Abstract
Background
Major depressive disorder (MDD) is a serious psychiatric illness, associated with an increasing rate of suicide. The pathogenesis of depression may be associated with the disruption of zinc (Zn) homeostasis. In the brain, several proteins that regulate Zn homeostasis are present, including Zn transporters (ZnTs) which remove Zn from the cytosol. The present study was designed to investigate whether depression and suicide are associated with alterations in the expression of the ZnTs protein.
Methods
Protein levels of ZnT1, ZnT3, ZnT4, ZnT5 and ZnT6 were measured in postmortem brain tissue from two different cohorts. Cohort A contained 10 subjects diagnosed with MDD (7 were suicide victims) and 10 psychiatrically-normal control subjects and cohort B contained 11 non-diagnosed suicide victims and 8 sudden-death control subjects. Moreover, in cohort A we measured protein level of NMDA (GluN2A subunit), AMPA (GluA1 subunit) and 5-HT1A receptors and PSD-95. Proteins were measured in the prefrontal cortex (PFC) using Western blotting. In addition, Zn concentration was measured using a voltammetric method.
Results
There was a significant increase in protein levels of ZnT1, ZnT4, ZnT5 in the PFC in MDD, relative to control subjects, while ZnT3 protein level was decreased in MDD. There was no significant difference in the Zn concentration in the PFC between control and MDD subjects. Similarly, in the PFC of suicide victims (non-diagnosed), an increase in protein levels of ZnT1, ZnT4, ZnT5 and ZnT6 was observed. Conversely, protein levels of ZnT3 were decreased in both suicide victims and subjects with MDD, in comparison with control subjects. There was also a significant decrease in the protein level of GluA1, GluN2A, PSD-95 and 5-HT1A in MDD.
Conclusions
Our studies suggest that alterations in Zn transport proteins are associated with the pathophysiology of MDD and suicide.
Keywords: Zinc, Zn transporters, Suicide, Major depressive disorder, postmortem brain tissue
1. Introduction
Major depressive disorder (MDD) is a serious mental illness with a lifetime prevalence of about 15%. One of the major causes of death in MDD is suicide. Approximately 15 – 25% of MDD patients attempt suicide (Mann et al., 1999; Sokero et al., 2003). Recent clinical studies report that MDD is accompanied by lower serum levels of Zn and reveal that low levels of Zn are often negatively correlated with the intensity and duration of depressive symptoms (McLoughlin and Hodge, 1990; Maes et al., 1994; Maes et al., 1997; Maes et al., 1999; Nowak and Schlegel-Zawadzka, 1999; Siwek et al., 2010)(Siwek et al., 2013). Moreover, successful antidepressant therapy led to a normalization of the serum levels of Zn, suggesting that Zn levels may be state dependent (McLoughlin and Hodge, 1990; Maes et al., 1994; Siwek et al., 2010). Furthermore, both clinical and preclinical studies showed that a dietary deficiency in Zn induces depressive symptoms (Marcellini et al., 2006; Tassabehji et al., 2008; Tamano et al., 2009; Whittle et al., 2009; Roy et al., 2010; Watanabe et al., 2010; Jacka et al., 2012; Maserejian et al., 2012; Mlyniec and Nowak, 2012; Doboszewska et al., 2015a; Markiewicz-Zukowska et al., 2015). These results support the hypothesis that the pathogenesis of depression is associated with disruptions of Zn homeostasis.
There are no data so far concerning levels of serum Zn in suicide. Our previous studies performed in postmortem tissue of suicide victims showed no siginificant difference in total Zn levels in the prefrontal cortex (PFC) and hippocampus as compared to control subjects (Nowak et al., 2003; Sowa-Kucma et al., 2013). However, another study revealed alterations in the Zn interaction with N-methyl-D-aspartate receptors (NMDA) (Nowak et al., 2003; Sowa-Kucma et al., 2013) and a decreased in levels of GPR39-Zn(2+)-sensing receptor in the brain of suicide victims (Mlyniec et al., 2014). Thus, there is indirect evidence for a disruption in Zn homeostasis in the pathophysiology of suicide.
Zn is an essential trace element required for proper cellular function. It is a structural component of numerous proteins such as growth factors, enzymes, transcription factors and receptors, and it is important for their biological activity (Fukada et al., 2011). Hence, it is important to maintain a homeostatic intracellular concentration of Zn, since either its reduction or increase leads to the disruption of cellular functions and consequently to a variety of health problems (Pfaender and Grabrucker, 2014; Pochwat et al., 2015). Zn homeostasis is regulated by different groups of proteins including the metalotransporter family ZnT. These proteins are encoded by the solute-linked carrier (SLC) gene family: ZnT (SLC30A). Ten members of ZnT have so far been identified (Kambe et al., 2014). The ZnT family facilitates Zn efflux from the cytosol out of the cells or into intracellular compartments (Huang and Reichardt, 2001). Of 10 known ZnTs, ZnT1, 3, 4, 5 and 6 are more highly expressed in the brain. ZnT1 is localized in the plasma membrane and is responsible for the export of Zn from the cytosol to the extracellular space. ZnT3 sequesters Zn into synaptic vesicles and ZnT4 is involved in the transport of Zn to cellular compartments (Huang and Tepaamorndech, 2013). ZnT5 and ZnT6 are localized on the membrane of the Golgi apparatus as well as cytoplasmic vesicles. ZnT5 may be responsible for the transport of Zn ions across the cellular membrane and ZnT6 is involved in the trafficking of Zn during acute immune responses (Kambe et al., 2002).
The crucial role that Zn plays in the pathophysiology of depression and that most suicide attempters suffer from depression led us to design the present study to examine the levels of Zn homeostasis-regulating proteins – Zn transporters – in the pathophysiology of depression and suicide. Protein levels of ZnT1, 3, 4, 5 and 6, (transporters that are more highly expressed in the brain) were assessed in the PFC (Brodmann’s area, BA10) of subjects diagnosed with MDD, in the PFC of suicide victims of unknown psychiatric history and in matched psychiatrically normal control subjects and in non-suicide control subjects. Furthermore, Zn concentration was determined in the PFC of subjects with MDD and normal control subjects.
Preclinical and clinical studies have demonstrated abnormalities in glutamatergic and serotonergic transmission in MDD (for reviews see Freudenberg et al., 2015; Kaufman et al., 2016; Ghasemi et al., 2014). On the other hand, one action of Zn is in the modulation of both glutamate (especially NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, AMPA) and serotonin (5-HT1A) receptor function (Nowak et al., 2015). Thus, an additional aim of the present study was to examine the expression of the NMDA receptor subunit GluN2A, AMPA receptor subunit GluA1and postsynaptic density protein-95 (PSD-95). We choose these particular proteins (GluN2A, GluA1 and PSD-95) based on reports of their important role in the function of NMDA and AMPA receptors, involvement in the pathophysiology of depression and Zn action (Feyissa et al., 2009, Freudenberg et al., 2015, Kalappa et al., 2015, Popescu, 2015, Szewczyk et al., 2015). We also mesured the level of 5-HT1A receptor protein in the PFC of subjects with MDD and matched normal control subjects (cohort A). The potential correlation between the ZnTs and the glutamate receptor subunits and 5-HT1A were analyzed.
2. Materials and methods
2.1. Subjects and tissue collection
Cohort (A)
Postmortem brain tissue from 10 male subjects diagnosed with MDD (mean age ± SEM, 44.8 ± 3.8) and 10 psychiatrically-normal control subjects (mean age ± SEM, 45.9 ± 3.5) (Table 1) were collected at autopsy at the Cuyahoga County Medical Examiner’s Office, Cleveland, OH. The causes of death was ruled by the Medical Examiner. The Institutional Review Boards of University Hospitals Case Medical Center and the University of Mississippi Medical Center approved the protocol for recruitment, tissue collection, and interviews of the next-of-kin. Written informed consent was obtained from the legally-defined next-of-kin for collecting tissue and for informant-based diagnostic interviews regarding the deceased. Cases with a clinical history or evidence of a neurological disorder or injury were excluded. The Medical Examiner’s Office examined blood and urine samples from all subjects for measurement of psychotropic medications and substances of abuse. Retrospective, informant-based psychiatric assessments were performed for all subjects as previously described (Cobb et al., 2016). A trained interviewer administered the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID; First et al., 1996) to knowledgeable next-of-kin of all subjects to retrospectively assess the presence or absence of Axis I diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (APA, 1994). Interview notes and clinical histories were reviewed independently by two licensed mental health clinicians, who assigned consensus diagnoses in conference. There is a high degree of diagnostic agreement between assessments based on interviewing next-of-kin vs. living subjects (Dejong and Overholser, 2009). Ten male control subjects did not meet criteria for a current or lifetime major mental illness. Ten male subjects were diagnosed with major depressive disorder (MDD) and were experiencing a major depressive episode in the last month of life. Four subjects had experienced one depressive episode and six had experienced 2 or more such episodes. The average age (mean ± SEM) of onset of depression was 38.0 ± 3.3 years and the average duration of illness was 4.7 ± 2.6 years (Table 2). Among the subjects with MDD, one had a prescription for an antidepressant medication in the last month of life, although no such medications were present in toxicology screening (Table 3). Subjects with MDD were comorbid for alcohol dependence with prior history of amphetamine abuse (1), dysthymia (1), and prior history of obsessive compulsive disorder (1). One control subject met criteria for a prior history of alcohol dependence. During processing, tissue from each depressed subject was yoked with a control subject matched for gender, age (± 5 years) and postmortem interval (Table 3). Gray matter was dissected with a scalpel from frozen blocks of prefrontal cortex (PFC; BA10), and placed in an RNAse- and DNAse-free cold centrifuge tube on dry ice and then stored at −80° C.
Table 1.
Demographic characteristics of control and suicide/MDD subjects (cohort A)
| Parameter | Control n=10 |
Major depression (MDD) n=10 |
|
|---|---|---|---|
| Sex | Male | Male | – |
| Age (years) | 45.9 ± 3.50 | 44.8 ± 3.77 | p=0.371 |
| PMI (h) | 22.13 ± 6.77 | 23.78 ± 3.05 | p=0.637 |
| pH | 6.74 ± 0.07 | 6.60 ± 0.0 | p=0.107 |
Values are mean ±S.E.M. PMI-postmortem interval; The average ages, PMI and pH values of control subjects and those with MDD were not statistically different.
Table 2.
Clinical characteristics of subjects with MDD
| Parameters | MDD subjects (n=10) |
|---|---|
| Age of onset of depression (years) | 38.0 ± 3.3 (n=9); unknown (n=1) |
| Duration of depression (years) | 4.7 ± 2.6 (n=9); episodic (n=1) |
| Suicide (yes/no/not known) | 7/2/1 |
| MDD diagnosis in last month | 10/10 |
| Number of depressive episodes | 1-1; 2-1; 3-2; 4-1; 5-multiple; 6-2; 7-1; 8-at least 10; 9-multiple at least 2; 10-2. |
| Antidepressants in last month | 1-No; 2-No; 3-No; 4- Paxil; 5- No (Ritalin two months before death); 6-No; 7-No, 8-No; 9-No, 10-No |
| Comorbid axis I disorder | 1- No; 2-No; 3-No; 4-No; 5-alcohol dependence, past history of amphetamine abuse; 6-No; 7-No; 8- dysthymia; 9- past history of OCD; 10- No |
OCD- obsessive compulsive disorder; Mean age of control subjects (45.9 ± 3.5); mean age of MDD subjects (44.8 ± 3.8).
Table 3.
Features of control and MDD subjects
| Control Pair no. |
Sex | Age (years) |
PMI (h) |
Brain tissue pH |
Smoking | Toxicology Medications/Ethanol |
Cause of death |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | M | 27 | 17 | 6.88 | Yes | NO | Homicide gsw chest |
| 2 | M | 34 | 24 | 6.61 | Unknown | Ethanol | Thrombophlebitis |
| 3 | M | 35 | 25 | 6.74 | Yes | NO | Cardiac related |
| 4 | M | 41 | 28.5 | 7.14 | Yes | NO | HCS |
| 5 | M | 48 | 9 | 6.98 | Yes | NO | Cardiac related |
| 6 | M | 51 | 17 | 6.76 | Yes | NO | ACD |
| 7 | M | 52 | 17 | 6.28 | No | NO | Cardiac related |
| 8 | M | 54 | 26.3 | 6.50 | No | NO | Fibrinopurulent peritonitis |
| 9 | M | 56 | 27.2 | 6.87 | History | NO | Motor vehicle accident: multiple injuries |
| 10 | M | 61 | 30.3 | 6.61 | No | NO | ACD |
|
| |||||||
|
MDD Pair no. |
Sex |
Age (years) |
PMI (h) |
Brain tissue pH |
Smoking |
Toxicology Medications/Ethanol |
Cause of death |
|
| |||||||
| 1 | M | 20 | 20 | 6.73 | No | NO | SIGSW to chest |
| 2 | M | 37 | 31 | 6.71 | No | Ethanol | SIGSW to chest |
| 3 | M | 36 | 11 | 6.97 | No | NO | Undetermined |
| 4 | M | 42 | 44.3 | 6.68 | No | NO | Hanging |
| 5 | M | 45 | 29 | 6.86 | History | Ethanol | SIGSW to chest |
| 6 | M | 55 | 17.5 | 6.56 | History | NO | SIGSW to chest |
| 7 | M | 46 | 17 | 6.26 | No | NO | Homicide |
| 8 | M | 52 | 17 | 6.48 | No | NO | CO poisoning |
| 9 | M | 53 | 29 | 6.73 | No | NO | SIGSW to chest |
| 10 | M | 62 | 22 | 6.06 | Yes | NO | HCS |
M- male; ACD- atherosclerotic cardiovascular disease; CO – carbon monoxide; gsw – gunshot wound; HCS – Hypertensive coronary sclerotic heart disease w/ cardiomegaly; PMI – postmortem interval; SIGSW – self-inflicted gunshot wound;
Cohort (B)
Brain tissue from 11 non-diagnosed suicide victims (mean age ± SEM, 38.5 ± 6.1) and 8 sudden death controls (mean age ± SEM, 31.0 ± 4.9) was obtained as discarded tissue at the time of autopsy at the Department of Forensic Medicine, Jagiellonian University Medicum College [Grant no. 6P05B 142 20 from the State Committee for Scientific Research, approved by the Ethics Committee]. According to the obtained medical history, both suicide and control subjects were not treated for any chronic diseases of the CNS. Demographic characteristics of each subject are described in Table 4.
Table 4.
Demographic characteristics of control and suicide victims (cohort B)
| Age (years) | Sex | Cause of death | |
|---|---|---|---|
| Control | |||
| 1 | 17 | M | Cranial/brain injuries |
| 2 | 44 | F | Road accident |
| 3 | 20 | M | CO poisoning |
| 4 | 54 | M | Myocardial infarction |
| 5 | 29 | M | Homicide |
| 6 | 42 | M | Myocardial infarction |
| 7 | 21 | F | Road accident |
| 8 | 21 | F | Homicide |
|
| |||
| Suicide | |||
| 1 | 29 | M | Hanging |
| 2 | 17 | M | Hanging |
| 3 | 47 | M | Hanging |
| 4 | 19 | M | Jump under train |
| 5 | 75 | F | Self-mutilation |
| 6 | 29 | F | Jumping |
| 7 | 20 | M | Poisoning/drug overdose |
| 8 | 58 | M | Poisoning/drug overdose |
| 9 | 55 | M | Hanging |
| 10 | 55 | F | Poisoning/drug overdose |
| 11 | 19 | M | Hanging |
Mean age of control subjects (31.0 ± 4.9); mean age of suicide subjects (38.5 ± 6.1). F – female; M – male.
2.2. Western blot analysis
Western blot was used for measurement of protein level of ZnTs. The tissue was prepared as published previously (Sowa-Kucma et al., 2013). Briefly, tissue samples were homogenized in 2% solution of sodium dodecyl sulfate (SDS). The homogenates were then centrifuged for 5 min at 10,000 rpm at 4 °C. After centrifugation, the supernatant was collected. Bicinchoninic acid was used (Pierce Biotechnology, Inc., Rockford, IL, USA) for determination of protein content. Samples containing 50 μg of protein and mixed with sample buffer (Invitrogen, Paisley, UK) were fractionated by 10.0% SDS-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane (Invitrogen, Paisley, UK). 1% blocking solution (BM Chemiluminescence Western Blotting Kit;Mouse/Rabbit, Roche, Switzerland) was used to block non-specific binding. After blocking, the membranes were incubated overnight at 4 °C with primery antibodies. The following antibodies were used: polyclonal rabbit anti-ZnT1 (diluted 1:1000; Synaptic System), monoclonal mouse anti-ZnT3 (diluted 1:1000; Synaptic System), polyclonal rabbit anti-ZnT4 (diluted 1:1000; GeneTex); polyclonal goat anti-ZnT5 and anti-ZnT6 (diluted 1:1000; Santa Cruz); polyclonal rabit anti-glutamate receptor 1 (AMPA subtype) (diluted 1:1000; Abcam); polyclonal rabbit anti-GluN2A (diluted 1:1000; Abcam); polyclonal goat anti-PSD-95 (diluted 1:1000; Abcam); polyclonal rabbit anti-5-HT1A receptor (diluted 1:1000; Abcam). All used antibodies were dissolved in 0.5% blocking reagent (Roche). After incubation the membranes were washed three times for 10 min in Tris-buffered saline with Tween (TBS-T) and incubated for 60 min with an anti-mouse IgG-peroxidase conjugated/anti-rabbit IgG-peroxidase conjugated (BM Chemiluminescence Western Blotting Kit (Mouse/Rabbit, Roche) or rabbit anti-goat IgG-peroxidase conjugated antibodies (Abcam) respectively (diluted 1:7000). After incubation with secondary antibodies the membranes were washed three times for 10 min with TBS-T and then incubated with detection reagent (Roche). The signal from the tested proteins was visualized and measured using a Fuji-Las 1000 system and Fuji Image Gauge v.4.0 software. As a control for transfer and loading, GAPDH or β-actin was assessed on each blot. For this, a mouse monoclonal anti-GAPDH antibody (diluted 1:300; Santa Cruz Biotechnology; Santa Cruz, CA) or mouse monoclonal β-actin antibody (diluted 1:7000; Sigma-Aldrich) was used. Final results represent the ratio of the optical density of a particular protein to the optical density of GAPDH or β-actin present in the same sample. Representative Western blots are shown in Fig.1f, 3e and 4f.
Fig. 1.
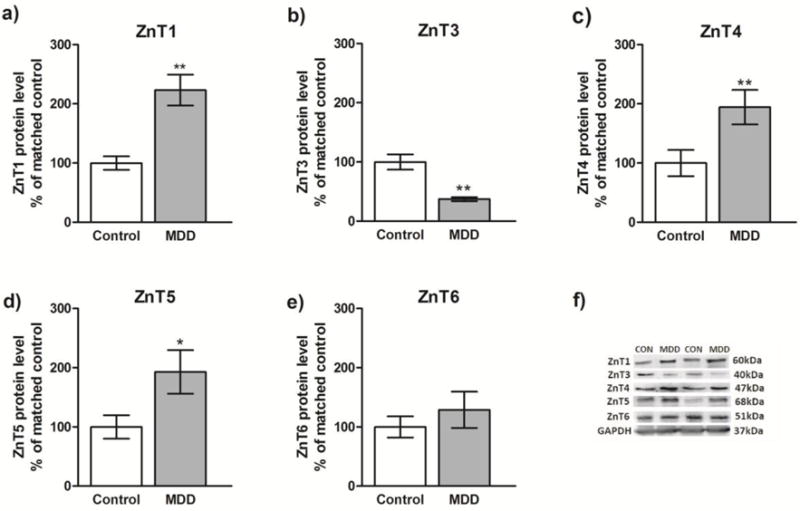
Analysis of the protein levels of ZnT1, ZnT3, ZnT4, ZnT5 and ZnT6 in the prefrontal cortex (BA10) of subjects with major depressive disorder (MDD) (n=10) and psychiatrically-normal control subjects (n=10). Data are expressed as protein level of investigated ZnTs in relation to GAPDH (mean R.O.D values ± SEM; % of matched control). Data were analyzed using paired Student’s t-test. *p<0.05, **p<0.01.
Fig. 3.
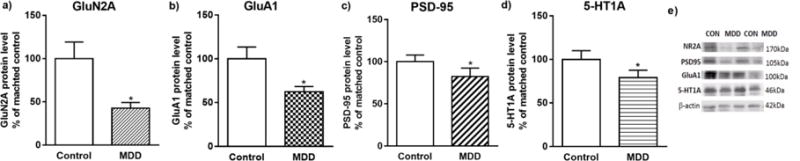
Analysis of the protein levels of GluN2A, GluA1, PSD-95 and 5-HT1A in the prefrontal cortex (BA10) of subjects with major depressive disorder (MDD) (n=10) and psychiatrically-normal control subjects (n=10). Data are expressed as protein level of GluN2A, GluA1, PSD-95 and 5-HT1A in relation to β-actin (mean R.O.D values ± SEM; % of matched control). Data were analyzed using paired Student’s t-test. *p<0.05.
Fig. 4.
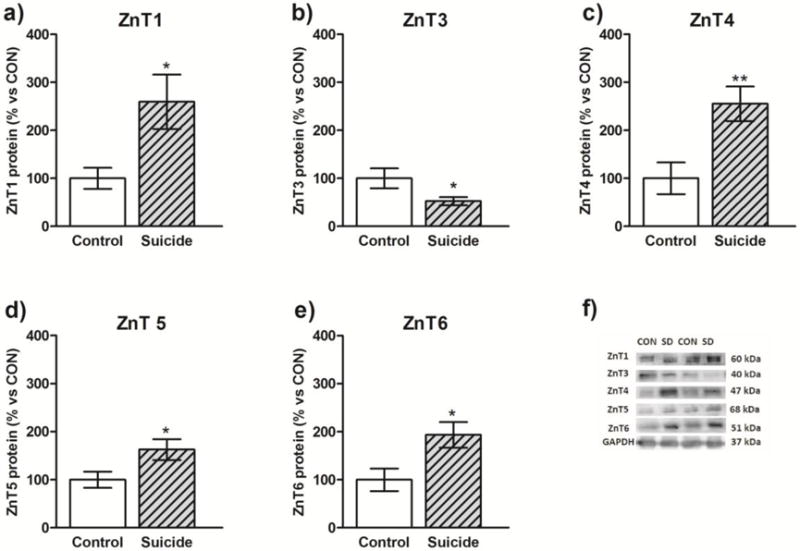
Protein levels of ZnT1, ZnT3, ZnT4, ZnT5 and ZnT6 in the prefrontal cortex of suicide subjects (n=11) and non-suicide control subjects (n=8). Data are expressed as protein level of investigated ZnTs in relation to GAPDH (R.O.D values ± SEM; % of control). Data were analyzed using unpaired Student’s t-test. *p<0.05, **p<0.01.
2.2.1. Statistical analysis
Data represent protein level of studied ZnTs in relation to GAPDH (R.O.D values ± SEM) and are expressed on the Fig. 1 and 4 as % of changes vs control. Protein levels of GluN2A, PSD-95, GluA1 and 5-HT1A are expressed in relation to β-actin (R.O.D. values ± SEM) and presented on Fig. 3 as % of change vs control. Differences between psychiatrically-normal control subjects and subjects with MDD (Cohort A) were analysed using paired Student’s t-test, while differences between suicide victims and non-suicide control subjects (Cohort B) were analyzed by unpaired Student’s t-test (Statistica 12), due to the different number of subjects in both groups. Furthermore, Pearson correlations were determined to identify potential correlations between variables such as age, postmortem interval (PMI), tissue pH, and duration and onset of depression (in MDD) and the studied proteins in Cohort A. In Cohort B, potential correlations were only determined between age and studied proteins (we were not able to obtain other data for these samples). A value of p<0.05 was considered statistically significant.
2.3. Voltammetric method
2.3.1. Instrumentation and Software
The Multipurpose Electrochemical Analyzer M161 with an electrode stand M164 (both mtm-anko, Poland) was used for all voltammetric measurements of Zn concentrations. The classical three-electrode cell, consisted of a controlled growth mercury drop electrode (CGMDE) as the working electrode (surface area 0.9 mm2), with a double junction reference electrode Ag/AgCl/3M KCl with replaceable outer junction (3M KNO3) and platinum wire as an auxiliary electrode. Stirring was performed using a magnetic Teflon®-coated bar rotating at approximately 600 rpm during the accumulation period. All experiments were carried out at room temperature. The mtm-anko EAGRAPH software was used for electrochemical measurements, data acquisition, and advanced processing of the results.
2.3.2. Reagents and Solutions
Prior to use, glassware was cleaned by immersion in a double-diluted aqueous solution of 65% HNO3, followed by copious rinsing in quadruple distilled water to avoid contamination. All solutions and the sample preparation were realized with quadruple distilled water. Nitric acid (65%), hydrogen peroxide (30%) and potassium nitrate (Merck, Suprapur®) were used for the preparation of samples and supporting electrolyte. Also, a Zn(II) standard stock solution (1.000 mg L−1, OUM Ł) was applied.
2.3.3. Sample Preparation
The tissue samples were thawed to room temperature, weighed (approximately 40 mg per sample) and digested in a mixed solution of 65% nitric acid (4.5 mL) and 30% hydrogen peroxide (0.5 mL) using Magnum II microwave digestion system (Ertec, Poland). All samples underwent a three-stage programmed digestion: stage 1- 17–20 bar for 12 min, 300W; stage 2- 30–33 bar for 8 min, 420W; stage 3- 42–45 bar for 28 min, 600W; all stages at 295°C). After 10 min of cooling, a totally clear solution of the sample was placed in a quartz evaporation vessel on a heated plate to evaporate and to remove the nitrate. The almost dry residue was then dissolved in quadruple distilled water and quantitatively transferred into a volumetric flask (5 mL) and filled up to the mark. In addition, blank analysis was carried out using the same procedure.
2.3.4. Voltammetric procedure
Before each measurement, 5 ml of the supporting electrolyte (0.2M KNO3) was placed in the voltammetric cell and the solution was purged with high-purity argon for 5 min. The voltammograms were then recorded to establish the baseline. All of the measurements of zinc content were performed using differential pulse anodic stripping voltammetry (DP ASV) with controlled growth mercury drop electrode. Preconcentration was carried at an Eacc= −1,35V vs the Ag/AgCl electrode with stirring for 15 s. After a rest period of 5 s, voltammograms were recorded from −1,3 V to −1 V with a signal amplitude of 20 mV and a step of 4mV, 40 ms (20 ms waiting + 20 ms sampling time). Then 200 μL of a sample solution was added to the cell while maintaining an argon atmosphere over the solution and three consequent standard additions were performed. Voltammograms corresponding to individual additions were recorded three times.
2.3.5. Statistical analysis
Differences in the Zn concentration between control subjects and those with MDD were analysed by Student t-test (GraphPad, Prism Software). A value of p<0.05 was considered statistically significant.
3. Results
3.1. ZnTs protein levels in the PFC of MDD and control subjects (cohort A)
The results of the Western blot analyses of ZnT1, ZnT3, ZnT4, ZnT5, and ZnT6 proteins in the PFC of depressed subjects and control subjects are summarized in Fig. 1 (a–e). The level of ZnTs for each subject was determined as the ratio of the optical density of ZnTs band to the optical density of the GAPDH band. Student’s t-test indicated significant increases in the levels of ZnT1 (t=4.68, df=9, p=0.0011), ZnT4 (t=4.035, df=9, p=0.0029) and ZnT5 (t=3,176, df=9, p=0.0112) in subjects with MDD vs. controls. In contrast, there was a significant decrease in the level of ZnT3 (t=4,215, df=9, p=0.0022) in subjects with MDD vs. conrtols. The level of ZnT6 remained unchanged (t=0.871, df=9, p=0.4063). There were no significant correlations between age, PMI or tissue pH and the protein level of investigated transporters in either control or MDD group. However, there was a trend for a positive correlation between age and the amount of ZnT1 (r=0.46) and for a negative correlation between age and the amount of ZnT3 (r= −0.21) in MDD.
3.2. Zinc concentration in the PFC of MDD and control subjects (cohort A)
Measurement of Zn using the voltammetric method revealed no significant difference in the concentration of Zn in the PFC between control and MDD subjects (t=0.9282, df=9, p=0.3775; Fig. 2).
Fig. 2.
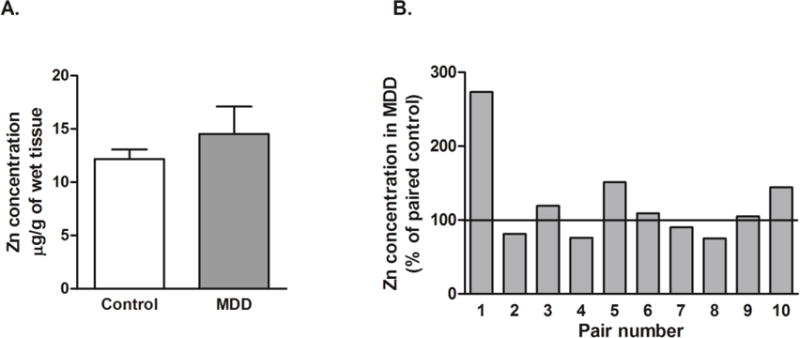
Concentration of Zn in the PFC of subjects with MDD (n=10) and psychiatrically-normal control subjects (n=10). Data are expressed as mean ± SD of Zn2+ μg/g wet tissue and were analyzed using paired Student’s t-test.
3.3. Levels of GluN2A, GluA1, PSD-95 and 5-HT1A receptor protein in the PFC of MDD and control subjects (cohort A)
The results of the Western blot analyses of GluN2A, GluA1, PSD-95 and 5-HT1A proteins in the PFC of depressed versus control subjects are summarized in Fig. 3 (a–d). For each subject, the level of each of these proteins was expressed as the ratio of the optical density of studied protein band to the optical density of the β-actin band. Student’s t-test indicated significant decreases in the levels of GluA1 (t=2.471, df=9, p=0.0354); GluN2A (t=2.521, df=9, p=0.0326); PSD-95 (t=2.839, df=9, p=0.0194) and 5-HT1A (t=3.428, df=9, p=0.0075) in subjects with MDD. There were no significant correlations between age, PMI or tissue pH and the expression of protein levels of GluN2A, GluA1, PSD-95 or 5-HT1A in either control or MDD subjects. There were also no significant correlations between GluN2A, GluA1, PSD-95 and 5-HT1A receptor and studied ZnTs in either control or MDD subjects.
3.4. ZnTs protein levels in the PFC of suicide victims and control subjects (cohort B)
The results of the Western blot analyses of ZnT1, ZnT3, ZnT4, ZnT5, ZnT6 proteins in the PFC of suicide victims and control subjects (cohort B) are summarized in Fig. 4 (a–e). Student’s t-test indicated significant increases in the level of ZnT1 (t=2.294, df=17, p=0.034), ZnT4 (t=3.037, df=17, p=0.007); ZnT5 (t=2.130, df=17, p=0.048) and ZnT6 (t=2.514, df=17, p=0.022) in suicide victims vs. control subjects. In contrast, there was a significant decrease in the level of ZnT3 (t=2.36, df=17, p=0.030) in suicide victims vs. control subjects. Analysis of the effect of the variables showed that there was no significant correlaton between age and the protein level of investigated transporters in either control or MDD group.
Zn levels were not measured in cohort B since our previous work in postmortem tissue from suicide victims (including the subjects from cohort B) did not reveal significant changes in the level of Zn in either the prefrontal cortex or hippocampus (Nowak et al., 2003; Sowa-Kucma et al., 2013).
4. Discussion
4.1. ZnTs in MDD and suicide
Our postmortem studies revealed significant changes in the level of zinc transporter proteins in the PFC of subjects with MDD and suicide victims compared to their respective control subjects. Overall, our data demonstrated an increase in the expression of ZnT1, ZnT4 and ZnT5 protein and a decrease in the expression of ZnT3 protein in the PFC of MDD and suicide victims. In addition, a statistically significant increase was noted in the expression of ZnT6 protein in the PFC of suicide victims, while there was only a trend for this protein to be increased in MDD.
Only one other study, performed in the PFC (BA9) of depressed patients with different types of dementia, has documented changes in expression levels of ZnT protein in depression. Elevated depression scores were significantly associated with reductions in ZnT3 protein levels (Whitfield et al., 2015), an report consistent with our observations of a decrease in the level of ZnT3 protein in the PFC of subjects diagnosed with MDD and suicide victims. Since ZnT3 is responsible for loading Zn into synaptic vesicles, reduced ZnT3 may correspond to reduced level of synaptic Zn and disruption in the downstream effects of Zn (Palmiter et al., 1996; Cole et al., 1999; Linkous et al., 2008;).
Other Zn transporter evaluated in the present study was ZnT1 for which a significant increase in both subjects with MDD and suicide victims was found. ZnT1 is the only transporter localized to the plasma membrane and functions mainly to export cytosolic Zn into the extracellular space and to protect cells from excess Zn influx under pathological conditions (Palmiter et al., 1996; Nolte et al., 2004). The expression of ZnT1 protein is highly regulated by Zn and is induced in the presence of increased cytoplasmic Zn via the metal-responsive transcription factor 1 (MTF-1) (Langmade et al., 2000). Considering that ZnT1 is sensitive to changes in cytosolic Zn concentration and is expressed in different brain areas (Sekler et al., 2002), it is possible that elevated levels of ZnT1 protein indicate increased levels of intracellular Zn in the PFC in MDD and in suicide. The increase in cytosolic Zn may occur via translocation of extracellular Zn into the cell due to sustained excitation of gluatamatergic neurons and may contribute to neurodegenerative processes which often accompany depression (Maes et al., 2011; Weisenbach and Kumar, 2014). Recently a new role has been proposed for ZnT1 at the excitatory glutamatergic synapse. Sindreu et al. (2014) demonstrated that ZnT1 is enriched in postsynaptic densities and Mellone et al. (2015) identified a direct interaction between ZnT1 and the GluN2A subunit of the NMDA receptor at the hippocampal excitatory synapse (Sindreu et al., 2014; Mellone et al., 2015). These findings and our results indicating changes in ZnT1 in MDD and suicide further suggest the importance of Zn-modulation of the central nervous system as a crucial mechanism involved in the pathophysiology of depression.
In our studies, ZnT4, ZnT5 and ZnT6 proteins were likewise increased in MDD and suicide. These ZnTs can also sequester Zn into different cellular organelles when intracellular Zn levels are elevated (Kambe et al., 2015). ZnT4 is localized to intracellular vesicles, trans-Golgi network (TGN) and endosomal compartments (Kambe et al., 2015). ZnT5 and ZnT6 form heterodimers and have been localized to early secretory pathway (Kambe, 2011). ZnT5 and ZnT6 transport activities are not potent and these transporters may play a role in the loading Zn to Zn-requiring enzymes rather in sequestering or detoxifying Zn, unlike the role of ZnT4 (Palmiter and Huang, 2004). Our recent preclinical data showed no changes in the protein level of ZnT4, 5 or 6 in the olfactory bulbectomy model of depression in the rat (Rafalo et. al., 2016). Thus, more studies are needed to determine the exact role of these ZnTs in the pathophysiology of depression. One may speculate that the increased expression of ZnT4, 5 and 6 observed in PFC in MDD and suicide may be a homeostatic response to reduce an excess of Zn in the cytosol and protect cells from its toxicity.
More generally, these studies highlight the role of Zn homeostasis as the relevant mechanisms involved in the pathophysiology of depression and paws the way for investigation processes and proteins reguling Zn status as the targets in the preventing and theraphy of this diseases.
4.2. NMDA, AMPA and 5-HT1A receptors and ZnTs in MDD
One of the main roles of Zn at the synapse is the modulation of the glutamatergic system, particularly through the inhibition of NMDA receptors (Paoletti et al., 1997; Smart et al., 2004). Zn2+ binding to the GluN2A subunit of the NMDA receptor seems to play a crucial role in the Zn-dependent modulation of NMDA receptor signaling (Paoletti et al., 1997). However, recent data also provide evidence that synaptically released Zn inhibits AMPA receptors (Kalappa et al., 2015), thus further suggesting an important role of Zn homeostasis in synaptic function.
As noted above, abnormalities in glutamatergic transmission are involved in the pathophysiology of depression. Several studies have clearly noted changes in the levels of NMDA receptors subunits, including GluN2A, in postmortem brain tissue from subjects with MDD and suicide victims (Smart et al., 2004; Feyissa et al., 2009; Karolewicz et al., 2009; Sowa-Kucma et al., 2013). In addition to the NMDA receptors, AMPA receptors are also involved in the etiology of depression. There are reports of a reduction in the mRNA and protein level of AMPA receptor subunits (including GluA1) in the brain of depressed patients (Beneyto et al., 2007, Meador-Woodruff et al., 2003) and in animals subjected to stress (Yuen et al., 2012, Toth et al., 2008). A decrease in PSD-95 protein, which is a marker of postsynaptic density (Yudowski et al., 2013) implicated in the trafficking and stabilization of NMDA and AMPA receptors at postsynaptic sites, was also noted in the the postmortem brain of MDD subjects (Feyissa et al., 2009, Karolewicz et al., 2009). Our present studies demonstrating a decrease in GluN2A subunits of NMDA receptors, GluA1 subunits of AMPA receptors and PSD-95 in the PFC of MDD subjects are thus consistent with earlier report. However, we did not detect a significant correlation between the decreased levels of these proteins and either decresed levels of ZnT3 or increased levels of ZnT4, 5 and 6. There was only a trend for a negative correlation between GluN2A or PSD-95 and ZnT1 protein expression. One of the limitations of our studies is that we measured the protein level of all studied proteins in the total homogenate of the PFC but not at the specific synaptic fractions. Moreover, most studies on the role of Zn in the modulation of NMDA or AMPA receptors were performed in the hippocampus. Thus further studies are needed to determine whether the NMDA/AMPA receptor-related pathophysiology observed in depression is also correlated with the release of presynaptic Zn and/or changes in the function of ZnTs in brain regions other than PFC.
A number of studies have also demonstrated a reduction in the protein levels of the 5-HT1A receptor and radioligand binding to this receptor in the prefrontal cortex of subjects with MDD (Stockmeier et al., 2009; Szewczyk et al., 2009; Szewczyk et al., 2010). The role of Zn in modulating of 5-HT1A receptors has been described (see Nowak et al., 2015; Satala et al., 2016). Thus, we speculate that changes observed in these receptors in the PFC of subjects with MDD may correlate with the alterations observed in ZnTs. Similarly, we found a decrease in the protein level of 5-HT1A receptors in the PFC of subjects with MDD vs matched controls; however no significant correlation was observed between 5-HT1A receptors and the studied ZnTs.
The lack of correlations between changes in the expression of receptors and ZnTs shown in our study does not rule out the important role of Zn homeostasis in the depression. Rather, there is a need for further research to determine the functional significance of Zn transporters in the pathophysiology of depression and to describe any mechanisms underlying the observed changes.
5. Conclusions and limitations
In summary, the present study measured the protein levels of five out of ten known ZnTs, namely ZnT1, ZnT3, ZnT4, ZnT5 and ZnT6 in the PFC of MDD and suicide victims. All of these Zn transporters are highly expressed in the brain. We observed a significant increase in the levels of ZnT1, ZnT4, ZnT5, and a significant decrease in the level of ZnT3 in the PFC (BA10) in MDD and in suicide. Levels of ZnT6 were significantly increased in suicide but there was only a trend for an increase in MDD. Our data suggest that changes in the expression of Zn transporters are associated with the pathophysiology of depression and suicide and could reflect altered cortical distribution of Zn.
About 60 percent of suicide victims met criteria for depression (Bradvik, 2002; Bradvik and Berglund, 2005). Moreover, it has been reported that compared with suicide attempters, suicide completers more likely suffered from MDD (Coryell and Young, 2005; Srivastava and Kumar, 2005; Pompili et al., 2008). Seven of the ten subjects with MDD in our study also died by suicide. It is quite likely that depression was present in many of our suicide victims for whom no diagnostic information was available. However, lacking of psychiatric diagnosis for the suicide victims in cohort B, it cannot be ruled out that the changes observed in ZnTs were related to factors other than depression.
Three of the eleven suicide subjects (cohort B) died from a drug overdose. Although there are no published data to suggest otherwise, we cannot exclude the possibility that changes in the expression of ZnTs proteins in MDD may result in part from the effects of antidepressant medications.
The present study using the voltammetric method demonstrated no significant difference in the total concentration of Zn in the PFC when comparing subjects with MDD and control subjects. Neither of our previous studies using postmortem brain tissue of suicide victims (including subjects from cohort B) showed a change in Zn concentration in either the hippocampus (Sowa-Kucma et al., 2013) or both frontal cortex and hippocampus (Nowak et al., 2003). While these data suggest that depression does not change the total amount of Zn in the brain, these methods are not able to detect any differences that may occur in MDD at the synaptic or intracellular level.
The influence of a number of potentially confounding variables on ZnTs were examined. There were no significant correlations between age, PMI, or tissue pH on the expression of Zn transporters examined in this study. However, there was a trend for a correlation of age and two Zn transporters in subjects with MDD (positive for ZnT1 and negative for ZnT3). Additional subjects are needed to rule out an interaction between these transporters and age.
Recent studies in postmortem brain tissue revealed changes in the expression of both glutamatergic and serotonergic receptors in MDD (Beneyto et al., 2007; Feyissa et al., 2009; Stockmeier et al., 2009; Szewczyk et al., 2009). Our present studies demonstrated significantly decreased levels of the GluN2A subunit of NMDA receptor, GluA1 subunit of AMPA-receptor and 5-HT1A receptors subjects with MDD. However we did not find any significant correlations between the level of these receptors and ZnTs in depression. Since the level of ZnTs may only indirectly reflect changes in the level of Zn in the brain, further studies directly examining the correlation between the synaptic or intracellular Zn level and the expression of these receptors are warrented. Moreover, expending these studies into other brain regions should also be considered.
Acknowledgments
The authors deeply appreciate the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. We also gratefully acknowledge the support of the staff of the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio. We acknowledge the expert assistance of Drs. James C. Overholser, George Jurjus, and Lisa C. Konick, and of Lesa Dieter and Gouri Mahajan in establishing the psychiatric diagnoses, acquiring written consent and in collecting the tissues.
Role of the funding source
This study was supported by: the Foundation for Polish Science “POMOST” Program (POMOST/2012-6/12), which was cofinanced by the European Regional Development Fund (Innovative Economy Operational Program 2007–2013), statutory funds of the Institute of Pharmacology PAS, and, in part by the Imaging and Postmortem Brain Cores of the COBRE Center for Psychiatric Neuroscience (P30 GM103328) and by The National Institute of Mental Health (MH67996).
Footnotes
Contributors
Anna Rafalo-Ulinska: conducted all Western blot studies and create the first version of manuscript.
Joanna Piotrowska, Agata Kryczyk and Wlodzimierz Opoka: conducted Zn concentration measurement.
Magdalena Sowa-Kucma, Paulina Misztak M, Grazyna Rajkowska and Craig A. Stockmeier: prepared post-mortem brain tissues.
Wojciech Datka: performed statistical analysis of results;
Grazyna Rajkowska, Craig A. Stockmeier and Gabriel Nowak: contributed to the revising of the last version of the manuscript.
Bernadeta Szewczyk: initiated the present studies, designed experiments and created the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest
References
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4th. Washington: APA; 1994. [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32(9):1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Bosomworth HJ, Adlard PA, Ford D, Valentine RA. Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS One. 2013;8(5):e65475. doi: 10.1371/journal.pone.0065475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradvik L. The occurrence of suicide in severe depression related to the months of the year and the days of the week. Eur Arch Psychiatry Clin Neurosci. 2002;252(1):28–32. doi: 10.1007/s004060200005. [DOI] [PubMed] [Google Scholar]
- Bradvik L, Berglund M. Suicide in severe depression related to treatment: depressive characteristics and rate of antidepressant overdose. Eur Arch Psychiatry Clin Neurosci. 2005;255(4):245–250. doi: 10.1007/s00406-004-0553-7. [DOI] [PubMed] [Google Scholar]
- Cobb JA, O’Neill K, Milner J, Mahajan GJ, Lawrence TJ, May WL, Miguel-Hidalgo J, Rajkowska G, Stockmeier CA. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209–220. doi: 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A. 1999;96(4):1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Young EA. Clinical predictors of suicide in primary major depressive disorder. J Clin Psychiatry. 2005;66(4):412–417. doi: 10.4088/jcp.v66n0401. [DOI] [PubMed] [Google Scholar]
- Dejong TM, Overholser JC. Assessment of depression and suicidal actions: agreement between suicide attempters and informant reports. Suicide Life Threat Behav. 2009;39(1):38–46. doi: 10.1521/suli.2009.39.1.38. [DOI] [PubMed] [Google Scholar]
- Doboszewska U, Sowa-Kucma M, Mlyniec K, Pochwat B, Holuj M, Ostachowicz B, Pilc A, Nowak G, Szewczyk B. Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2015a;56:254–263. doi: 10.1016/j.pnpbp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Doboszewska U, Szewczyk B, Sowa-Kucma M, Mlyniec K, Rafalo A, Ostachowicz B, Lankosz M, Nowak G. Antidepressant activity of fluoxetine in the zinc deficiency model in rats involves the NMDA receptor complex. Behav Brain Res. 2015b;287:323–330. doi: 10.1016/j.bbr.2015.03.064. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of GLUN2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for the DSM-IV Axis I disorders (SCID patient edition), version 2.0. New York State Psychiatric Institute; 1995. [Google Scholar]
- Freudenberg F, Celikel T, Reif A. The role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev. 2015;52:193–206. doi: 10.1016/j.neubiorev.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem. 2011;16(7):1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Phillips C, Trillo L, De Miguel Z, Das D, Salehi A. The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci Biobehav Rev. 2014;47:336–58. doi: 10.1016/j.neubiorev.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Tepaamorndech S. The SLC30 family of zinc transporters – a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med. 2013;34(2–3):548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Maes M, Pasco JA, Williams LJ, Berk M. Nutrient intakes and the common mental disorders in women. J Affect Disord. 2012;141(1):79–85. doi: 10.1016/j.jad.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Anderson CT, Goldberg JM, Lippard SJ, Tzounopoulos T. AMPA receptor inhibition by synaptically released zinc. Proc Natl Acad Sci U S A. 2015;112(51):15749–15754. doi: 10.1073/pnas.1512296112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem. 2011;75(6):1036–1043. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci. 2014;71(17):3281–3295. doi: 10.1007/s00018-014-1617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277(21):19049–19055. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- Kambe T, Tsuji T, Hashimoto A, Itsumura N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol Rev. 2015;95(3):749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of GLUN2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol. 2009;12(2):143–153. doi: 10.1017/S1461145708008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol. 2016;26(3):397–410. doi: 10.1016/j.euroneuro.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10(10):808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275(44):34803–34809. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- Linkous DH, Flinn JM, Koh JY, Lanzirotti A, Bertsch PM, Jones BF, Giblin LJ, Frederickson CJ. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J Histochem Cytochem. 2008;56(1):3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De JR, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, D’Haese PC, Scharpe S, D’Hondt P, Cosyns P, De Broe ME. Hypozincemia in depression. J Affect Disord. 1994;31(2):135–140. doi: 10.1016/0165-0327(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Maes M, De VN, Demedts P, Wauters A, Neels H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J Affect Disord. 1999;56(2–3):189–194. doi: 10.1016/s0165-0327(99)00011-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32(1):7–24. [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Marcellini F, Giuli C, Papa R, Gagliardi C, Dedoussis G, Herbein G, Fulop T, Monti D, Rink L, Jajte J, Mocchegiani E. Zinc status, psychological and nutritional assessment in old people recruited in five European countries: Zincage study. Biogerontology. 2006;7(5–6):339–345. doi: 10.1007/s10522-006-9048-4. [DOI] [PubMed] [Google Scholar]
- Markiewicz-Zukowska R, Gutowska A, Borawska MH. Serum zinc concentrations correlate with mental and physical status of nursing home residents. PLoS One. 2015;10(1):e0117257. doi: 10.1371/journal.pone.0117257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserejian NN, Hall SA, McKinlay JB. Low dietary or supplemental zinc is associated with depression symptoms among women, but not men, in a population-based epidemiological survey. J Affect Disord. 2012;136(3):781–788. doi: 10.1016/j.jad.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin IJ, Hodge JS. Zinc in depressive disorder. Acta Psychiatr Scand. 1990;82(6):451–453. doi: 10.1111/j.1600-0447.1990.tb03077.x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Hogg AJ, Jr, Smith RE. Striatal ionotropic glutamate receptor expression in schizophrenia, bipolar disorder, and major depressive disorder. Brain Res Bull. 2001;55(5):631–640. doi: 10.1016/s0361-9230(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Mellone M, Pelucchi S, Alberti L, Genazzani AA, Di LM, Gardoni F. Zinc transporter-1: a novel NMDA receptor-binding protein at the postsynaptic density. J Neurochem. 2015;132(2):159–168. doi: 10.1111/jnc.12968. [DOI] [PubMed] [Google Scholar]
- Mlyniec K, Nowak G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants Pharmacol Rep. 2012;64(2):249–255. doi: 10.1016/s1734-1140(12)70762-4. [DOI] [PubMed] [Google Scholar]
- Mlyniec K, Doboszewska U, Szewczyk B, Sowa-Kucma M, Misztak P, Piekoszewski W, Trela F, Ostachowicz B, Nowak G. The involvement of the GPR39-Zn (2+)-sensing receptor in the pathophysiology of depression. Studies in rodent and suicide victims Neuropharmacology. 2014;79:290–297. doi: 10.1016/j.neuropharm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Nolte C, Gore A, Sekler I, Kresse W, Hershfinkel M, Hoffmann A, Kettenmann H, Moran A. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia. 2004;48(2):145–155. doi: 10.1002/glia.20065. [DOI] [PubMed] [Google Scholar]
- Nowak G. Zinc, future mono/adjunctive therapy for depression: Mechanisms of antidepressant action. Pharmacol Rep. 2015;67(3):659–662. doi: 10.1016/j.pharep.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Nowak G, Schlegel-Zawadzka M. Alterations in serum and brain trace element levels after antidepressant treatment: part I. Zinc Biol Trace Elem Res. 1999;67(1):85–92. doi: 10.1007/BF02784278. [DOI] [PubMed] [Google Scholar]
- Nowak G, Szewczyk B, Sadlik K, Piekoszewski W, Trela F, Florek E, Pilc A. Reduced potency of zinc to interact with NMDA receptors in hippocampal tissue of suicide victims. Pol J Pharmacol. 2003;55(3):455–459. [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93(25):14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447(5):744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-GLUN2A receptors. J Neurosci. 1997;17(15):5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender S, Grabrucker AM. Characterization of biometal profiles in neurological disorders. Metallomics. 2014;6(5):960–977. doi: 10.1039/c4mt00008k. [DOI] [PubMed] [Google Scholar]
- Pochwat B, Nowak G, Szewczyk B. Relationship between Zinc (Zn (2+)) and Glutamate Receptors in the Processes Underlying Neurodegeneration. Neural Plast. 2015;2015:591563. doi: 10.1155/2015/591563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili M, Innamorati M, Raja M, Falcone I, Ducci G, Angeletti G, Lester D, Girardi P, Tatarelli R, De PE. Suicide risk in depression and bipolar disorder: Do impulsiveness-aggressiveness and pharmacotherapy predict suicidal intent? Neuropsychiatr Dis Treat. 2008;4(1):247–255. doi: 10.2147/ndt.s2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu GK. Zinc transporter found attached to N-methyl-D-aspartate receptors. J Neurochem. 2015;132(2):155–158. doi: 10.1111/jnc.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalo A, Zadrozna M, Nowak B, Kotarska K, Wiatrowska K, Pochwat B, Sowa-Kucma M, Misztak P, Nowak G, Szewczyk B. The level of the zinc homeostasis regulating proteins in the brain of rats subjected to olfactory bulbectomy model of depression. 2016 doi: 10.1016/j.pnpbp.2016.08.009. doi: 0.1016/j.pnpbp.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Roy A, Evers SE, Avison WR, Campbell MK. Higher zinc intake buffers the impact of stress on depressive symptoms in pregnancy. Nutr Res. 2010;30(10):695–704. doi: 10.1016/j.nutres.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Sekler I, Moran A, Hershfinkel M, Dori A, Margulis A, Birenzweig N, Nitzan Y, Silverman WF. Distribution of the zinc transporter ZnT-1 in comparison with chelatable zinc in the mouse brain. J Comp Neurol. 2002;447(3):201–209. doi: 10.1002/cne.10224. [DOI] [PubMed] [Google Scholar]
- Sindreu C, Bayes A, Altafaj X, Perez-Clausell J. Zinc transporter-1 concentrates at the postsynaptic density of hippocampal synapses. Mol Brain. 2014;7:16. doi: 10.1186/1756-6606-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek M, Dudek D, Schlegel-Zawadzka M, Morawska A, Piekoszewski W, Opoka W, Zieba A, Pilc A, Popik P, Nowak G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord. 2010;126(3):447–452. doi: 10.1016/j.jad.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10(5):432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- Sokero TP, Melartin TK, Rytsala HJ, Leskela US, Lestela-Mielonen PS, Isometsa ET. Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry. 2003;64(9):1094–1100. doi: 10.4088/jcp.v64n0916. [DOI] [PubMed] [Google Scholar]
- Sowa-Kucma M, Szewczyk B, Sadlik K, Piekoszewski W, Trela F, Opoka W, Poleszak E, Pilc A, Nowak G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J Affect Disord. 2013;151(3):924–931. doi: 10.1016/j.jad.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Srivastava AS, Kumar R. Suicidal ideation and attempts in patients with major depression: Sociodemographic and clinical variables. Indian J Psychiatry. 2005;47(4):225–228. doi: 10.4103/0019-5545.43059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi X, Sobanska A, Clarke G, Friedman L, Rajkowska G. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J Psychiatr Res. 2009;43(10):887–94. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12(2):155–68. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Rogaeva A, Fitzgibbon H, May WL, Rajkowska G, Miguel-Hidalgo JJ, Stockmeier CA, Woolverton WL, Kyle PB, Wang Z, Austin MC. Decreased expression of Freud-1/CC2D1A, a transcriptional repressor of the 5-HT1A receptor, in the prefrontal cortex of subjects with major depression. Int J Neuropsychopharmacol. 2010;13(8):1089–101. doi: 10.1017/S1461145710000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Tamano H. Insight into zinc signaling from dietary zinc deficiency. Brain Res Rev. 2009;62(1):33–44. doi: 10.1016/j.brainresrev.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Tamano H, Kan F, Kawamura M, Oku N, Takeda A. Behavior in the forced swim test and neurochemical changes in the hippocampus in young rats after 2-week zinc deprivation. Neurochem Int. 2009;55(7):536–541. doi: 10.1016/j.neuint.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav. 2008;95(3):365–369. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107(2):522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tamano H, Kikuchi T, Takeda A. Susceptibility to stress in young rats after 2-week zinc deprivation. Neurochem Int. 2010;56(3):410–416. doi: 10.1016/j.neuint.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463. doi: 10.1007/s11920-014-0463-y. [DOI] [PubMed] [Google Scholar]
- Whitfield DR, Vallortigara J, Alghamdi A, Hortobagyi T, Ballard C, Thomas AJ, O’Brien JT, Aarsland D, Francis PT. Depression and synaptic zinc regulation in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia. Am J Geriatr Psychiatry. 2015;23(2):141–148. doi: 10.1016/j.jagp.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Whittle N, Lubec G, Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36(1):147–158. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73(5):962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


