/18 | 1.525 +/− .249 | 1.502 +/− .288 | 1.762 +/− .287 | F(2,118) = 10.964, p = 4.29 × 10-5 | 0.93 | 0.000313 | 0.000225 |
| 85: R occipital, BA 19/39 | 97: L occipital (BA19) | .826 +/− .312 | 1.17 +/− .282 | .928 +/− .289 | F(2,118) = 14.084, p = 3.27 × 10-6 | 2.12E-06 | 0.252 | 0.0014 |
| 85: R occipital, BA 19/39 | 114: L Parieto-Occipital, BA7/19 | .511 +/− .276 | .838 +/− .347 | .499 +/− .326 | F(2,118) = 13.9, p = 3.80 × 10-6 | 2.79E-05 | 0.983 | 2.56E-05 |
| 85: R occipital, BA 19/39 | 132: R Parieto-Occipital, BA7/19 | .488 +/− .334 | .792 +/− .319 | .415 +/− .313 | F(2,118) = 14.143, p = 3.12 × 10-6 | 0.000154 | 0.556 | 5.14E-06 |
| 85: R occipital, BA 19/39 | 170: R Occipital, BA19 | .902 +/− .306 | 1.20 +/− .282 | 1.01 +/− .271 | F(2,118) = 10.595, p = 5.86 × 10-5 | 3.35E-05 | 0.207 | 0.0141 |
| 97: L occipital (BA19) | 102: R occipital, BA 18/19 | .512 +/− .278 | .836 +/− .316 | .688 +/− .302 | F(2,118) = 11.971, p = 1.85 × 10-5 | 1.12E-05 | 0.02 | 0.084 |
| 97: L occipital (BA19) | 138: R Occipital, BA19 | .522 +/− .293 | .850 +/− .301 | .763 +/− .227 | F(2,118) = 15.721, p= 8.85 × 10-7 | 1.72E-06 | 0.000282 | 0.369 |
| 97: L occipital (BA19) | 172: R temporal, BA37 | .622 +/− .345 | .965 +/− .276 | .817 +/− .228 | F(2,118) = 14.196, p = 2.99 × 10-6 | 1.93E-06 | 0.007 | 0.076 |
| 97: L occipital (BA19) | 177: L Occipital BA18/19 | .500 +/− .322 | .786 +/− .289 | .740 +/− .212 | F(2,118) = 12.646, p = 1.06 × 10-5 | 3.98E-05 | 0.000371 | 0.763 |
| 97: L occipital (BA19) | 189: L occipital/temporal, BA 19/37 | .601 +/− .349 | .997 +/− .290 | .859 +/− .204 | F(2,118) = 19.559, p = 4.61 × 10-8 | 5.46E-08 | 2.29E-04 | 0.105 |
| 114: L Parieto - Occipital, BA7/19 | 172: R temporal, BA37 | .447 +/− .293 | .747 +/− .322 | .503 +/− .263 | F(2,118) = 11.272, p = 3.31 × 10-5 | 3.68E-05 | 0.658 | 0.00132 |
| 114: L Parieto - Occipital, BA7/19 | 189: L occipital/temporal, BA 19/37 | .434 +/− .304 | .764 +/− .327 | .532 +/− .295 | F(2,118) = 11.737, p = 2.24 × 10-5 | 1.48E-05 | 0.309 | 0.00407 |
| cells in green are significant at p<.05 | ||||||||
Abbreviations: ANOVA: ANalysis Of Variance, BA: Brodmann Area, BP: Bipolar, CPZE: Chlorpromazine equivalents, FC: Functional Connectivity, FDR: False Discovery Rate, HC: Healthy Comparison, HSD: Honest Significant Difference, L: Left, R: Right, ROI: Region of Interest, SD: Standard Deviation..
Examining the twenty-two ROI pairs for between-group differences in functional connectivity, several patterns emerge:
Within the group of subjects with bipolar disorder type I, mania is differentiated from euthymia by increased functional connectivity in two patterns: First, increased functional connectivity is seen in mania between bilateral occipital / parieto-occipital ROIs (ROI85, ROI97, and ROI 114) and multiple other occipital / parieto-occipital ROIs.
A second pattern of increased functional connectivity in mania (compared to euthymia) is observed between a pair of bilateral ROIs located in the dorsal frontal lobe (ROI 38 and ROI 61) and multiple other ROIs distributed across the temporal lobe (ROIs 39 and 140), the precuneus (ROI 174), and medial prefrontal cortex (mPFC) (ROIs 186 and 187).
Comparing bipolar euthymia to a HC population reveals a variation on the two patterns described above. First, the occipital / parieto-occipital FC differences observed between bipolar mania and euthymia are observed to differentiate bipolar euthymia from a HC population with most ROI pairs showing increased FC in the HC population when compared to euthymia. These differences are less pronounced than the differences between bipolar mania and bipolar-euthymia. For most ROI pairs, the HC population shows FC values intermediate between low FC values in bipolar euthymia and high FC values in bipolar mania.
The second pattern of FC differences between bilateral dorsal frontal ROIs (ROI 38 and 61) and a distributed set of ROIs across frontal, parietal, and temporal lobes is also observed to differentiate bipolar euthymia from a HC population. Here again the HC group is observed to demonstrate increased FC relative to the euthymia group. For these ROIs however, the bipolar-mania and HC populations are not significantly different with the exception of the relationship between ROI 38 and ROI 116 located at the intra-parietal sulcus (IPS). This ROI pair did not demonstrate correlated activity in the HC population but showed significant correlation in both bipolar populations.
Functional connectivity changes related to medication prescription
Given the presence of significant differences in prescribed antipsychotic dosage between the bipolar mania and bipolar euthymia groups, we measured the correlation between zFC and CPZE for all 200×200 ROI-ROI zFC values across all subjects with bipolar disorder. None of the ROI pairs showed a significant (p-FDR <.05) correlation between CPZE and zFC. We repeated this analysis with the bipolar manic and bipolar euthymic groups individually and similarly failed to find significant associations in ROI pair connectivity and CPZE.
ROI-Voxel based changes in functional connectivity related to bipolar diagnosis and mood state
To better place the results of the ROI to ROI analysis in the context of published fMRI activation maps that display results in a voxel-wise format, we conducted exploratory ROI to voxel analyses using the ROIs most frequently showing across group differences in FC (ROIs 85, 97, 38, and 61). We chose these ROIs because they were represented in 17 of the 22 ROI pairs identified in the previous analysis.
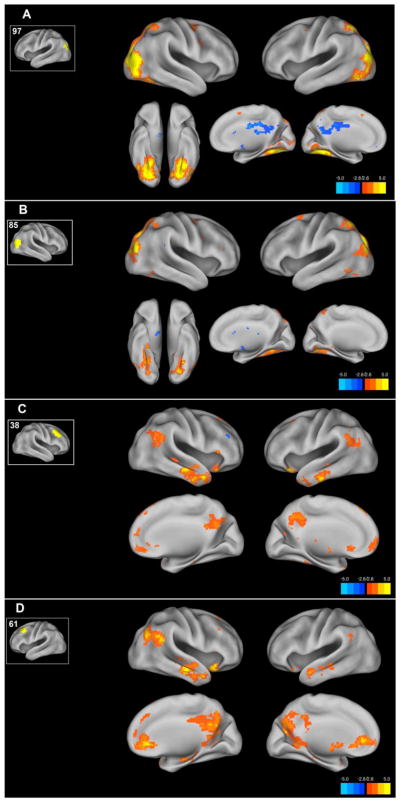
Comparing FC in bipolar mania to euthymia using the occipital ROIs 97 and 85 (Figure 1A &B), reveals a pattern of occipital / parieto-occipital increased FC to these ROIs in mania compared to euthymia. This map of increased connectivity in mania corresponds to published maps of the posterior portions of the dorsal attention network (DAN). When this map is combined with the finding of increased connectivity between ROI 34 and ROI 115 in the frontal lobe in mania, the result closely matches the canonical distribution of the DAN.
Figure 1. ROI to voxel maps of functional connectivity differences between bipolar mania and bipolar euthymia.
Using ROIs identified in the ROI to ROI connectivity analysis we examined whole brain functional connectivity differences between the bipolar mania and bipolar euthymia groups. The resulting maps of the bipolar mania-bipolar euthymia contrast are shown projected onto the cortical surface. For each figure A–D the inset shows the ROI number and the ROI location on the cortical surface. Voxels here are thresholded at the voxel P<.005 level. Color Bar indicates T statistic.
Comparing FC in bipolar mania to euthymia using the dorsal frontal ROIs 38 and 61 (Figure 1C and 1D) reveals increased FC in mania in a distributed pattern across the mPFC, temporal-parietal junction, temporal lobes and the precuneus. This map of increased connectivity in mania corresponds to published maps of the default mode network (DMN).
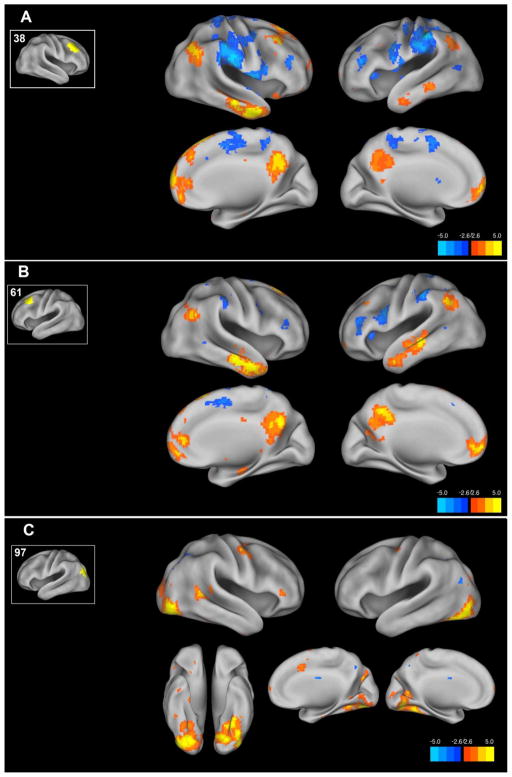
Comparing FC in the HC population to a bipolar euthymia population using the dorsal frontal ROIs 38 and 61 (Figure 2A and 2B), reveals a similar distributed pattern of increased connectivity in the HC population across the mPFC, temporal-parietal junction, temporal lobes and the precuneus corresponding to the distribution of the DMN. Also observed in a significant decrease in FC between ROI 38 and the IPS corresponding to ROI 116 as observed in the ROI-ROI analysis above. The occipital ROI 97 demonstrates increased FC in the HC group compared to bipolar euthymia in the DAN in a similar pattern to that observed in the mania versus euthymia comparison but in a more limited spatial extent (figure 2C).
Figure 2. ROI to voxel maps of functional connectivity differences between the healthy comparison and bipolar euthymia groups.
Using ROIs identified in the ROI to ROI connectivity analysis we examined whole brain functional connectivity differences between the healthy comparison and bipolar euthymia groups. The resulting maps of the healthy comparison-bipolar euthymia contrast are shown projected onto the cortical surface. For each figure A–C the inset shows the ROI number and the ROI location on the cortical surface. Voxels here are thresholded at the voxel P<.005 level. Color Bar indicates T statistic.
As an exploratory analysis, we sought to supplement the between-group mania versus euthymia comparison with a correlation between functional connectivity and a continuous measure of manic symptomatology (YMRS) across all 47 bipolar subjects (manic and euthymic). We observed a highly significant correlation between YMRS and zFC values for the ROI pairs selected for analysis (Supplemental Methods, Supplemental Table 1 and Supplemental Figure 2). Although the bipolar patient groups did not show significant differences in the presence or absence of medications other than antipsychotics in their prescribed regimens, it is possible that some of the observed connectivity differences are attributable to these medications. We therefore re-calculated the correlation between YMRS and zFC values for these ROI pairs with the presence or absence of medication classes as a covariate (supplemental methods). We still observed highly significant correlations between mania symptomatology and zFC values even with medication covariates added (Supplemental Table 1).
Discussion
To our knowledge, this is the first study to examine whole brain functional connectivity across the manic and euthymic states in bipolar disorder. In this data driven approach to whole brain connectivity, we observe two patterns of altered functional connectivity that differentiate mood states in bipolar disorder as well as distinguishing bipolar euthymia from a HC population:
The first of these patterns is altered functional connectivity within the dorsal attention network. For most nodes within this network, the bipolar euthymia group demonstrates hypo-connectivity and the mania group demonstrates hyper-connectivity relative to a HC population. Our current understanding of the role of this brain network suggests its importance in the allocation of cognitive resources either in pursuit of internally motivated goals or in response to stimuli (Recently reviewed in (Vossel et al., 2014)). Most studies of the dorsal attention network have examined activity in response to cognitive tasks rather than resting state activity. How does resting state connectivity in this network correlate with cognition or behavior? The data collected in this study does not include cognitive testing and performance measures to correlate with DAN connectivity so we can only offer hypotheses. Drawing on existing literature of DAN connectivity in patient groups, it seems unlikely that DAN hyperconnectivity reflects distractibility in mania. Existing studies of resting state FC in adult attention deficit disorder (ADD) demonstrates hypoconnectivity in the DAN in this population that correlates with ADD symptomatology (McCarthy et al., 2013).
We instead hypothesize that the DAN hyperconnectivity observed may reflect the heightened activation / arousal to external stimuli or “vital sense” experienced by subjects in a manic state. This circuit dysfunction could additionally be implicated in the emotional reactivity observed in bipolar mania. We base this on the finding that emotional processing is an attention-dependent process (Pessoa et al., 2002) and the observation that the DAN is reliably and differentially activated in response to the emotional valence of external stimuli e.g. cortical activation in response to pictures with a negative emotional valence compared to neutral pictures (e.g. figure 2 from (Uchida et al., 2015)). Testing this hypothesis may be accomplished by follow-on studies that examine how FC in this network predicts responses to emotionally valenced stimuli in subjects with bipolar disorder and HC subjects.
The second observed pattern of functional connectivity changes across groups involves the default mode network (DMN). Here we observe that the bipolar euthymic state can be differentiated from both the manic state and a HC population by connectivity changes between dorsal frontal nodes of the DMN and the rest of the DMN. Notably, the euthymic state shows hypo-connectivity, while bipolar mania demonstrates connectivity comparable to a HC population. In our prior study, we observed a similar pattern of amygdala connectivity with the HC showing connectivity that was intermediate between bipolar mania and euthymia and we conjectured that the difference between HC and bipolar euthymia may represent a compensatory mechanism rather than a disease process(Brady et al., 2016). Does the dorsal frontal hypoconnectivity in the DMN in euthymia in our current study represent a primary disease process or compensatory state? We note that the location of the disconnected DMN nodes corresponds to the standard left dorso-lateral prefrontal cortex target for transcranial magnetic stimulation (TMS) for treatment of depression (Fox et al., 2012). While there are multiple reports of treatment emergent mania in TMS treatment of bipolar depression, it remains unclear to what extent TMS treatment contributes to this risk (Xia et al., 2008). Our results provide a potential mechanistic link between TMS and treatment emergent mania in that TMS causing increased functional connectivity between other areas of the DMN and the TMS target area would potentially move a bipolar subject towards a pattern of FC that resembles the manic state. None of the participants in this experiment were depressed so this hypothesis remains conjecture until studies of TMS treatment of bipolar depression are accompanied by functional MRI imaging.
Taken together, our findings join a growing body of neuroimaging literature that has identified network dysfunction as a hallmark of several disease processes e.g. (Baker et al., 2014; Seeley et al., 2009). In stark contrast to degenerative disorders such as Alzheimer’s disease and fronto-temporal dementia, we here relate behavioral pathology not to circuit disruption but rather increased coherence (connectivity).
The brain regions identified in these experiments represent comparatively novel circuits, not previously implicated in the pathophysiology of bipolar disorder, as compared to those already well characterized, such as amygdala-prefrontal circuits implicated in emotion regulation. How can this finding be integrated with extant literature? Heretofore, most studies of spontaneous neural activity have only made comparisons between bipolar disorder and HC populations or have specified a priori regions of interest. There are notable exceptions to this: Meda et al. conducted an ICA based examination of between-network connectivity and demonstrated reduced connectivity between frontal DMN regions and posterior networks(Meda et al., 2012). Teng et al. examined whole brain connectivity to multiple ROIs from the DMN and striatal-thalamic regions (Teng et al., 2014). This study utilized a different set of ROIs than our study but in their analysis, dorsal medial regions of the DMN (e.g. ROI 29 Left MFG/SFG) was observed to show decreased functional connectivity to multiple non-frontal DMN nodes in bipolar disorder compared to a HC population. Lv et al. compared whole brain connectivity using a different whole brain parcellation between bipolar and HC groups. They observed that dorsal medial frontal nodes (e.g. medial superior frontal gyrus ROI) demonstrate significantly decreased connectivity to other portions of the DMN (angular gyrus and anterior cingulate ROIs) in euthymic bipolar disorder compared to HC subjects (supplementary materials in (Lv et al., 2016)). These results are consistent with our own findings described above.
Limitations
Several limitations of this study should be noted. First, this study is hypothesis free and does not test existing models of bipolar disorder. Rather, it may serve to complement these models. Methodological limitations include the following: In a comparison of mood states in which one state (mania) is identified with motor hyperactivity, movement artifacts are a potential confound. We have attempted to minimize the effects of movement through a combination of procedures including volume editing, global signal regression, and the use of advanced models for head motion during preprocessing. Another potential limitation of this study is medication effects. While all bipolar subjects were prescribed medications, the identities and dosages used varied significantly between states. However, a recent review suggests that concerns about these effects may be overstated(Hafeman et al., 2012). We looked for, but failed to find significant correlations between medications and FC values in the study population. Furthermore, the pattern of FC differences observed here frequently demonstrate that the unmedicated HC population shows connectivity intermediate between the two medicated bipolar groups, a result that seems entirely inconsistent with medication effect. Another potential confound is that all bipolar subjects in this study had a history of psychosis during manic episodes. While this is consistent with the finding that 88% of patients hospitalized for a first episode of mania had psychotic features(Tohen et al., 2003), there is evidence that brain connectivity differs between bipolar subjects with versus without a psychosis history(Anticevic et al., 2015). The results of this study may not be generalizable to subjects without a psychosis history.
Another limitation is that patients were not each studied longitudinally. This study compared two cohorts of bipolar subjects in two different mood states rather than performing a within subject comparison of subjects imaged in each mood state.
Finally, the nature of this experiment (data driven exploration) necessitates a correction for multiple comparisons that likely result in type 2 errors. For this reason, we do not suggest the presented results represent a comprehensive atlas of bipolar state and trait brain connectivity differences. Rather, the presented results represent the most prominent and most likely to be reproduced differences between patient groups. As an example of this, our current approach did not identify the ACC-Amygdala disconnectivity in mania that we previously identified in a hypothesis driven examination of amygdala connectivity (Brady et al., 2016). Examining our imaging data after processing in the same method as that publication, we observe that connectivity between the ROIs that most closely correspond to the R amygdala (ROI 155) and the ACC (ROI 22) is different across groups at a p-FDR corrected value of .062 and therefore, while showing a trend consistent with past findings, did not meet the threshold for significance in the current analysis.
Future inquiries will include testing the hypothesis that FC in the dorsal attention network predicts behavioral performance including studies of emotional reactivity. Other experiments include: Replicating these findings in a single cohort of subjects longitudinally across multiple mood states as well as expanding the study populations to include non-psychotic bipolar disorders and schizoaffective disorders, all in order to best delineate the neural circuit changes of mania from markers of disease trait. Finally, the demonstration of mood related variations in activity in networks accessible to transcranial magnetic stimulation (e.g. (Halko et al., 2014)) provides potential new targets for neuromodulation in scientific investigation and possible clinical intervention.
Supplementary Material
Highlights.
We performed data driven analyses of functional connectivity in bipolar disorder
Whole brain connectivity patterns differentiate bipolar trait and mood state
Dorsal Attention Network connectivity differentiates mania and euthymia
Frontal connectivity in the Default Mode Network also differentiates state & trait
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anticevic A, Savic A, Repovs G, Yang G, McKay DR, Sprooten E, Knowles EE, Krystal JH, Pearlson GD, Glahn DC. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophrenia bulletin. 2015;41:133–143. doi: 10.1093/schbul/sbu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Ongur D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Jr, Cooper A, Jensen JE, Tandon N, Cohen B, Renshaw P, Keshavan M, Ongur D. A longitudinal pilot proton MRS investigation of the manic and euthymic states of bipolar disorder. Transl Psychiatry. 2012;2:e160. doi: 10.1038/tp.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Masters GA, Mathew IT, Margolis A, Cohen BM, Öngür D, MK State Dependent Cortico-Amygdala Circuit Dysfunction in Bipolar Disorder. Journal of Affective Disorders. 2016;201:79–87. doi: 10.1016/j.jad.2016.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Phillips ML. Elucidating Neural Network Functional Connectivity Abnormalities in Bipolar Disorder: Toward a Harmonized Methodological Approach. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1:288–298. doi: 10.1016/j.bpsc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33:1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB New York State Psychiatric Institute. Biometrics Research, D. Structured clinical interview for DSM-IV-TR axis I disorders : SCID-I. Biometrics Research Dept., New York State Psychiatric Institutute; New York, N.Y: 2007. [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 2014;34:12049–12056. doi: 10.1523/JNEUROSCI.1776-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois G, Linke J, Wessa M. Altered functional connectivity between emotional and cognitive resting state networks in euthymic bipolar I disorder patients. PLoS One. 2014;9:e107829. doi: 10.1371/journal.pone.0107829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv D, Lin W, Xue Z, Pu W, Yang Q, Huang X, Zhou L, Yang L, Liu Z. Decreased functional connectivity in the language regions in bipolar patients during depressive episodes but not remission. J Affect Disord. 2016;197:116–124. doi: 10.1016/j.jad.2016.03.026. [DOI] [PubMed] [Google Scholar]
- McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, Johnson K, Fagan A, Gill M, Meaney J, Frodl T. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, Thaker G, Pearlson GD. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, Delbello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Lu CF, Wang PS, Li CT, Tu PC, Hung CI, Su TP, Wu YT. Altered resting-state functional connectivity of striatal-thalamic circuit in bipolar disorder. PLoS One. 2014;9:e96422. doi: 10.1371/journal.pone.0096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohen M, Zarate CA, Jr, Hennen J, Khalsa HM, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- Uchida M, Biederman J, Gabrieli JD, Micco J, de Los Angeles C, Brown A, Kenworthy T, Kagan E, Whitfield-Gabrieli S. Emotion regulation ability varies in relation to intrinsic functional brain architecture. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Xia G, Gajwani P, Muzina DJ, Kemp DE, Gao K, Ganocy SJ, Calabrese JR. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11:119–130. doi: 10.1017/S1461145707007699. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Seeley WW. Network dysfunction in Alzheimer’s disease and frontotemporal dementia: implications for psychiatry. Biol Psychiatry. 2014;75:565–573. doi: 10.1016/j.biopsych.2014.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.