Abstract
When nitrosothiols react with excess hydrogen sulfide, H2S, they form several intermediates including nitrosopersulfide (SSNO−). The stability and importance of this species has been debated. While some data suggest SSNO− can be a relatively stable source of NO activity, others suggest that the species degrades too quickly. We find the species to be relatively stable in isolation. Due to the abundance and prominence of iron-containing proteins throughout the human body, it is important to establish the interaction of ferrous- and ferric-iron containing proteins with SSNO−. Study of the reactions of SSNO− with heme proteins can also provide information about the potential in vivo stability and spontaneous reactivity of this species. We have used time-resolved electron paramagnetic resonance and UV-Vis absorption spectroscopy to study the reactions of SSNO− with heme proteins. Iron-nitrosyl hemoglobin is formed when SSNO− is reacted with deoxyhemoglobin and deoxygenated methemoglobin, suggesting NO formation from SSNO−. However, the yields of nitrosyl hemoglobin in reactions of SSNO− with deoxyhemoglobin are much less than when SSNO− is reacted with deoxygenated methemoglobin. Very little to no nitrosyl hemoglobin is formed when SSNO−is reacted carboxyhemoglobin, HbCO, and when SSNO− is reacted with oxygenated hemoglobin, minimal methemoglobin is formed Taken together, these data confirm the release of NO, but indicate a vacant heme is necessary to facilitate a direct heme-SSNO− reaction to form substantial NO. These data also suggest that the ferric iron in methemoglobin potentiates SSNO− reactivity. These results could potentially impact NO and sulfide bioavailability and reactivity.
Keywords: Hydrogen sulfide, nitric oxide, nitroxyl, nitrosopersulfide, nitrosothiol
Introduction
Nitric oxide (NO) produced by nitric oxide synthase (NOS) in the endothelium must diffuse into the smooth muscle to activate its heme-containing target, soluble guanylate cyclase (sGC). The reactivity of NO with heme-containing proteins is implicated in the inhibition of mitochondrial respiration and NO cytoxicity where the targets are cytochrome c oxidase and catalase, respectively [1-3]. NO bioavailability is diminished by its reaction with oxyhemoglobin [4]. In the presence of oxygen, NO can form nitrosothiols which have been thought to be more stable than NO and still carry NO activity [5]. NO reactivity with ferrous and ferric heme proteins influences its signaling in substantial ways [6-8].
When the ferriheme of methemoglobin (metHb) reacts with NO, it binds to and subsequently reduces the heme at both acidic and alkaline pH values, but is fastest at higher pH [9, 10]. Hence, nitrosyl hemoglobin (HbNO) formation from metHb requires two NO, one to reduce and the other to bind, and the rate of reduction is dependent on [OH−] (Eqs. 1-4) [9, 10],
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
| Eq. 4, |
where Hb(Fe3+) is methemoglobin, Hb(Fe3+)NO is the ferriheme-nitrosyl adduct, Hb(Fe2+) is reduced ferrous hemoglobin, and Hb(Fe2+)NO is iron-nitrosyl hemoglobin. The equilibrium constant, K, at pH 7.4 is 1.3 ± 0.1 × 104 M−1, the rate constant, kH2O, is 1.1 × 103 M−1 s−1 and the rate constant, kOH, is 3.2 × 103 M−1 s−1 [9]. In the case of myoglobin, metmyoglobin binds NO to form the ferric nitrosyl species with an equilibrium constant K = 1.3. ± 0.1 × 104 M−1, but subsequent reduction of the ferric nitrosyl does not occur between pH 6.0 and 7.2. [9]. Only the hydroxyl ion facilitates the reduction of metmyoglobin, not water like Hb as in Eq. 3.
Nitroxyl (HNO) also reduces ferriheme containing globins to yield nitrosyl species, as shown in Eq. 5,
| Eq. 5, |
where Hb (Fe3+) is metHb, HNO is nitroxyl, and Hb(Fe2+)NO is HbNO. The reduction of ferriheme depicted in Eq. 5 is expected to occur with a rate constant similar to that for metmyoglobin which has been reported to be 8 × 105 M−1 s−1 [11]. The reaction of HNO with oxyhemoglobin (oxyHb) yields metHb and NO, which leads to NO dioxygenation by excess oxyHb (Eqs. 6, 7) [12, 13]. NO dioxygenation occurs when the O2-ligated ferrous heme of oxyHb reacts with NO, yielding metHb and nitrate (Eq. 7) [13-15],
| Eq. 6 |
| Eq. 7 |
where oxyhemoglobin, Hb(Fe2+)O2 reacts with HNO or NO on the order of 107 M−1 s−1 [13].
H2S, like NO, is a gasotransmitter that has many biological effects, including the potentiation of NO signaling [16, 17]. Endogenously produced H2S is stored in sulfane sulfur, such as, polysulfides, persulfides, thiosulfate, etc. [18]. At neutral pH, unstored H2S is predominantly HS− due to a pKa of 6.8 [19]. Although NO and HS− are likely nonreactive, HS− and NO+, which occurs in S-nitrosothiol (RSNO), or sulfide radical (HS•) and NO are reactive [19-21].
As a product of the chemical reaction of RSNO with HS− and a long-term activator of sGC, nitrosopersulfide (SSNO−) has gained interest for its physiological potential [21]. As shown in Eqs 8 and 9,
| Eq. 8 |
| Eq. 9 |
nucleophilic attack of HS− on the sulfur of RSNO may result in thionitrous acid (HSNO) formation and HNO formation upon a subsequent nucleophilic attack of HS− on HSNO (Eqs. 8, 9) [20] [21]. Although HSNO is likely a product of this reaction, its labile nature excludes it as a candidate for the prolonged sGC activation observed by Cortese-Krott and colleagues[20]. The prevalence of persulfide (HSS-) at high concentrations of sulfide leads to formation SSNO−,
| Eq. 10 |
where nucleophilic attack of HSS− on the RSNO results in the formation of SSNO− (Eq. 10) [20]. For this reason, SSNO− formation is prevalent at 1:1 or higher HS− to RSNO concentration ratios [20]. When concentration ratios of 1:1 and 2:1 (HS−:RSNO) are used, SSNO− is formed within 10 minutes and there is substantial NO released when compared to RSNO alone [20]. Cortese-Krott et al. show sustained sGC activation and cGMP accumulation upon SSNO− decomposition along with a gradual increase in absorbance at 290-300 nm, indicating polysulfide formation [20]. These data support homolytic cleavage of SSNO− as a mechanism to obtain NO from SSNO−. SSNO− decomposition by homolytic cleavage as shown by Eq. 11,
| Eq. 11, |
results in NO and the disulfide radical (SS•), which form polysulfides [20]. In this way, the once oxidized redox congener of NO, RSNO, can behave as a reservoir for NO bioactivity.
Precontracted aortic rings that were preincubated with a sGC inhibitor showed diminished relaxation in response to GSNO and SSNO−[21], confirming a role for sGC in GSNO and SSNO−-induced relaxation. Although application of an NO scavenger effectively mitigated GSNO-induced relaxation, it had no effect on SSNO−-induced relaxation [21]. These data suggest a mechanism for SSNO−-induced relaxation in which NO is not “released” from SSNO−, but sGC activation is obtained. A possible explanation may be HNO formation or direct SSNO− and sGC interaction. sGC is a heme containing protein and direct interaction between SSNO−and sGC cannot be ruled out. Although some believe SSNO− is physiologically relevant, still others suggest that SSNO− is too unstable to be physiologically relevant [20, 22]. In this study, we sought to determine the stability and spontaneous reactivity of SSNO− in the presence of heme-containing proteins.
Experimental Procedures
Chemicals
MAHMANONOate and Angeli’s salt were purchased from Cayman Chemical Company. Equine skeletal muscle myoglobin, lyophilized bovine liver catalase, TRIZMA hydrochloride (Tris HCl), phosphate buffered saline (PBS) pH 7.4, monosodium phosphate, disodium phosphate, diethylenetriaminepentaacetic acid (DTPA) and potassium hexacyanoferrate (III) (K3Fe(CN)6) were purchased from Sigma. Sodium hydroxide (NaOH) solution, 1 N, was purchased from Fisher Scientific. Na2S 9H2O was obtained form Acros Organics. S-nitrosoglutathione (GSNO) was formed from the protocol by T.W. Hart [23] with adaptations.
Reagent Preparation
Methemoglobin and metmyoglobin were prepared by mixing oxyhemoglobin or equine skeletal muscle myoglobin, respectively, with K3Fe(CN)6 in 10-fold excess in PBS, pH 7.4. A stock of K3Fe(CN)6 was prepared in PBS pH 7.4 and the globin/K3Fe(CN)6 mixtures were gently agitated for 20 minutes to ensure thorough mixing. Then, the mixtures were filtered through a sephadex G-25 M column containing 0.15% Kathon. Concentration was determined by UV-visible spectroscopy. Lyophilized bovine liver ferricatalase stocks were prepared in 100 mM Phosphate Buffer, pH 7.4 and centrifuged at 13,000 × g to remove any debris. Concentration was determined spectrally, ε405 = 1.49 × 105 M−1 cm−1 [24].Stocks of 10 mM NaOH with and without 0.1 mM DTPA were made in deionized (DI) water and degassed with N2 or Ar gas. Stocks of PBS pH 7.4 with and without 0.1 mM DTPA were made and degassed with N2 or Ar. DI water and 20 mM TRIS HCl pH7.4 were also degassed.
In a 5 mL polypropylene tube capped with a rubber stopper, Na2S crystals were rinsed with DI water and immediately degassed with N2 or Ar until dried. The addition of 3-4 mL of degassed 10 mM NaOH with 0.1 mM DTPA to the degassed Na2S crystals yielded stock concentrations that ranged between 10-25 mM. Na2S stock concentrations were ascertained using UV-visible spectroscopy by diluting the stock into 2 mL of degassed DI water in a degassed 1 cm quartz cuvette, ε232 = 7700 M−1 cm−1[21]. Stocks were freshly made and used within 6 hours.
GSNO stocks were prepared in glass, amber-tinted vials, capped with a rubber septum by adding 400-500 μL of degassed PBS with 0.1 mM DTPA to fully degassed GSNO. Concentration of the GSNO stocks were ascertained by UV-visible spectroscopy by diluting the stock into 2 mL of degassed PBS in a degassed 1 cm quartz cuvette, ε338 = 980 M−1 cm−1[25]. GSNO stock concentration ranged between 20-40 mM.
Experimental Methods
SSNO− mixture stocks were prepared by reacting Na2S and GSNO at a 2:1 molar ratio in degassed PBS pH 7.4 without DTPA in a degassed, rubber stoppered, polypropylene tube covered in aluminum foil. After allowing the Na2S and GSNO to react for 10 or 30 minutes, SSNO− stocks were then degassed with N2 or Ar for 30 minutes. SSNO− mixture stocks were used immediately after being degassed. Throughout this work, “SSNO− at 10 (or 30) minutes” or “SSNO− preincubated for 10 (or 30) minutes” refers to Na2S and GSNO coincubated for 10 (or 30) minutes prior to using for any reactions with proteins. Since we were unable to spectrally detect any other nitroso or nitrogen oxide species in the reaction product, the concentration of SSNO− was based on the concentration of GSNO measured before adding sulfide. It is possible that there are other NO-based products that we did not detect spectrally, so the concentrations of SSNO− referred to are potentially higher than actual concentrations. SSNO− reactions with proteins were carried out in anaerobic or aerobic 1 cm quartz cuvettes. UV-vis spectroscopy was obtained for 10 minutes at one scan every 30 seconds using a Cary 50 or Cary 100 spectrophotometer.
OxyHb was prepared from red cell lysates. Concentration and purity was determined by spectral deconvolution of a UV/vis absorption spectrum. MAHAMANOnoate stocks were prepared in anaerobic 10 mM NaOH and the concentration was determined by absorbance, ε250=7,250 M−1 cm−1 [26]. HbNO yield from reacting deoxyHb with MAHMANOnoate was used to determine NO yield. MAHMANOnoate final concentrations were corrected according to the NO yield. OxyHb, 0.1 mM, was reacted with an equimolar SSNO− mixture or equimolar NO and followed with UV/visible spectroscopy for 10 minutes prior to freezing 400 μL of each sample and scanning for metHb formation using EPR.
HbNO yield from SSNO− and metHb under anaerobic and aerobic conditions was obtained by electron paramagnetic resonance (EPR). After reacting H2S and GSNO for 10 or 30 minutes, observing SSNO− formation spectroscopically, and degassing SSNO− for 30 minutes, degassed 0.1 mM metHb was reacted with the SSNO− mixture at equimolar concentrations in anaerobic or aerobic 20 mM TRIS HCl, pH 7.4. Reactions were followed for 10 minutes using UV-visible spectroscopy. EPR spectra of HbNO were taken using a Bruker EMX 10/12 spectrometer operated at 110K using a modulation amplitude of 5.0 Gauss, 9.38 GHz microwave frequency, and a microwave power of 13.0 decibels. EPR of metHb was collected similarly except that the temperature was 5K and we used a modulation amplitude of 15.0 G.
DeoxyHb was prepared with gentle agitation during degassing to obtain a stock that was >95% deoxygenated. Concentration and purity was determined by spectral deconvolution of a UV/vis spectroscopic scan. MAHAMANOnoate stocks were prepared in anaerobic 10 mM NaOH and concentrations determined by absorbance, ε250=7,250 M−1 cm−1[26]. HbNO yield from reacting deoxyHb with MAHMANOnoate was used to determine NO yield from the NOnoate. MAHMANOnoate final concentrations were corrected according to the NO yield. Reactions of equimolar SSNO− mixture with equimolar 0.1 mM deoxyHb or 0.1 mM metMb and equimolar NO from MAHMANOnoate with 0.1 mM deoxyHb or 0.1 mM metMb were followed by absorption spectroscopy for 10 minutes in anaerobic 20 mM TRIS HCl pH 7.4. After allowing the protein and SSNO− to react for 10 minutes, 400 μL of each sample was frozen and scanned for nitrosyl formation using EPR.
A basis spectrum of ferrous nitrosyl catalase was created by reacting 50-fold excess, 0.1 mM, HNO donor with 2 μM ferricatalase. A nitrosyl ferricatalase basis spectrum was created by reacting 50-fold excess NO donor with 2 μM ferricatalase. Stocks of Angeli’s Salt were prepared in anaerobic 10 mM NaOH and were used within 4-6 hours of preparation. HbNO yield from reacting metHb with Angeli’s Salt was used to determine HNO yield. Angeli’s Salt final concentrations were corrected according to HNO yield. Spectral features of ferricatalase, nitrosyl ferricatalase, and ferrous nitrosyl catalase correspond to those demonstrated by Huang et al [27]. Excess SSNO− at 10 minutes was reacted with 2 μM ferricatalase and the spectrum of the final product was deconvoluted by fitting to the basis spectra. All reactions were carried out in anaerobic 100 mM Phosphate Buffer pH 7.4 in Argon or Nitrogen purged 1 cm quartz cuvettes.
The rate of HbNO formation from metHb and SSNO− was obtained by reacting metHb with 4-, 8-, 12-, and 16-fold excess SSNO− in 20 mM TRIS HCl pH 7.4. Reaction kinetics were recorded by absorption spectroscopy using 1 scan every 6 seconds for 8 minutes. The observed rate constant was obtained by fitting to a single exponential function and plotted v. [SSNO−]. The biomolecular rate constant for SSNO− formation was obtained by reacting Na2S and GSNO at fixed 2:1 ratios in PBS pH 7.4. Reaction kinetics were recorded by absorption spectroscopy at 1 scan every 6 seconds for 10 minutes. The initial rate of the decomposition of GSNO, ε338 = 980 M−1 cm−1, assumed to be concomitant with SSNO− formation, was found from the slope of the absorbance decay. From this, the bimolecular rate constant for SSNO− formation was obtained by dividing by the initial concentrations of GSNO and Na2S. The stability of 200 μM SSNO− was determined by absorption spectroscopy, λmax = 412 nm, using 1 scan every 5 minutes for 3 hours in N2 saturated, 5% oxygenated, and ambient air equilibrated PBS pH 7.4. The absorbance at 5 minutes was used as the maximal absorbance in order to plot the change in absorbance at 412 nm as a percent of maximal absorbance.
Results
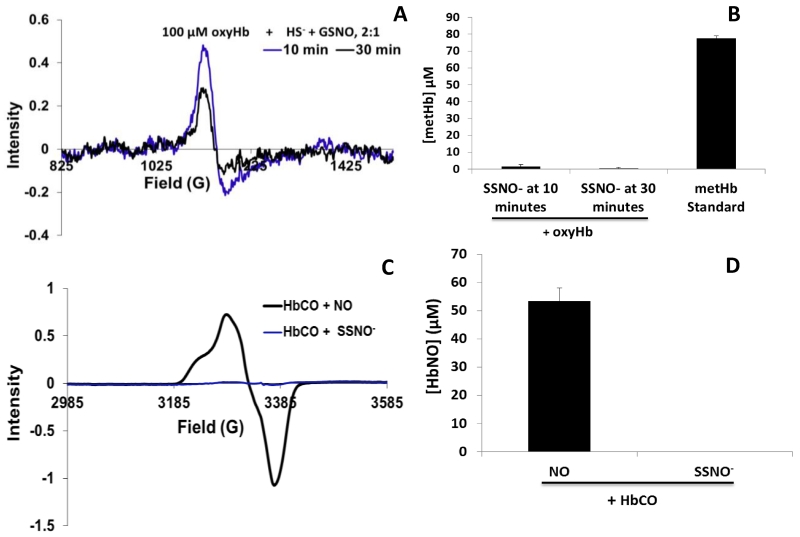
As it pertains to the generation of NO or its congeners, current data consisting of chemiluminescent NO detection, sGC activity, and cGMP formation indicate SSNO− to be a potent NO generator [28]. There is an inverse correlation between SSNO− decomposition and NO formation [20]. However, our data show that, in the presence of heme containing proteins under aerobic conditions, SSNO− appears to release only small amounts of NO (Fig. 1A). NO reacts with oxyHb in a 1:1 ratio to yield 1 metHb (Eq. 5) [13]. The intensity of the EPR signal of metHb yield from the reaction of 100 μM SSNO− mixture and 100 μM oxyHb is small (Fig. 1A). Fig. 1B shows metHb yields from reactions of SSNO− and oxyHb. In reactions of oxyHb with SSNO−, SSNO− mixtures were preincubated for 10 or 30 minutes and degassed for 30 minutes, prior to reacting with oxyHb. In reactions using SSNO− preincubated for 10 minutes, metHb yields were 1.3 ± 1.3 μM, after subtracting basal metHb in oxyHb. In reactions using SSNO− preincubated for 30 minutes, metHb yields were 0.5 ± 0.5 μM, after subtracting basal metHb in oxyHb (Fig. 1B). The decrease in metHb yield is not significant, p=0.41. A 100 μM metHb standard is also shown (Fig. 1B). As shown in Fig. 1C, reacting a SSNO− mixture with carboxyhemoglobin (HbCO) forms no HbNO although the use of MAHMANOnoate produces HbNO (Fig. 1C) with an average yield of 53.3 ± 4.8 μM (Fig. 1D). In cases where heme is ligated, here shown with oxygen or carbon monoxide, the absence of nitrosyl formation from a HbCO and SSNO− mixture and the minimal metHb yield from oxyHb and SSNO− suggests little-to-no NO is spontaneously released from SSNO− during the time periods studied.
Fig. 1. Diminished NO yield from SSNO− in the presence of ligated hemoglobin.
A EPR representative spectra of metHb yielded from the reaction of 100 μM SSNO− mixture preincubated for 10 (blue) or 30 (black) minutes, degassed, and then, reacted with 100 μM oxyHb for 10 minutes. Throughout this manuscript, “SSNO− at 10 (or 30) minutes” or “SSNO− preincubated for 10 (or 30) minutes” refers to Na2S and GSNO coincubated for 10 (or 30) minutes prior to using for any reactions with proteins. B Average metHb yields when 100 μM SSNO− mixture is reacted with 100 μM oxyHb. Yields were 1.3 ± 1.3 at 10 minutes and 0.5 ± 0.5 at 30 minutes (n = 3). Also shown is a 100 μM metHb standard. C EPR representative spectra of HbNO yield from reactions of MAHMANONOate (black) or SSNO− aged to 10 minutes (blue) with HbCO for 10 minutes D Average HbNO yields when 100 μM SSNO− mixture after 10 minutes of reacting or MAHMANOnoate (NO yield 100 μM) is reacted with 100 μM HbCO for 10 minutes (n=3). HbNO yields from 100 μM of HbCO and NO donor were 53.3 ± 4.8 μM.
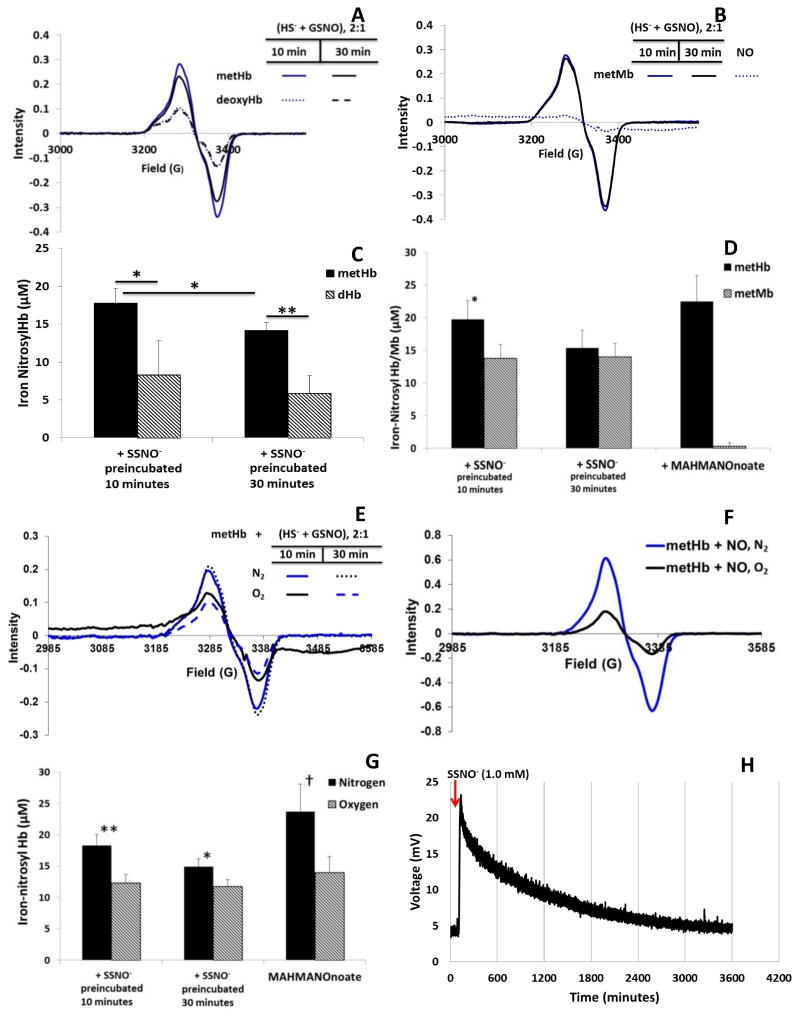
On the contrary, nitrosyl ferrous heme is obtained from the reaction of SSNO− and vacant ferriheme containing proteins under anaerobic conditions (Fig. 2). Representative EPR spectra demonstrate enhanced HbNO yield from reactions of deoxygenated metHb and SSNO− when compared to reactions of deoxyHb and SSNO− (Fig. 2A). Deoxygenated metMb and SSNO− also produces nitrosyl myoglobin (MbNO) with no significant changes when SSNO− is preincubated for 10 or 30 minutes prior to use, as shown by representative EPR spectra (Fig. 2B). Fig. 2C shows substantial average HbNO yields from reactions of deoxygentated hemoglobin and methemoglobin reacted with SSNO− preincubated for 10 and 30 minutes. The change in average HbNO yield from deoxygenated metHb reacted with SSNO− at 10 and 30 minutes is significantly different, p= 0.04. The HbNO yield from deoxygenated metHb and SSNO− is significantly different from deoxyHb and SSNO− at identical SSNO− preincubation times, p=0.026 at 10 minutes and p=0.005 at 30 minutes. The fact that so little or no NO formation is evident in reactions of SSNO− mixtures with oxygenated or carboxyHb (Fig. 1), yet substantial HbNO is made from deoxyHb and metHb (Fig. 2A,C), suggests that a vacant heme facilitates or is necessary for substantial NO liberation. Differences in HbNO yield, when SSNO− mixtures are combined with deoxyHb compared to when they are combined with metHb (Fig. 2A, 2C) suggests a mechanism that is due to either nitroxyl formation or enhanced reactivity of ferriheme with SSNO−. The reduction of metMb by NO is fairly slow, as the rate constant, kOH (see Eq 2), for Mb2+ formation is 8.8 ± 0.2 × 10−4 M−1 s−1 at pH 8.3 [9]. This rate constant,kOH, increases as pH increases; hence, at neutral pH, MbNO yield from reacting NO and MetMb should be very small (Fig. 2B, 2D). Indeed, at pH 6.5 Hoshino et al find that there is no reductive nitrosylation of metMb by NO, just reversible binding of NO to the ferriheme[9]. Average MbNO yields from metMb and SSNO−, shown in Fig. 2D, along with NO donor controls, are 13.8 ± 2.07 μM and 13.9 ± 2.08 μM at 10 and 30 minutes, respectively, and 0.3 ± 0.6 μM for the NO donor. The absence of MbNO formation from metMb and an NO donor (Fig. 2B, 2D) supports this notion that reductive nitrosylation of metMb does not occur to a measurable extent at pH 7.4. On the other hand, the formation MbNO when metMb is reacted with SSNO−, as shown in Fig. 2B and 2D, suggests that there is a direct reaction of metMb and SSNO−. The NO donor does, however, form substantial HbNO when reacted with metHb (Fig. 2D) and this is expected as ferric nitrosyl Hb reacts with hydroxyl ion and water (Eq. 3) so it is much more efficient in recductive nitrosylation at neutral pH than ferric nitrsoyl Mb which only reacts with the hydroxyl ion [9].
Fig. 2. Nitrosyl yield from unliganded hemes.
A Representative EPR spectra of HbNO yields from reactions of 100 μM deoxyHb (dHb) or 100 μM deoxygenated metHb and equimolar SSNO− mixture preincubated for 10 and 30 minutes. B Representative EPR spectra of MbNO yields from 100 μM of metMb and equimolar SSNO− and 100 μM of metMb with MAHMANOnoate yielding 100 μM NO. C Average HbNO yield from a 100 μM SSNO− mixture preincubated for 10 or 30 minutes, degassed, and reacted with 100 μM metHb in anaerobic buffer and 100 μM deoxyHb (n=3). Average nitrosyl yields from metHb and SSNO− at 10 and 30 minutes were 17.8 ± 1.9 μM and 14.2 ± 1.0 μM, p < 0.05. Average nitrosyl yields from deoxyHb and SSNO− at 10 and 30 minutes were 8.3 ± 4.5 μM and 5.9 ± 2.3 μM, p=0.27. Nitrosyl yields from deoxyHb + SSNO− at 10 and 30 minutes are significantly less than yields from metHb + SSNO− at the same preincubation times. *= p-value < 0.050 and **= p-value = 0.005. D Nitrosyl yield from 100 μM SSNO− mixture at 10 or 30 minutes, degassed, and reacted with 100 μM metHb (n=6) and 100 μM metMb (n=4) for 10 minutes (*= p-value < 0.005). MAHMANOnoate (yield 100 μM NO) reacted with 100 μM metHb and metMb for 10 minutes in deoxygenated buffer for 10 minutes showed significantly different nitrosyl yields. E Representative EPR spectra of HbNO yield from the reaction of 100 μM SSNO− pre-incubated for 10 minutes and 100 μM metHb in the absence (solid blue) and presence of (solid black) O2. Using the SSNO− incubated for 30 minutes, HbNO yield is shown in the absence (black dot) and presence (blue dash) of O2. F Representative EPR spectra of HbNO yield from 100 μM metHb and MAHMANOnoate (100 μM NO yield) reacted under aerobic and anaerobic conditions for 10 minutes. G Average HbNO yield from 10 minute SSNO− mixture (100 μM) and metHb (100 μM) in aerobic and anaerobic conditions(n=6, n=3, respectively). Average HbNO yield from 30 minute SSNO− mixture (100 μM) and metHb (100 μM) in aerobic and anaerobic conditions (n=6, n=3, respectively). Also shown is MAHMANOnoate (100 μM NO yield) and 100 μM metHb reacted for 10 minutes in aerobic and anaerobic conditions (n=3). At 10 and 30 minutes, nitrosyl yields were significantly different between atmospheric conditions. Likewise, nitrosyl yields were significantly different between atmospheric treatments of metHb and MAHMANOnoate (†= p-value = 0.05, *= p-value < 0.01, **= p-value = 0.001,). H Spontaneous release of NO from 1.0 mM SSNO− mixture stock. Injection was 5 μL into PBS pH 7.4.
The reaction of SSNO− with O2 may be an alternate explanation for the diminished metHb yields observed in Fig. 1. However, analysis of representative HbNO EPR spectra from SSNO− and metHb under aerobic conditions (Fig. 2E) suggests it is an unlikely explanation. Although Fig. 2E shows a smaller yield under aerobic conditions, this diminished yield is not likely due to a reaction of SSNO− with O2. Fig. 2F shows the HbNO formation from a mixture of metHb (100 μM) and NO donor MAHMANOnoate (NO yield of 100 μM) under aerobic and anaerobic conditions. The lower nitrosyl yield under aerobic conditions is likely due to NO dissociation followed by dioxygenation with an oxygenated heme to form metHb and nitrate. As summarized in Fig. 2G, the effect of oxygen on HbNO yield is as great when NO is administered through a donor or via SSNO−. Thus, the low metHb yield in Fig. 1A is unlikely to be due to an oxygen/SSNO− reaction.
In order to further characterize spontaneous NO yield from SSNO−, we observed NO liberation using a nitric oxide analyzer. A 5 uL injection of a 1.0 mM SSNO− stock solution, prepared and injected anaerobically, gave a broad peak that returned to baseline over the course of 1 hour (Fig. 2H). Calculating the area under the curve using a nitrite standard, the NO yield was 59.2 μM at 10 minutes. Assuming linearity, this NO yields suggest about 6 μM NO liberation at 10 minutes for 100 μM SSNO−, much less than the amount of nitrosyl species formed when SSNO− is reacted with vacant heme proteins. The data in Fig. 2H supports the notion that spontaneous NO release from SSNO− is much less than that produced from a reaction of SSNO− with a vacant heme.
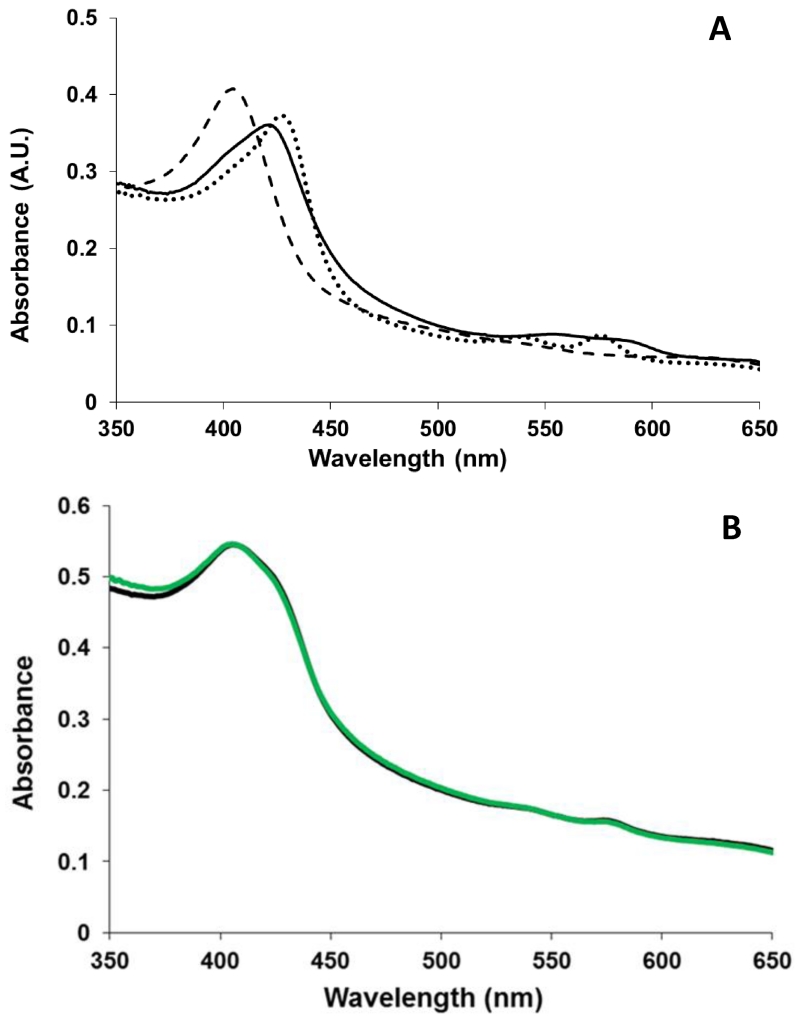
One alternative explanation for the increased yield using the ferric heme is that SSNO− produces HNO, which reacts with the ferric heme to form HbNO [12]. To explore this possibility, we studied the ferrous nitrosyl yield when SSNO− is reacted with ferricatalase. The formation of ferrous nitrosyl catalase from the reaction of ferricatalase and NO is very inefficient [27]. However, ferrous nitrosyl catalase is efficiently made when HNO reacts with ferricatalase (Fig. 3) [27]. With the addition of Angeli’s Salt (an HNO donor), the absorbance peak of ferricatalase at 405 nm shifts to 421 nm and a new band at 555-580 nm appears, indicating irreversible formation of ferrous nitrosyl-catalase (Fig. 3A) [27]. An example of the absorption spectrum obtained when excess SSNO− is added to catalase is shown in Fig. 3B. The final products from this reaction are ferricatalase and nitrosyl ferricatalase. The average of several trials yielded 42.0% ± 17.2% nitrosyl ferricatalase, 2.0% ± 2.8% nitrosyl ferrous catalase, and 56.0%, ± 14.4% ferricatalase, n = 3. Hence, diminished HbNO formation in the reaction of deoxyHb and SSNO− is likely not due to HNO formation. These data are in agreement with current literature that shows low HNO yield from SSNO− [28].
Fig. 3. Nitrosyl ferrous catalase formation in the presence of HNO.
A Representative UV-visible absorbance spectra of 2 μM ferricatalase (dashed line) with a Soret absorbance peak at 405, nitrosyl ferricatalase with absorbance peaks at 427, 540, and 576nm (dotted line) formed in the reaction of 2 μM ferricatalase and excess MAHMANOnoate or ProliNOnoate, and nitrosyl ferrous catalase with absorbance peaks at 421 and 555-580 nm (solid line) formed in the reaction of 2 μM ferricatalase and excess Angeli’s Salt. B Representative spectra of the final product of the reaction of 2μM ferricatalase and excess SSNO−(green) fit to the spectra of ferricatalase, nitrosyl ferricatalase, and ferrous nitrosyl catalase showing some nitrosyl ferricatalase (56.6%), but no nitrosyl ferrous catalase formation.
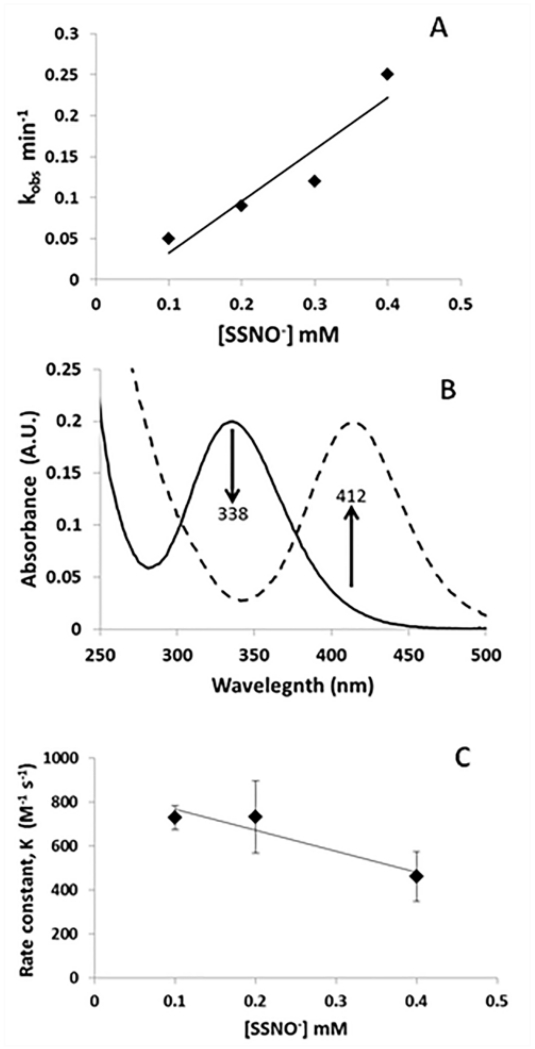
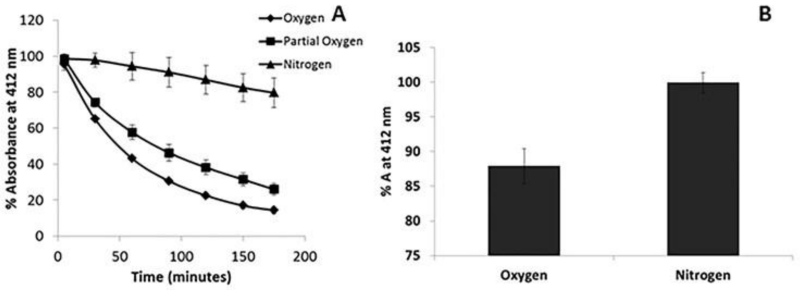
In order to assess potential significance of the reactions of SSNO− with ferric hemes, it is important to know the kinetics of the reaction. Thus, we measured the observed rate constant of the reaction of excess SSNO− with metHb (25 μM) (Fig. 4A). The observed rate constant appeared to be linear in SSNO− [20], suggesting a 2nd order reaction, with a bimolecular rate constant of 11 M−1 s−1. The formation of SSNO− from GSNO and H2S is evidenced by a decrease in absorption at 338 nm (due to GSNO) with concomitant increase in absorption at 412 nm (characteristic of SSNO−, Fig. 4B) By maintaining a constant ratio of H2S:GSNO of 2:1, we found the bimolecular rate constant for SSNO− formation from GSNO and H2S to be 640 ± 200 M−1 s−1 (Fig. 4C). The decrease at 0.4 mM SSNO− is not significant, p=0.063. SSNO− is less stable in oxygen (Fig. 5A). Fig. 5A shows the decomposition of 200 μM SSNO− under aerobic and anaerobic conditions over the course of 3 hours as well as that at an intermediate oxygen pressure (5%). Maximal absorbance was taken 5 minutes after the initiation of the reaction and the absorbance is plotted as a percent of maximal absorbance. Fig. 4D shows that after ten minutes, substantial SSNO− is present under aerobic or anaerobic conditions.
Fig. 4. Kinetics involving SSNO−.
A The reaction of excess SSNO− and metHb was observed using absorption spectroscopy and the observed rate constant plotted vs [SSNO−]. The bimolecular rate constant for HbNO formation from the reaction of 25 μM metHb and excess SSNO−, k = 11 M−1 s−1 and . R2=0.88. B Changes in the absorption spectra of GSNO when H2S is added. When 200 μM GSNO is reacted with 400 μM H2S in PBS pH 7.4, the absorbance at 338 nm decreases (solid line) as the absorbance at 412 nm increases (dashed line). For GSNO, λmax = 338 nm. For SSNO−, λmax = 412. C The reaction between GSNO and sulfide was monitored by absorption spectroscopy for fixed 2:1 ratios of sulfide (800, 400, 200 μM) to GSNO (400, 200, 100 μM). The initial rate of the reaction was used to calculated a second order rate constant (dividing by the concentrations of GSNO and sulfide). The bimolecular rate constant for SSNO− formation, k, is 640 ± 200 M−1 s−1; R2=0.89, n=3. Reactions performed anaerobically, in 20 mM TRIS buffer pH 7.4 at room temperature.
Fig. 5. Stability of SSNO−.
A Decomposition of 200 μM SSNO− in N2 saturated, ambient air equilibrated, and 5% oxygenated PBS pH 7.4 over the course of 3 hours. The absorbance at 5 minutes was used as the maximal absorbance. The absorbance is plotted as a percent of maximal absorbance. (n=3) B Average absorbance at 412 nm at 10 minutes for 200 μM SSNO− in ambient air equilibrated and N2 saturated conditions. The average % absorbance (% A) under O2 and N2 are 88.0 ± 2.5% and 100.0 ± 1.5%, respectively. (n=3)
Discussion
It has been suggested that SSNO− is the main, stable, long-term NO-donating reaction product of the RSNO/HS− reaction [20]. However, another group has suggested that SSNO− is too unstable to be of any physiological relevance [22]. We find that SSNO− is relatively stable in the absence of heme-proteins. Our data suggest that NO is not efficiently formed from SSNO− in the presence of ligated heme, but is formed when there is a vacant heme, indicating a direct reaction between the vacant heme and SSNO− is required to get NO from SSNO−. Furthermore, ferriheme is more efficient at acquiring NO from SSNO− than ferrous heme [22]. We also find that in the presence of heme-containing proteins, SSNO− does not yield HNO.
In previous work the compound SSNO− was shown to be unstable in the presence of light [22], decaying within a minute of exposure to 55 mW of light at 470 nm. It also was shown to decay completely within 10 minutes when exposed to water [22]. However, in our hands, SSNO− is very stable under anaerobic conditions and decays slowly under aerobic conditions (half life of about 40 minutes) in aqueous buffer (Fig. 4C,D). In aerobic conditions, SSNO− is stable enough that it could react with heme proteins as studied here, over 85% of SSNO− remains after ten minutes. It has been posited that during the incubation of SNAP and HS− (time < 10 minutes) enhanced oxygen consumption is due to the formation radical intermediates[20]. These data are consistent with those of the Feelisch group [20] Our data may differ from that suggesting SSNO− is unstable [22] due to differences in preparation and solution conditions. Importantly, we made SSNO− by simply mixing sulfide and nitrosothiols in PBS.
In the reactions of SSNO− with oxyHb and HbCO, we show the absence of the expected products of NO and heme, when heme is ligated (Fig. 1). NO dioxygenation is expected to yield metHb and nitrate. The absence of the metHb formation from the reaction of SSNO− and oxyHb demonstrates that SSNO− does not release substantial amounts of free NO in the time-frame and conditions of our experiments. Also, HNO and oxyHb produce NO upon reaction. Hence, the absence of metHb formation also indicates that HNO is not freely released from SSNO− in the time-frame of our experiments. Previous work has shown spontaneous release of NO from SSNO− using chemiluminescence detection [20], but the yields in those experiments are not apparent. Our data do not preclude some NO or HNO being spontaneously released from SSNO−, but we do find that the yields are small. Indeed, we demonstrate spontaneous release of NO in Fig. 2H, but the yield is much less than that when vacant hemes are present.
The formation of nitrosyl species from SSNO− and vacant heme proteins confirms the necessity of a vacant heme in order to acquire substantial yields of NO from SSNO− (Fig. 2). In the case of a ferric heme, one possible pathway that could lead to NO formation from metHb would be from a reaction of GSNO and a reduced heme [29]. However, in our hands, immediate reduction of metHb by HS− occurs only when HS− is in extreme excess, 12×, to heme (data not shown). Otherwise, at ratios up to 4:1 HS−:RSNO the sulfide-heme adduct is stable for over 15 hrs, and at ratios in between, reduction is delayed. Thus, at ratios of 2:1 HS−:RSNO, reduction of metHb followed by iron nitrosylation is unlikely.
The reaction of metMb and an NO donor demonstrates the ineffective reductive nitrosylation of metMb by NO (Fig. 2B). Hence, the formation of MbNO from metMb and SSNO− is likely not the result of free NO, but of a direct reaction with the heme. Reacting metHb with SSNO− under aerobic conditions did not eliminate HbNO formation, as might occur if SSNO− and O2 reacted directly (Fig. 2E). Rather, HbNO formation from metHb under oxygenated conditions and NO donor is mitigated due to dioxygenation secondary to NO release from HbNO.
It has been suggested that HNO is at least partially responsible for the vasorelaxant properties of RSNO/HS− reaction products[21]. However, the two main, stable intermediates of the RSNO/HS− reaction are lackluster HNO donors[28]. Our work with catalase (Fig. 3) supports a minimal role of HNO in potential SSNO− activity. HNO reacts with ferricatalase to form nitrosyl ferrous catalase [27]. NO does not efficiently reduce ferricatalase, but forms nitrosyl ferricatalase. Therefore, the formation of nitrosyl ferricatalase when SSNO− is added to ferricatalase indicates NO, but not HNO formation. However, we could not detect nitrosyl ferricatalase with EPR.
Although we cannot define mechanisms for the observed reactions in this work with certainty, we can propose a couple of potential pathways. MetHb could react with SSNO− to form ferrous nitrosyl Hb and elemental sulfur,
| Eq. 12 |
and the elemental sulfur could further react to form polysulfides. For the reaction with deoxyHb we suggest the simple pathway
| Eq. 13 |
where the decomposition of SSNO− into NO and HSS•, previously suggested to occur spontaneously [30], is facilitated by the vacant ferrous heme. An alternative mechanism would include oxidation of the ferrous heme, but we did not observe any MetHb formation in this reaction (data not shown). Further work is required to establish the mechanisms of these reactions.
Our kinetic data (Fig. 4) provide some information that can be useful in assessing potential physiological relevance of SSNO−. The rate constant we obtained for SSNO− formation was 640 M−1s−1. Based in the stoichiometry, we originally thought that the rate constant would be third order, so the rate would depend on [HS−]2[GSNO]. However, we found that the rate of the reaction depended more on just [HS−][GSNO] (Fig. 4B). Perhaps there is an initial, rate-limiting, reaction of one HS− with GSNO forming an intermediate species that quickly reacts with another HS−. The rate constant for SSNO− formation from GSNO compares favorably with other reactions of GSNO including transnitrosation to from S-nitrosohemoglobin (0.1 M−1s−1 [31]) and oxidation of deoxygenated ferrous Hb (<0.05 M−1s−1 [29]). Thus, concentrations of sulfide that are about 3 orders of magnitude below that of these (and probably similar) heme proteins can compete for GSNO to form SSNO−. Once formed, we found that SSNO− reacts with metHb in a reaction described by the rate constant of 11 M−1s−1. In conditions of the experiments performed here, SSNO− is very stable under anaerobic conditions. Under aerobic conditions, taking the lifetime of SSNO− to be 90 minutes (Fig. 4C), 17 μM or more excess ferric heme would compete successfully with spontaneous decay of the SSNO−. These rough calculations suggest that SSNO− and its reaction with ferric heme proteins may be of physiological relevance, but much more work is needed to see if this is the case. Of particular interest is the possibility that, like with Mb and Hb, SSNO− could react sGC to form a nitrosyl species and thereby activate the enzyme.
Highlights.
Nitrosopersulfide is a stable reaction product of sulfide and GSNO.
Spontaneous release of NO from nitrosopersulfide is minimal.
The presence of ligated heme proteins does not increase NO release.
Substantial NO is formed in the presence unliganded, heme proteins.
Ferric heme results in more NO release than ferrous heme.
Acknowledgements
This work was supported by NIH grant HL058091.
We thank Daniel Griffin and Julien-Fabrice Momo for help with performing experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brown GC. Reversible Binding and Inhibition of Catalase by Nitric Oxide. European Journal of Biochemistry. 1995;232(1):188–191. doi: 10.1111/j.1432-1033.1995.tb20798.x. [DOI] [PubMed] [Google Scholar]
- [2].Brunori M, Giuffrè A, Forte E, Mastronicola D, Barone MC, Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochimica et biophysica acta. 2004;1655(1-3):365–71. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- [3].Sarti P, Forte E, Mastronicola D, Giuffrè A, Arese M. Cytochrome c oxidase and nitric oxide in action: Molecular mechanisms and pathophysiological implications. Biochimica et Biophysica Acta - Bioenergetics. 2012;1817(4):610–619. doi: 10.1016/j.bbabio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- [4].Thomas DD, Liu ZP, Kantrow SP, Lancaster JR. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O-2. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang YH, Hogg N. S-nitrosothiols: cellular formation and transport. Free Radic. Biol. Med. 2005;38(7):831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- [6].Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin [agr] regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Straub AC, Zeigler AC, Isakson BE. The Myoendothelial Junction: Connections That Deliver the Message. Physiology. 2014;29(4):242–249. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Helms C, Kim-Shapiro DB. Hemoglobin-mediated nitric oxide signaling. Free Radic. Biol. Med. 2013;61:464–472. doi: 10.1016/j.freeradbiomed.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoshino M, Maeda M, Konishi R, Seki H, Ford PC. Studies on the Reaction Mechanism for Reductive Nitrosylation of Ferrihemoproteins in Buffer Solutions. Journal of the American Chemical Society. 1996;118(24):5702–5707. [Google Scholar]
- [10].Tejero J, Basu S, Helms C, Hogg N, King SB, Kim-Shapiro DB, Gladwin MT. Low NO concentration dependence of reductive nitrosylation reaction of hemoglobin. The Journal of biological chemistry. 2012;287(22):18262–74. doi: 10.1074/jbc.M111.298927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proceedings of the National Academy of Sciences. 2003;100(16):9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and reduction of hemoproteins by trioxodinitrate(II). The role of nitrosyl hydride and nitrite. Journal of the American Chemical Society. 1988;110(2):593–599. [Google Scholar]
- [13].Bellavia L, DuMond JF, Perlegas A, King SB, Kim-Shapiro DB. Nitroxyl accelerates the oxidation of oxyhemoglobin by nitrite. Nitric Oxide. 2013;31 doi: 10.1016/j.niox.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS. Mechanism of NO-Induced Oxidation of Myoglobin and Hemoglobin. Biochemistry. 1996;35(22):6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- [15].Herold S, Exner M, Nauser T. Kinetic and Mechanistic Studies of the NO•-Mediated Oxidation of Oxymyoglobin and Oxyhemoglobin. Biochemistry. 2001;40(11):3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- [16].Wang R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiological Reviews. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- [17].Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(10):1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- [18].Toohey JI. Sulphane sulphur in biological systems: a possible regulatory role. Biochemical Journal. 1989;264(3):625–632. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].King SB. Potential Biological Chemistry of Hydrogen Sulfide (H2S) with the Nitrogen Oxides. Free Radical Biology and Medicine. 2013:1–7. doi: 10.1016/j.freeradbiomed.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cortese-Krott M, Fernandez B, Santos J, Mergia E, Grman M, Nagy P, Kelm M, Butler A, Feelisch M. Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol. 2014 doi: 10.1016/j.redox.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berenyiova A, Grman M, Mijuskovic A, Stasko A, Misak A, Nagy P, Ondriasova E, Cacanyiova S, Brezova V, Feelisch M, Ondrias K. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide. 2014;46:123–130. doi: 10.1016/j.niox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- [22].Wedmann R, Zahl A, Shubina TE, Duerr M, Heinemann FW, Bugenhagen BEC, Burger P, Ivanovic-Burmazovic I, Filipovic MR. Does Perthionitrite (SSNO−) Account for Sustained Bioactivity of NO? A (Bio)chemical Characterization. Inorg Chem. 2015;54(19):9367–9380. doi: 10.1021/acs.inorgchem.5b00831. [DOI] [PubMed] [Google Scholar]
- [23].Hart TW. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of L-cysteine and glutathione. Tetrahedron Letters. 1985;26(16):2013–2016. [Google Scholar]
- [24].Kim YS, Kim SM, Han S. Nitric oxide converts catalase compounds II and III to ferricatalase. Bulletin of the Korean Chemical Society. 2002;23(11):1664–1666. [Google Scholar]
- [25].Basu S, Wang X, Gladwin MT, Kim-Shapiro DB. Chemiluminescent detection of S-nitrosated proteins: comparison of tri-iodide, copper/CO/cysteine, and modified copper/cysteine methods. Methods in enzymology. 2008;440:137–56. doi: 10.1016/S0076-6879(07)00808-7. [DOI] [PubMed] [Google Scholar]
- [26].Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. The Journal of Organic Chemistry. 1993;58(6):1472–1476. [Google Scholar]
- [27].Huang J, Kim-Shapiro DB, King SB. Catalase-mediated nitric oxide formation from hydroxyurea. Journal of Medicinal Chemistry. 2004;47(14):3495–3501. doi: 10.1021/jm030547z. [DOI] [PubMed] [Google Scholar]
- [28].Cortese-Krott M, Kuhnle G, Dyson A, Fernandez B, Grman M, DuMond J, Barrow M, McLeod G, Nakagawa H, Ondrias K, Nagy P, King S, Saavedra J, Keefer L, Singer M, Kelm M, Butler A, Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. PNAS. 2015;(34):E4651–60. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Spencer NY, Zeng H, Patel RP, Hogg N. Reaction of S-nitrosoglutathione with the heme group of deoxyhemoglobin. J. Biol. Chem. 2000;275(47):36562–36567. doi: 10.1074/jbc.M005347200. [DOI] [PubMed] [Google Scholar]
- [30].Cortese-Krott MM, Fernandez BO, Kelm M, Butler AR, Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide-Biol Ch. 2015;46:14–24. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- [31].Patel RP, Hogg N, Spencer NY, Kalyanaraman B, Matalon S, Darley-Usmar VM. Biochemical characterization of human S-nitrosohemoglobin - Effects on oxygen binding and transnitrosation. J. Biol. Chem. 1999;274(22):15487–15492. doi: 10.1074/jbc.274.22.15487. [DOI] [PubMed] [Google Scholar]