Abstract
It is well established that environmental toxins, such as exposure to arsenic, are risk factors in the development of urinary bladder cancer, yet recent genome-wide association studies (GWAS) provide compelling evidence that there is a strong genetic component associated with disease predisposition. A single nucleotide polymorphism (SNP), rs8102137, was identified on chromosome 19q12, residing 6 kb upstream of the important cell cycle regulator and proto-oncogene, Cyclin E1 (CCNE1). However, the functional role of this variant in bladder cancer predisposition has been unclear since it lies within a non-coding region of the genome. Here, it is demonstrated that bladder cancer cells heterozygous for this SNP exhibit biased allelic expression of CCNE1 with 1.5-fold more transcription occurring from the risk allele. Furthermore, using chromatin immunoprecipitation assays, a novel enhancer element was identified within the first intron of CCNE1 that binds Kruppel-like Factor 5 (KLF5), a known transcriptional activator in bladder cancer. Moreover, the data reveal that the presence of rs200996365, a SNP in high linkage disequilibrium with rs8102137 residing in the center of a KLF5 motif, alters KLF5 binding to this genomic region. Through luciferase assays and CRISPR-Cas9 genome editing, a novel polymorphic intronic regulatory element controlling CCNE1 transcription is characterized. These studies uncover how a cancer-associated polymorphism mechanistically contributes to an increased predisposition for bladder cancer development.
Implications
A polymorphic KLF5 binding site near the CCNE1 gene explains genetic risk identified through genome wide association studies.
Keywords: Urinary bladder cancer, Cyclin E1, intronic enhancers, genetic predisposition
Introduction
Urinary bladder cancer (UBC) is one of the most prevalent cancers worldwide, ranking 8th in the United States for leading cancer deaths in men (NCI, www.cancer.gov; ACS, www.cancer.org). This disease typically presents at the superficial, low-grade stage when surgical methods are the best treatment options, however, the odds for recurrence are alarmingly high even when full resections are successful (1, 2). Although the molecular mechanisms are not fully elucidated with either the superficial or more aggressive subtypes of UBC, the driving oncogenic mutations associated with low-grade tumors are often enhanced upon the inactivation of certain tumor suppressors (2). This synergism leads to the progression and recurrence of muscle invasive tumors (3-6). Therefore, further understanding of the underlying molecular pathways involved in bladder cancer pathogenesis is crucial for better diagnosis and long-term treatment of this disease.
An emerging concept in bladder cancer is that genetic background significantly contributes to tumor onset and invasiveness. Previously, the greatest contributors to UBC development were environmental risk factors, including smoking and exposure to arsenic (7-10). Somatic activating mutations in H-Ras and FGFR3, as well as other large chromosomal deletions throughout the genome, also drive the development of this disease (2, 11-16). By contrast, recent genome-wide association studies (GWAS) performed in Icelandic, European, and Asian populations revealed a number of inherited polymorphisms within the genome that lead to an increased susceptibility for development of UBC (17-21). These studies highlighted variants that are both specific to UBC onset and associated with several proto-oncogenic loci, including Cyclin E1 (CCNE1), which harbors few other disease-associated SNPs. However, given that the CCNE1 variant and the other GWAS-identified polymorphisms lie in intergenic and non-coding regions, their biological significance is currently unknown.
CCNE1, a member of the cyclin family of proteins, has been implicated in the development of multiple cancer types, specifically bladder tumors (14, 20, 22-28). CCNE1 is also critical for faithful centrosome duplication, initiation of DNA replication, and chromosomal stability (29-32). Tight transcriptional control of CCNE1 guides cell cycle regulation, such that inappropriate expression confers rapid proliferation and genome instability as is seen in low grade, superficial UBC (27, 32, 33). This indicates that dysregulation of CCNE1 expression is likely an early pathogenic event in the development of UBC, working synergistically with other driving oncogenic mutations. Interestingly, while CCNE1 amplification is seen in UBC tumors (14, 27, 34), somatic mutations within the gene do not frequently occur, suggesting that enhanced expression is associated with misregulation of the gene. It is well established that E2F-binding sites and polymorphic regions within the CCNE1 promoter contribute to transcription regulation (23, 29, 35), however very little is known about additional CCNE1 DNA regulatory regions, particularly those involved in UBC development.
In light of the GWAS in UBC patients and the implications of CCNE1 in bladder cancer, we sought to mechanistically connect the genetic variant near the CCNE1 locus to the increased risk of UBC development. Fortunately, the presence of polymorphisms within the genome allows for assessment of allele-specific contribution, expression, and physiology from genotypically distinct alleles. This provides a powerful tool to study genetic variants and to understand the altered activity occurring on different alleles within a cancer cell. Here we combined human UBC SNP data with high-throughput sequencing methods to characterize a novel enhancer active in bladder cancer cells to explain the functional significance of one particular genetic component in the development of UBC.
Materials and methods
Cell culture
Bladder cancer cell lines were a generous gift from Dr. David Robbins (Sylvester Comprehensive Cancer Center, University of Miami, Florida). All cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Pen-Strep antibiotics.
SNP Linkage Disequilibrium (LD) Analysis
LD analysis was performed using the SNP Annotation and Proxy Search (SNAP) algorithms from the Broad Institute (MIT, www.broadinstitute.org/mpg/snap) and an r2 ≥ 0.8 was recognized as significant.
Heterogeneous Nuclear RNA (hnRNA) Extraction
Cells were harvested and lysed in 0.5% NP-40 lysis buffer to isolate nuclei, and then nuclear RNA was extracted using the Trizol protocol (Ambion). RT-PCR products were gel extracted and submitted for sequencing in parallel with PCR fragments from genomic DNA from the same cell line to control for dye profiles within each sequencing run. For allele-specific expression analysis, qRT-PCR was performed using the mismatch amplification mutation assay (MAMA) (36).
Real-Time PCR (qPCR) and Reverse Transcriptase-PCR (qRT-PCR)
qPCR and qRT-PCR were performed using the iQ SYBR Green Supermix (BioRad) and analyzed on a C1000 Thermal Cycler (BioRad) using the BioRad CFX Manager 2.0 software. Due to the high GC content within the CCNE1 intronic region, PCR was performed using HotStarTaq DNA Polymerase (Qiagen). The HotStarTaq Master Mix together with Q solution (Qiagen) and appropriate locus-specific primers allowed for amplification of the GC-rich intronic region. For quantitative analyses, the QuantiFast SYBR Green PCR Kit was utilized per manufacturer's instructions using the Q solution.
Chromatin Immunoprecipitation (ChIP)
ChIPs were performed according to the Upstate ChIP Kit protocol (Millipore) using antibodies against an IgG control (sc-2027, Santa Cruz Biotechnology), H3K27ac (ab4729, Abcam), and KLF5 (07-1580, Millipore). Briefly, 1-2 × 106 cells were crosslinked at 37°C in 1% formaldehyde and sonicated twice after lysis using a water bath sonicator (BioRuptor) on high intensity for 10 minutes at 30-second intervals. qPCR was used to measure enrichment compared to input relative to either a no Antibody or IgG control. Graphs represent three independent experiments.
ChIP-sequencing Analysis
DNA was pooled from two replicate H3K27ac ChIP assays, and input DNA was run in parallel to the ChIP to account for any discrepancies in ploidy within the cell lines. 30 ng of ChIP DNA from three UBC cell lines (5637, T24, and J82) were submitted for library preparation and sequencing in a 50 base pair, single-end, multiplexed run, yielding 20-30 million reads per sample. Upon completion of the sequencing run, sequencing reads were aligned against the human reference genome (hg19) using BOWTIE (37). A MACS analysis was performed to determine peaks of enrichment of H3K27ac throughout the genome (38). The MACS analysis was conducted as recommended by comparing the ChIP DNA reads to the Input reads using a tutorial (http://liulab.dfci.harvard.edu/MACS/) (38, 39). Sequences and processed datasets have been submitted to Gene Expression Omnibus under accession number GSE75286.
siRNA Mediated Knockdown
Two Silencer Select siRNAs (Life Technologies) against KLF5 or a scrambled negative control were used for RNAi experiments. Reverse transfections were performed according to manufacturer's protocol using Lipofectamine RNAiMAX Reagent. Cells were transfected with 10 nM concentrations of siRNA, and RNA was harvested 48 hours post transfection. qRT-PCR and Western blotting were performed to confirm efficient depletion using the following antibodies: GAPDH (sc-47724, Santa Cruz Biotechnology) and KLF5 (ab24331, Abcam). The data depict three independent experiments.
Dual-Luciferase Assays
Enhancer capabilities of the CCNE1 intronic region were assessed using the Dual-Luciferase expression assay (Promega) according to manufacturer's instructions. Briefly, enhancer constructs were cloned into the pGL3-promoter vector, and 5637 cells were transiently transfected with a pGL3 vector, a Renilla control vector (using a 1:10 dilution), and with or without the pcDNA3 empty vector or the pcDNA3-KLF5 expression vector (Addgene). Transfections were performed in 24-well plates using Lipofectamine LTX and 0.5 ug DNA per well. After 48 hours, cells were harvested and Firefly and Renilla luciferase activities were measured using a microplate luminometer. Data are presented as relative luciferase units (RLUs) indicating Firefly activity/Renilla activity and represent an average of three independent transfections.
Genome Editing Using CRISPR-Cas9
Dual sgRNA/Cas9 CRISPR vectors were designed to target the CCNE1 intronic region using lenticrispr v2 (40). Target sequences were GCGCAAAGGGGGAGGGGTAC and CGCAAAGGGGGAAGGGGTAC. 5637 cells were infected with a lentivirus carrying an sgRNA/Cas9 construct and infected cells were selected in 1 ug/ml puromycin media. Single-cell clones were expanded, and RNA and DNA were harvested from each clone for subsequent analyses on genome editing efficiency. DNA surrounding the CCNE1 intron was PCR amplified from individual cell isolates and cloned to characterize individual alleles. Multiple clones were sequenced to determine the allelic complement of specific clonal cell lines.
Statistics
All experiments were performed three independent times, and error bars denote standard error of the mean (SEM) unless otherwise noted. Student's t-tests were used to calculate significance.
Results
Biased allelic expression of CCNE1 occurs in bladder cancer cells heterozygous for the GWAS-identified SNP rs8102137
We were interested to address the connection between UBC risk and the rs8102137 SNP on chromosome 19q12 (17, 20). The GWAS-identified polymorphism falls 6 kb upstream from the transcription start site (TSS) of CCNE1 and exhibits an odds ratio of 1.13 (20). Upregulation of CCNE1 is typically associated with early events in UBC pathogenesis, particularly within superficial and low-grade lesions, undoubtedly affecting many cellular processes (27). Interestingly, rs8102137 falls within a linkage disequilibrium (LD) block that encompasses over 19 kb of coding and non-coding DNA (Fig. 1A), precluding ascribing any functional significance to the original identification. We used allele-specific expression analysis to explore this issue. First, we genotyped bladder cancer cell lines and fortuitously found four that are heterozygous for the rs8102137 SNP and the LD block at CCNE1. These four UBC cell lines were all derived from transitional cell carcinomas, varying from low to intermediate stages of disease (ATCC, www.atcc.org).
Figure 1.
Allele-biased expression of CCNE1 is observed in bladder cancer cell lines heterozygous for the GWAS SNP rs8102137. (A) Schematic of the CCNE1 locus and the LD block encompassing the GWAS SNP rs8102137 (indicated by the green plus) and two intronic SNPs in high LD. (B) Four heterozygous bladder cancer cell lines (J82, 5637, UMUC3, and T24) show biased allelic expression of CCNE1, while a prostate cancer cell line (LNCaP) heterozygous at this locus does not. Allelic expression is measured through qRT-PCR using MAMA primers specific to each allele. The ratio of the two alleles in the hnRNA population is relative to the ratio of the alleles in the genomic DNA. The right panel depicts a representation of the chromatograms of genomic DNA and RNA from the heterozygous UBC line, 5637. Error bars indicate SEM and asterisks indicate significance (*p < 0.05).
The heterozygosity of the bladder cancer cell lines allowed studies of individual allelic expression within common cellular contexts. The LD block encompassing the CCNE1 promoter and transcribed regions contains an intronic SNP, rs3218036, which is strongly linked to the GWAS SNP rs8102137 with r2 = 1.000 (Fig. 1A). To determine if there were differences in allelic expression of CCNE1 associated with the risk variant, we collected heterogeneous nuclear RNA (hnRNA) from the heterozygous bladder cell lines and assayed for expression of the intronic rs3218036 SNP in mRNA using allele-specific RT-PCR. This technique revealed the relative contribution of each individual allele (low or high risk) within the total unspliced RNA population (compared to genomic DNA). We found that in all four UBC lines, there was at least 1.5 fold more expression from the high risk allele versus the low risk allele, a bias not seen in a non-UBC cell line (Fig. 1B). To further visualize the allelic differences (41), we performed RT-PCR followed by direct sequencing and compared the profiles to genomic DNA from the same cells (Fig. 1B, right panel). This independent approach confirmed that the higher risk allele is expressed at a 1.5 fold higher level than the lower risk allele in common cellular backgrounds. We conclude that the rs8102137 SNP is associated with elevated CCNE1 transcription.
Enhancer marks and linked SNP rs200996365 highlight a KLF5 regulatory element within the first intron of CCNE1
The elevated levels of transcription associated with the higher risk allele suggest that the presence of the risk variant directly or indirectly alters endogenous transcriptional regulation of CCNE1, likely due to variation in a linked regulatory element. Thus, we sought to understand the DNA landscape at chromosome 19q12 in the bladder cancer genome. Initial studies compared the GWAS SNP to the Genbank and ENCODE databases which contain extensive information on evolutionary sequence conservation and epigenetic modifications. We observed no peaks of enriched sequence conservation or histone modifications characteristic of regulatory elements over the rs8102137 SNP, which resides 6 kb upstream of CCNE1 (Fig. 2). There were no other regions within the 5′ flanking region of CCNE1 that had any hallmarks of regulatory elements and which also contained SNPs linked to the rs8102137 bladder cancer risk GWAS SNP. Often the GWAS-reported polymorphism serves as a chromosomal marker for a risk locus and does not necessarily contribute to disease phenotypes. Therefore, we expanded our analysis to a more thorough examination of CCNE1 and 19q12.
Figure 2.
H3K27ac enrichment at the CCNE1 locus extends into the gene body. The top panel depicts a comparison of the ChIP-seq data generated from the bladder lines with ENCODE and other datasets available on the UCSC genome browser. DNase hypersensitivity (DHS) tracks from urothelial cells are shown below the ChIP-seq tracks and correspond to regions of H3K27ac enrichment. The bottom panel shows the H3K27ac enrichment peaks around the CCNE1 locus in 3 different bladder cancer cell lines. The yellow bar encompasses the UBC-specific peak of H3K27ac enrichment.
To identify active enhancers and regulatory elements within the CCNE1 locus of the bladder cancer genome, we performed histone H3 lysine 27 acetylation (H3K27ac) chromatin immunoprecipitation followed by high throughput sequencing (ChIP-seq) in three bladder cancer cell lines (5637, J82, and T24 cells). The H3K27ac mark indicates active regulatory regions, including promoters and both distal and proximal enhancers (42). We compared our data to other previously published H3K27ac ChIP-seq datasets available from the ENCODE project (www.genome.ucsc.edu) to identify any bladder-specific enhancer-like elements within the CCNE1 locus (Fig. 2). Interestingly, we found a bimodal peak of H3K27ac enrichment at the 5′ end of the gene that corresponds to DNase1-Hypersensitivity Sites (DHS) highlighted in a urothelial cell line dataset (Fig. 2, ENCODE, UCSC Genome Browser, www.genome.ucsc.edu). The first peak immediately upstream of the CCNE1 TSS (“Common”) is present in several ENCODE cell lines. However, the second peak of H3K27ac (“UBC” in our ChIP-seq dataset) maps within the first intron of the CCNE1 gene and is largely unique to the bladder cancer cell lines (Fig. 2). We therefore considered this putative intronic enhancer for a role in CCNE1 regulation.
We next overlaid our ChIP-seq dataset with the disease-associated GWAS SNP and the LD block at CCNE1 to determine any areas of overlap that could further highlight important regulatory regions in bladder cancer. While no H3K27ac peak coincides with the rs8102137 SNP 6 kb upstream of CCNE1 (Fig. 2), the nearby linked SNP rs200996365 (A/-) falls within the “UBC” enhancer signal. We therefore examined this SNP for a potential causal role in UBC risk.
The rs200996365 SNP (A/-) lies near the boundary of the second exon of CCNE1 and is linked to the rs8102137 GWAS SNP based on allele frequencies within the LD block with r2 = 0.890. Further analysis of this intronic DNA sequence revealed potential transcription factor binding sites in the vicinity. Specifically, motif analysis searches using the JASPAR database showed that rs200996365 falls in the middle of a consensus KLF5 motif (Fig. 3A). Moreover, the higher risk allele (a one base pair deletion) predicts a very strong consensus KLF5 binding site (predicted binding score of 12.471), whereas the lower risk A allele is predicted to have less favorable binding, with a predicted binding score of 4.094 (Fig. 3A). Intriguingly, KLF5 is a transcriptional activator in the bladder epithelium, influences differentiation and proliferation of urothelial cells, and is also implicated in the transformation of these cells to a malignant state (43, 44). KLF5 is also a known downstream mediator of important signaling pathways such as H-Ras (45), a frequently altered pathway in UBC. Thus, we set out to determine the functional significance of this intragenic region and the rs200996365 SNP through a candidate transcription factor approach beginning with KLF5.
Figure 3.
The GWAS-linked SNP rs200996365 alters binding of KLF5 to this CCNE1 intronic region. (A) rs200996365 maps to the center of a KLF5 binding site within the first intron of CCNE1. The presence of the high risk genotype at rs200996365 (-) predicts a higher affinity binding site – GGGAGGGG. Predicted binding scores as calculated by JASPAR are shown beside each allele. (B) ChIP analysis shows enrichment of KLF5 at the intronic enhancer region of CCNE1. The positive control region is within the CCND1 promoter and the negative control region is in an intergenic region on chromosome 11 devoid of histone modifications. Data are presented as percent of Input and compared to IgG controls in this qPCR analysis. (C) KLF5 preferentially binds the higher risk allele within CCNE1 as indicated by direct sequencing of the ChIP signal. Quantification of the ratio of sequencing dye traces at the bases indicated by a black star is depicted in the graph. Error bars represent SEM and asterisks indicate significance (*p < 0.05). (D) Depletion of KLF5 results in significantly reduced CCNE1 expression. 5637 cells were transfected with two independent siRNAs targeting KLF5 compared to a scrambled control for 48 hours. KLF5 and CCNE1 mRNA levels were compared to Actin by qRT-PCR analysis. Error bars indicate SEM and asterisks indicate significance (**p < 0.005). The Western blot depicts the resulting protein levels of KLF5 upon siRNA knockdown with the two different siRNAs.
We first assessed the occupancy of the KLF5 transcription factor within the CCNE1 intronic region through ChIP. We found that KLF5 binds avidly to the predicted site in bladder cancer cells (Fig. 3B, Supplemental Fig. 1). Notably, by assessing the allelic binding specificity, we found that there was a >2-fold enrichment in KLF5 binding to the higher risk allele (Fig. 3C). Furthermore, to determine if KLF5 contributes to CCNE1 expression, we depleted KLF5 levels via transient transfection of siRNAs and found that CCNE1 levels are reduced significantly by over 25% (Fig. 3D). These data strongly suggest that KLF5 contributes to CCNE1 transcriptional activation.
The CCNE1 intronic region exhibits intrinsic enhancer capabilities
To further assess the activity of the CCNE1 intronic regulatory element, we conducted dual-luciferase assays with pGL3 vectors containing the 350 bp region with the highest level of H3K27ac in UBCs. We cloned both the higher and lower risk intronic segments into the pGL3-promoter vector and tested for intrinsic enhancer activity. Normalized luciferase values revealed that both alleles (lower and higher risk genotypes) confer significant enhancer activity (Fig. 4A). Additionally, upon exogenous overexpression of KLF5, there was significant enrichment in luciferase activity from the higher risk allele (Supplemental Fig. 2).
Figure 4.
The CCNE1 intronic region exhibits enhancer capabilities, and mutation to this region results in a reduction in CCNE1 expression levels. (A) The activity of the CCNE1-enhancers containing either the low risk allele (rs200996365/A) or the high risk allele (rs200996365/-) was measured in a dual luciferase assay. Equal amounts of the pGL3 promoter only or pGL3 CCNE1-enhancer vectors were transfected into 5637 cells alongside a Renilla control. Firefly and Renilla activity were measured 48 hours post transfection and the graphs depict Firefly/Renilla measurements in relative luciferase units (RLUs). Error bars represent standard deviation (SD) and asterisks indicate significance, where *p < 0.05 (NS – not significant). (B) Schematic of the CRISPR-Cas9 experimental design and a representation of sequenced mutant alleles. WT denotes a wild type allele (r – high risk allele, n – low risk allele). (C) qRT-PCR analysis of CCNE1 mRNA levels compared to Actin in genotyped CRISPR-mutated cell clones. A2 contains a wild type sequence, therefore all expression levels are relative to this cell line. A mutant allele refers to a sequenced allele with at least a 2 base pair deletion.
The data above suggest that KLF5 regulates CCNE1 expression in bladder cells via a novel polymorphic enhancer. However, we sought to validate the function of the intronic region in CCNE1 gene regulation. To this end, we applied a more directed approach using the CRISPR-Cas9 mediated genome editing technique. Our goal was to mutate the enhancer in bladder cancer cells and determine any effect on CCNE1 expression. We designed two CRISPR constructs that target the rs200996365 SNP and introduced them into 5637 cells.
We found that CRISPR-Cas9 targeting was efficient in inducing mutation of the CCNE1 intronic region. Cell lines were characterized by PCR and sequencing individual alleles. We found up to five different alleles in clonal lines indicating that the cells are polyploid for the CCNE1 gene region. Mutations varied from 2 base pair deletions and single base pair mismatches up to 63 base pair deletions (Fig. 4B).
Next we analyzed CCNE1 mRNA levels in the mutated cell lines to determine if any alteration within the intronic regulatory region resulted in a change in overall expression. Since each CRISPR-mutated cell line harbored variable allelic genotypes, we observed varying levels of CCNE1 expression across the isolated clones (Fig. 4C). The A2 clone contained only two single base pair substitutions on two different alleles, neither of which disrupted the predicted KLF5 binding site, and this line expressed wild type levels of CCNE1 mRNA. In contrast, we found that the expression levels in the CRISPR-mutated cell lines were on average 30% lower than the control, mimicking the observations after KLF5 depletion with siRNA (Fig. 3D). CRISPR line D3 that contains no intact wild type alleles demonstrates that this element contributes to almost 40% of CCNE1 expression levels. Thus, CRISPR-mediated mutation of the intronic enhancer supports its regulatory role for CCNE1 transcription in bladder cells.
Discussion
Although extensive studies have established a relationship between UBC and environmental factors (7-9), the genetic components associated with disease progression still remain largely unresolved. Our approach identified a novel regulatory element within the bladder cancer genome and a putative causal genetic variant involved in disease onset (Fig. 5). Our model incorporates ChIP-seq analysis along with luciferase and CRISPR-Cas9 mutagenesis data that identify an intragenic enhancer that is crucial for proper CCNE1 transcriptional regulation. Our data also suggest how the UBC-associated SNP at the CCNE1 locus contributes to disease predisposition. The recent focus on inter- and intra-genic DNA regulatory elements and how these regions contribute to a more complex network of gene regulation further emphasizes the putative importance of this intronic SNP within the “UBC” peak of enhancer marks (46, 47). While the GWAS-reported SNP appears to serve as a marker of this chromosomal location due to the lack of sequence conservation and enhancer characteristics, we find that a linked SNP 6 kb away from the GWAS variant appears to contribute to disease predisposition. We show that there is consistently more expression from the higher risk variant allele in different UBC cell lines, indicating that the region harboring the higher risk polymorphism is a more potent transcriptional activator than the lower risk allele (Fig. 5). Upregulation of this cell cycle regulator may sensitize a normal cell to other oncogenic hits, ultimately leading to cellular transformation. This is presumably the consequence of higher affinity binding of KLF5 to the higher risk allele as shown by ChIP. Subtle enhancement of CCNE1 expression from the higher risk variant could lead to enhanced cell proliferation or genomic instability and predisposition to UBC (29, 32).
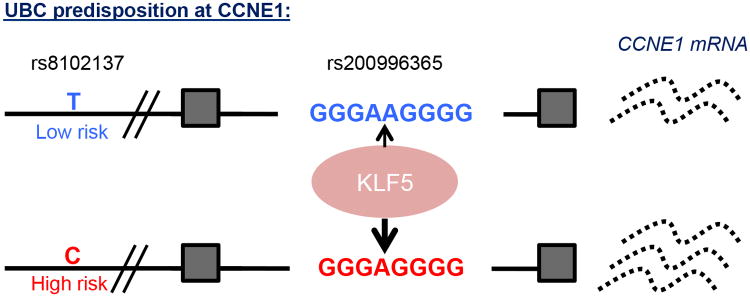
Figure 5.
A model for CCNE1 regulation by KLF5 in urothelial cells heterozygous for the GWAS SNP rs8102137. KLF5 binds both the low and high risk alleles of CCNE1 to drive gene transcription, yet there is increased occupancy on the high risk allele. This results in enhanced CCNE1 expression from the risk allele.
It is well-documented that KLF5 is a crucial downstream effector of oncogenic H-Ras (45) and specifically, KLF5 has been implicated in bladder cancer development and transformation, driving proliferation in urothelial cells (22, 43, 44). KLF5 interacts with chromatin modifiers and epigenetic mediators, such as CBP and p300, both of which help promote transcriptional activation and are also frequently mutated during oncogenesis (48, 49). Furthermore, in addition to being overexpressed in a variety of different cancer types, KLF5 is upregulated in 5% of bladder carcinomas and is frequently hypomethylated in cancers of the urinary tract (COSMIC database, www.cancer.sanger.ac.uk; ref. 50). Thus, elevated levels of KLF5 in conjunction with increased binding to this polymorphic intronic region subsequently drive enhanced CCNE1 expression (29, 32).
In understanding the contribution of the 19q12 risk variants to UBC predisposition, our molecular studies augment the growing knowledge on specific genetic variations and how they confer an increased risk for disease. Further correlation of these risk-associated variants with cis-regulatory mechanisms will provide a clearer image of the basic underpinnings of many cancers and other common diseases.
Supplementary Material
Acknowledgments
We thank David Robbins for generously providing cell lines. We also thank members of the Cole lab for thoughtful discussion and the Dartmouth Genomics Shared Resources core for help with the high throughput sequencing procedures. This work was supported by a grant from the National Cancer Institute (CA080320; MDC) and by a National Institutes of Health Training Grant Award (T32GM8704-12S1; JMP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Dinney CP, McConkey DJ, Millikan RE, Wu X, Bar-Eli M, Adam L, et al. Focus on bladder cancer. Cancer Cell. 2004 Aug;6(2):111–6. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg JE, Hahn WC. Bladder cancer: modeling and translation. Genes Dev. 2009 Mar 15;23(6):655–9. doi: 10.1101/gad.1789109. [DOI] [PubMed] [Google Scholar]
- 3.Cordon-Cardo C, Wartinger D, Petrylak D, Dalbagni G, Fair WR, Fuks Z, et al. Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992 Aug 19;84(16):1251–6. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- 4.Cote RJ, Dunn MD, Chatterjee SJ, Stein JP, Shi SR, Tran QC, et al. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998 Mar 15;58(6):1090–4. [PubMed] [Google Scholar]
- 5.Presti JC, Jr, Reuter VE, Galan T, Fair WR, Cordon-Cardo C. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991 Oct 1;51(19):5405–9. [PubMed] [Google Scholar]
- 6.Stein JP, Ginsberg DA, Grossfeld GD, Chatterjee SJ, Esrig D, Dickinson MG, et al. Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst. 1998 Jul 15;90(14):1072–9. doi: 10.1093/jnci/90.14.1072. [DOI] [PubMed] [Google Scholar]
- 7.Fei DL, Li H, Kozul CD, Black KE, Singh S, Gosse JA, et al. Activation of Hedgehog signaling by the environmental toxicant arsenic may contribute to the etiology of arsenic-induced tumors. Cancer Res. 2010 Mar 1;70(5):1981–8. doi: 10.1158/0008-5472.CAN-09-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagas MR, Tosteson TD, Morris JS, Demidenko E, Mott LA, Heaney J, et al. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control. 2004 Jun;15(5):465–72. doi: 10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- 9.Lesseur C, Gilbert-Diamond D, Andrew AS, Ekstrom RM, Li Z, Kelsey KT, et al. A case-control study of polymorphisms in xenobiotic and arsenic metabolism genes and arsenic-related bladder cancer in New Hampshire. Toxicol Lett. 2012 Apr 5;210(1):100–6. doi: 10.1016/j.toxlet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwender H, Selinski S, Blaszkewicz M, Marchan R, Ickstadt K, Golka K, et al. Distinct SNP combinations confer susceptibility to urinary bladder cancer in smokers and non-smokers. PLoS One. 2012;7(12):e51880. doi: 10.1371/journal.pone.0051880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billerey C, Chopin D, Aubriot-Lorton MH, Ricol D, Gil Diez de Medina S, Van Rhijn B, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001 Jun;158(6):1955–9. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999 Sep;23(1):18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 13.Czerniak B, Cohen GL, Etkind P, Deitch D, Simmons H, Herz F, et al. Concurrent mutations of coding and regulatory sequences of the Ha-ras gene in urinary bladder carcinomas. Hum Pathol. 1992 Nov;23(11):1199–204. doi: 10.1016/0046-8177(92)90285-b. [DOI] [PubMed] [Google Scholar]
- 14.Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013 Dec;45(12):1459–63. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zieger K, Dyrskjot L, Wiuf C, Jensen JL, Andersen CL, Jensen KM, et al. Role of activating fibroblast growth factor receptor 3 mutations in the development of bladder tumors. Clin Cancer Res. 2005 Nov 1;11(21):7709–19. doi: 10.1158/1078-0432.CCR-05-1130. [DOI] [PubMed] [Google Scholar]
- 16.Linnenbach AJ, Pressler LB, Seng BA, Kimmel BS, Tomaszewski JE, Malkowicz SB. Characterization of chromosome 9 deletions in transitional cell carcinoma by microsatellite assay. Hum Mol Genet. 1993 Sep;2(9):1407–11. doi: 10.1093/hmg/2.9.1407. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa JD, Ye Y, Siddiq A, Garcia-Closas M, Chatterjee N, Prokunina-Olsson L, et al. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Hum Mol Genet. 2014 Mar 1;23(5):1387–98. doi: 10.1093/hmg/ddt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortessis VK, Yuan JM, Van Den Berg D, Jiang X, Gago-Dominguez M, Stern MC, et al. Risk of urinary bladder cancer is associated with 8q24 variant rs9642880[T] in multiple racial/ethnic groups: results from the Los Angeles-Shanghai case-control study. Cancer Epidemiol Biomarkers Prev. 2010 Dec;19(12):3150–6. doi: 10.1158/1055-9965.EPI-10-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiemeney LA, Thorlacius S, Sulem P, Geller F, Aben KK, Stacey SN, et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet. 2008 Nov;40(11):1307–12. doi: 10.1038/ng.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010 Nov;42(11):978–84. doi: 10.1038/ng.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Ye Y, Kiemeney LA, Sulem P, Rafnar T, Matullo G, et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat Genet. 2009 Sep;41(9):991–5. doi: 10.1038/ng.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Pizzo JJ, Borkowski A, Jacobs SC, Kyprianou N. Loss of cell cycle regulators p27(Kip1) and cyclin E in transitional cell carcinoma of the bladder correlates with tumor grade and patient survival. Am J Pathol. 1999 Oct;155(4):1129–36. doi: 10.1016/S0002-9440(10)65216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu YP, Kohaar I, Moore LE, Lenz P, Figueroa JD, Tang W, et al. The 19q12 bladder cancer GWAS signal: association with cyclin E function and aggressive disease. Cancer Res. 2014 Oct 15;74(20):5808–18. doi: 10.1158/0008-5472.CAN-14-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitahara K, Yasui W, Kuniyasu H, Yokozaki H, Akama Y, Yunotani S, et al. Concurrent amplification of cyclin E and CDK2 genes in colorectal carcinomas. Int J Cancer. 1995 Jul 4;62(1):25–8. doi: 10.1002/ijc.2910620107. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Prescott MS, Zhu Z, Singh P, Chun SY, Kuick RD, et al. Identification and characterization of a 19q12 amplicon in esophageal adenocarcinomas reveals cyclin E as the best candidate gene for this amplicon. Cancer Res. 2000 Dec 15;60(24):7021–7. [PubMed] [Google Scholar]
- 26.Marone M, Scambia G, Giannitelli C, Ferrandina G, Masciullo V, Bellacosa A, et al. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int J Cancer. 1998 Jan 5;75(1):34–9. doi: 10.1002/(sici)1097-0215(19980105)75:1<34::aid-ijc6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Richter J, Wagner U, Kononen J, Fijan A, Bruderer J, Schmid U, et al. High-throughput tissue microarray analysis of cyclin E gene amplification and overexpression in urinary bladder cancer. Am J Pathol. 2000 Sep;157(3):787–94. doi: 10.1016/s0002-9440(10)64592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snijders AM, Nowee ME, Fridlyand J, Piek JM, Dorsman JC, Jain AN, et al. Genome-wide-array-based comparative genomic hybridization reveals genetic homogeneity and frequent copy number increases encompassing CCNE1 in fallopian tube carcinoma. Oncogene. 2003 Jul 3;22(27):4281–6. doi: 10.1038/sj.onc.1206621. [DOI] [PubMed] [Google Scholar]
- 29.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003 Aug 22;114(4):431–43. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 30.Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell. 2000 Sep 29;103(1):127–40. doi: 10.1016/s0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 31.Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, et al. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. Embo J. 2003 Sep 15;22(18):4794–803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999 Sep 16;401(6750):297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 33.Kamai T, Takagi K, Asami H, Ito Y, Oshima H, Yoshida KI. Decreasing of p27(Kip1) and cyclin E protein levels is associated with progression from superficial into invasive bladder cancer. Br J Cancer. 2001 May 4;84(9):1242–51. doi: 10.1054/bjoc.2000.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schraml P, Bucher C, Bissig H, Nocito A, Haas P, Wilber K, et al. Cyclin E overexpression and amplification in human tumours. J Pathol. 2003 Jul;200(3):375–82. doi: 10.1002/path.1356. [DOI] [PubMed] [Google Scholar]
- 35.Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12146–50. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha RS, Zarbl H, Keohavong P, Thilly WG. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 1992 Aug;2(1):14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- 37.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng J, Liu T, Zhang Y. Using MACS to identify peaks from ChIP- Seq data. Current Protocols in Bioinformatics. 2011;2(14):1–2. doi: 10.1002/0471250953.bi0214s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014 Aug;11(8):783–4. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright JB, Brown SJ, Cole MD. Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol Cell Biol. 2010 Mar;30(6):1411–20. doi: 10.1128/MCB.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009 May 7;459(7243):108–12. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell SM, Zhang L, Mendell A, Xu Y, Haitchi HM, Lessard JL, et al. Kruppel-like factor 5 is required for formation and differentiation of the bladder urothelium. Dev Biol. 2011 Oct 1;358(1):79–90. doi: 10.1016/j.ydbio.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong XY, et al. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006 Mar 15;118(6):1346–55. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 45.Nandan MO, Yoon HS, Zhao W, Ouko LA, Chanchevalap S, Yang VW. Kruppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004 Apr 22;23(19):3404–13. doi: 10.1038/sj.onc.1207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kowalczyk MS, Hughes JR, Garrick D, Lynch MD, Sharpe JA, Sloane-Stanley JA, et al. Intragenic enhancers act as alternative promoters. Mol Cell. 2012 Feb 24;45(4):447–58. doi: 10.1016/j.molcel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Ott CJ, Blackledge NP, Kerschner JL, Leir SH, Crawford GE, Cotton CU, et al. Intronic enhancers coordinate epithelial-specific looping of the active CFTR locus. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):19934–9. doi: 10.1073/pnas.0900946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011 Sep;43(9):875–8. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Teng CT. Phosphorylation of Kruppel-like factor 5 (KLF5/IKLF) at the CBP interaction region enhances its transactivation function. Nucleic Acids Res. 2003 Apr 15;31(8):2196–208. doi: 10.1093/nar/gkg310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015 Jan;43(Database issue):D805–11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.