Abstract
Intact cognitive control or executive function has characteristic patterns in both behavior and functional neurocircuitry. Functional neuroimaging studies have shown that a frontal-cingulate-parietal-insular (i.e., “multiple demand”) network forms a common functional substrate undergirding successful adaptation to diverse cognitive processing demands. Separate work on intact neurocognitive performance implicates a higher order factor that largely explains performance across domains and may reflect trait cognitive control capacity. In the current review we highlight findings from respective psychiatric disorders (i.e., psychotic, bipolar and unipolar depressive, anxiety, and substance use disorders) suggesting that cognitive control perturbations amidst psychopathology are most pronounced within these common brain and behavioral indices of adaptive cognitive functioning and moreover, are evident across disorders (i.e., transdiagnostically). Specifically, within each of the disorder classes impairments are consistent in the multiple demand network across a wide range of cognitive tasks. While severity varies between disorders, broad as opposed to domain-specific impairments consistently emerge in neurocognitive performance. Accumulating findings have revealed that phenotypically diverse psychiatric disorders share a common factor or vulnerability to dysfunction that is in turn related to broad neurocognitive deficits. Furthermore, we have observed that regions of the multiple demand network, which overlap with the salience network (dorsal anterior cingulate and bilateral anterior insula) are characterized by reduced gray matter transdiagnostically and predict weaker neurocognitive performance. In summary, transdiagnostic (as opposed to disorder-specific) patterns of symptomatic distress and neurocognitive performance deficits, concurrent with parallel anomalies of brain structure and function may largely contribute to the real-world socio-occupational impairment common across disorders.
Cognitive control or executive functions refer to those processes integral to the effortful deployment of cognitive resources for flexible, adaptive responding to shifting contingencies. As such, cognitive control undergirds the self-regulation imperative for successful, dynamic accommodation to the demands of daily life (cf. Diamond, 2013 for review). Even among healthy individuals, cognitive control capacity predicts endeavors and success in educational performance and attainment (Duncan et al., 2007), occupational stability and advancement (Foxall, 2014), health promotion (McClernon et al., 2015; Riggs et al., 2010), as well as broader measures of overall quality of life (Davis et al., 2010). Given the influence on functional status among healthy individuals, cognitive control/executive functions are likely integral to the development, resistance to, maintenance, and remediation of psychopathology. That is, as phasic (or prolonged) distress manifests in the context of mental health or illness, cognitive control neurocircuits are likely recruited in the service of symptom regulation. In fact, meta-analysis of functional neuroimaging studies of adaptive emotion regulation demonstrate the recruitment of neural networks characteristic of cognitive control—a frontoparietal network containing the dorsolateral prefrontal and posterior parietal cortices, and a cingulo-opercular network containing the dorsal anterior cingulate cortex (dACC), anterior insula, and the anterior prefrontal cortex (Kohn et al., 2014).
The foundation of intact cognitive control has characteristic patterns in both behavior and functional neurocircuitry. As such, cognitive control perturbations amidst psychopathology are potentially most pronounced within indices of these processes common to adaptive functioning and moreover, evident across disorders (i.e., transdiagnostically). In this review we first discuss behavioral evidence of cognitive dyscontrol transdiagnostically, focusing on psychotic, bipolar and unipolar depressive, anxiety, and substance use disorders. Second, to contextualize behavioral correlates of cognitive control anomalies in mental illness relative to underlying structure, we discuss our recent findings from a meta-analysis (Goodkind et al., 2015) demonstrating transdiagnostic gray matter reductions in a dorsal cingulate-anterior insula-based network. We also highlight the demonstrated functional relevance of this network in terms of impaired cognitive control performance. Next, we focus on the neurocircuitry implicated across psychotic, bipolar and unipolar depressive, anxiety, and substance use disorders in functional neuroimaging studies of cognitive dyscontrol. Finally, we briefly discuss the implications for future research and the potential to leverage cognitive control and its neurocircuitry for innovating powerful and broadly applicable transdiagnostic interventions to ameliorate distress and improve daily real-world functioning.
Clues to Core Cognitive Control Dysfunction Common Across Psychiatric Disorders
A Common Underlying Cognitive Control Factor: Behavior
Latent variable analysis of performance on a wide array of neuropsychological tasks has shown that intact cognition has a characteristic pattern of interrelated executive functions throughout the lifespan from childhood (Lehto et al., 2003) through middle (Miyake et al., 2000) and older adulthood (Adrover-Roig et al., 2012). For example, Miyake and colleagues (2000, 2012) have demonstrated that updating (i.e., monitoring and refreshing working memory store), inhibition (resisting prepotent responses), and shifting (switching between mental sets) largely explain cognitive processes. Alternatively, Alvaraz and Emory (2006) have highlighted the synergy of working memory, inhibition, and selective attention. The fact that diverse functions show coherence suggests that these heterogeneous processes may work in tandem to promote cognitive wellbeing, but may also share a vulnerability to dysfunction.
In fact, latent variable analysis of task batteries spanning multiple domains of cognitive control suggest that hallmark executive functions are explained not only as subprocesses such as updating, inhibition, and shifting, but also an underlying common factor reflecting general cognitive control capacity (Miyake & Friedman, 2012). The extent of impairment in this common factor has yet to be examined in most psychiatric disorders. Historically, hallmark symptoms of individual disorders have prompted hypotheses about domain-specific impairments in neuropsychological profiles per disorder (e.g., poor resistance to interference in PTSD due to intrusions). As such, investigations on respective disorders have typically focused on one or two exemplar tasks of a given domain, precluding latent variable analysis to discern contributions of a common cognitive control factor. However, taken together the extant literature on neuropsychological performance shows broad (i.e., domain non-specific) rather than distinct performance impairments in studies of individual psychiatric disorders.
Evidence of broad impairments for respective disorders and classes of related disorders, albeit with variations in severity, was clearly demonstrated by Snyder and colleagues (2015). The authors summarized the effect sizes of meta-analyses of cognitive control/executive functions by individual disorder. For consistency with prevailing latent variable models, results for individual tasks were aggregated into domains of shifting, inhibition, updating, and working memory manipulation and maintenance. Measures of planning and verbal fluency, which typically recruit multiple executive functions and thus do not clearly load one of the other factors, were also summarized across studies as they have been examined frequently in clinical samples. Schizophrenia showed the most pronounced and consistently cross-domain deficits, aligned with the severe functional impairment characteristic of the disorder (Harvey & Strassnig, 2012). Specifically, aggregating across eight meta-analyses, schizophrenia demonstrated impairments with large effect sizes on all measures except the relatively less demanding process of working memory maintenance, for which a medium effect sized impairment emerged. Slightly less severe, but nonetheless consistent cross-domain deficits were observed in the average of ten meta-analyses of bipolar disorder. Notably, eight of the ten meta-analyses examined euthymic bipolar disorder, suggesting that cognitive control deficits are present regardless of mood state but are likely more pronounced during acute depression or mania. Unipolar depression showed the same pattern but marked by medium effect sized deficits across all domains with the exception of a lesser impairment on working memory maintenance, a pattern that was similar to, but less severe than in schizophrenia. Aggregating across three meta-analyses of obsessive-compulsive disorder (OCD) and one of posttraumatic stress disorder (PTSD) showed medium to small effect sizes across all domains. Neurocognitive functioning in other (DSM-IV-defined) anxiety disorders (i.e., specific phobia, social anxiety, generalized anxiety, and panic disorders) have been the subject of few published investigations, possibly reflecting the file drawer problem (i.e., hurdles to publishing null results) or a presumption on the part of investigators that cognitive control is uninterrupted in these presentations and thus not examined. Finally, multiple substance use disorders demonstrated deficits foremost in inhibition, but also shifting and working memory. Notably, Stavro and colleagues (2013) revealed that deficits were typically moderate up to one year of abstinence, and lessened for samples abstinent for at least one year. Despite the paucity of work directly comparing different disorders, taken together these findings have prompted a growing appreciation of the likelihood of shared deficits in cognitive control capacity across a wide range of Axis I disorders (e.g., Cáceda et al., 2014; Cole, Repovs, & Anticevic, 2014; Etkin et al., 2013; Goschke, 2014; Snyder et al., 2015).
A Common Underlying Psychopathology Factor: Symptoms
A general liability for cognitive dyscontrol, which cuts across diagnostic boundaries may be related to transdiagnostic vulnerability to mental illness. The historic neo-Kraeplinian approach of the Diagnostic and Statistical Manual of Mental Disorders ((e.g., DSM-III, APA, 1980; DSM-5, APA, 2013) and the corresponding International Classification of Diseases of the World Health Organization (ICD-9, WHO, 1977 ICD-10, WHO, 2007) conceptualizes disorders as discontinuous entities with presumably distinct etiologies and biomarkers. However, burgeoning evidence from large-scale phenotypic studies has robustly demonstrated common liabilities across disorders (e.g., Krueger, 1999). For example, examination of disorder patterns in epidemiological samples has revealed a latent internalizing dimension, typically expressed as anxiety or unipolar depressive disorders. A latent externalizing dimension is manifest in substance use and conduct disorders while psychotic disorders load on a third independent thought disorder/psychosis dimension (Kotov et al., 2011; Markon, 2010; Wright et al., 2013). Bipolar disorders and symptoms, likely owing to the relative prominence of psychotic, depressive, and mania features in different samples have at times loaded on the internalizing dimension (Wright et al., 2013) and at others on the psychosis dimension (Kotov et al., 2011). Importantly, recent extensions of this work have shown that these dimensions are, in turn, strongly related to an underlying general psychopathology dimension (Carragher et al., 2015; Lahey et al., 2012). Adult twin studies have revealed a likely genetic basis to these higher- and lower-order dimensions (Kendler et al., 2003; Kendler et al., 2011). Furthermore, child and adolescent models have suggested general psychopathology is largely a function of genetic vulnerability, whereas disorder-specific features are more so a function of non-shared environmental influences (Rhee et al., 2015). Notably, the general psychopathology factor robustly accounts for lifespan functional impairment, family history of mental illness, and prospective psychopathology (Caspi et al., 2014; Kim & Eaton, 2015) above and beyond current symptom-based predictions. It is also a more robust predictor of disorder nonspecific factors such as childhood maltreatment, which likely catalyze disorder expression.
Of particular relevance to a potential transdiagnostic liability to cognitive dyscontrol, early childhood and adult neurocognitive functioning is far more strongly related to the underlying general psychopathology factor than the internalizing, externalizing, or thought disorder dimensions (Caspi et al., 2014). More specifically, higher loadings on the general psychopathology factor predict worse performance on neuropsychological tasks of recall, recognition, and working memory, sustained attention and vigilance, and motor planning. General psychopathology also predicts limited early academic achievement as well as lifespan IQ. Finally, at the extreme of this general psychopathology factor, neurocognitive impairments are concurrent with microvascular brain abnormalities, neurological soft signs, receptive language impairment, and IQ deficits (Caspi et al., 2014).
Common cognitive control liability appears distinct from rather than subsumed by the transdiagnostic vulnerability to psychopathology. That is, cognitive control deficits are not clearly a proxy for, cause, or consequence of psychopathology. For example, neurocognitive functioning has meaningfully predicted longitudinal socio-occupational functional outcomes across disorders above and beyond symptom-based predictions (Gyurak et al., 2015; Miller et al., 2015; Tabarés-Seisdedos et al., 2008). In cases of severely impairing symptomatology in acute episodes of late onset schizophrenia, neurocognitive function is preserved in relation to early onset schizophrenia (Rajji et al., 2009). Further, impairments are evident even prior to the prodromal phase of psychosis (Bora & Murray, 2014). Relatedly, poor cognitive control is not only a consequence of substance abuse, but also an antecedent (e.g., Boelema et al., 2016; Peeters et al., 2014). In the case of major depression, symptom remission following antidepressant treatment has shown no accompanying improvement in neurocognitive deficits spanning multiple domains (Shilyansky et al., 2016). Rather than relating to symptom severity or prior antidepressant trial failures, neurocognitive deficits were more pronounced the more chronic the depression. Furthermore, neurocognitive deficits are more pronounced in unaffected relatives of probands than the general population, foremost for schizophrenia (Sitskoorn et al., 2004; Snitz et al., 2006) but also bipolar disorder (Bora et al., 2009; Ivleva et al., 2012; Kim et al., 2015) and substance use disorder (Cservenka, 2016; Gierskei et al., 2013). In essence, acute symptom expression does not correspond clearly to manifested neurocognitive deficits. Instead, cognitive control deficits may be a risk factor, intermediate phenotype, or endophenotype of latent psychopathology vulnerability (e.g., Buckholtz & Meyer-Lindenberg, 2012; Kozak & Cuthbert, 2015; Nolen-Hoeksema & Watkins, 2011). Towards this end, individual differences in general cognitive control capability are almost completely (i.e., 99%) heritable, rendering this factor among the most heritable of psychological traits, exceeding even IQ (Friedman et al., 2008).
A Common Underlying Structure: Gray Matter Volume
The preponderance of evidence for common, largely heritable liabilities to experiencing general psychopathology as well as cognitive dyscontrol prompts the question of whether there are accompanying structural anomalies seated within the neurocircuitry subserving cognitive control. To explore the possibility of such a common substrate, we recently completed a meta-analysis of volumetric differences across Axis I patient and matched control groups (Goodkind et al., 2015). Structural neuroimaging meta-analyses have been reported for a number of psychiatric disorders (e.g., Hamilton et al., 2010; Rotge et al., 2010; Selvaraj et al., 2012). However, these have either focused on single disorders or compared two phenotypically related disorders such as unipolar and bipolar depressive disorders (e.g., Kempton et al., 2011), with an eye towards differences rather than similarities across diagnoses. As such, interpretation of findings has often reflected disorder-specific neural circuit models. Likewise other meta-analyses have not provided spatially unbiased information across the brain (e.g. manual volumetric tracing studies). In meta-analytically summarizing a more complete spectrum of psychopathology across the entire brain, we hypothesized that transdiagnostic commonalities might emerge.
In brief, studies were selected if they (1) used voxel-based morphometry (VBM) to analyze gray matter in patients with a psychiatric diagnosis, (2) included a comparison between these patients and matched healthy control participants, (3) performed a whole-brain analysis, and (4) reported coordinates in a defined stereotaxic space (e.g., Talaraich space or Montreal Neurological Institute space). A psychotic disorders category comprised studies of schizophrenia, schizoaffective, schizophreniform, and delusional disorders. A non-psychotic disorders category comprised studies of bipolar depressive disorders, unipolar depressive disorders (major depression, dysthymia), anxiety disorders, and substance use disorders (mixed substance abuse and/or dependence disorders). Studies were selected to capture lifespan patterns and thus included participants ranging from childhood through older adulthood. Included in the meta-analysis were peak voxel coordinates from published studies that compared a psychiatric group to healthy participants, which thus represented indicators of regional gray matter volume differences associated with that diagnosis. The final sample included nearly 200 peer-reviewed papers and 16,000 patients and matched healthy controls.
The revised activation likelihood estimation (ALE) algorithm was implemented to identify consistent patterns of gray matter differences between patients and controls across studies (Eickhoff et al., 2009; 2012). This algorithm aims to identify areas showing a convergence of reported coordinates across experiments higher than expected under a random spatial association.
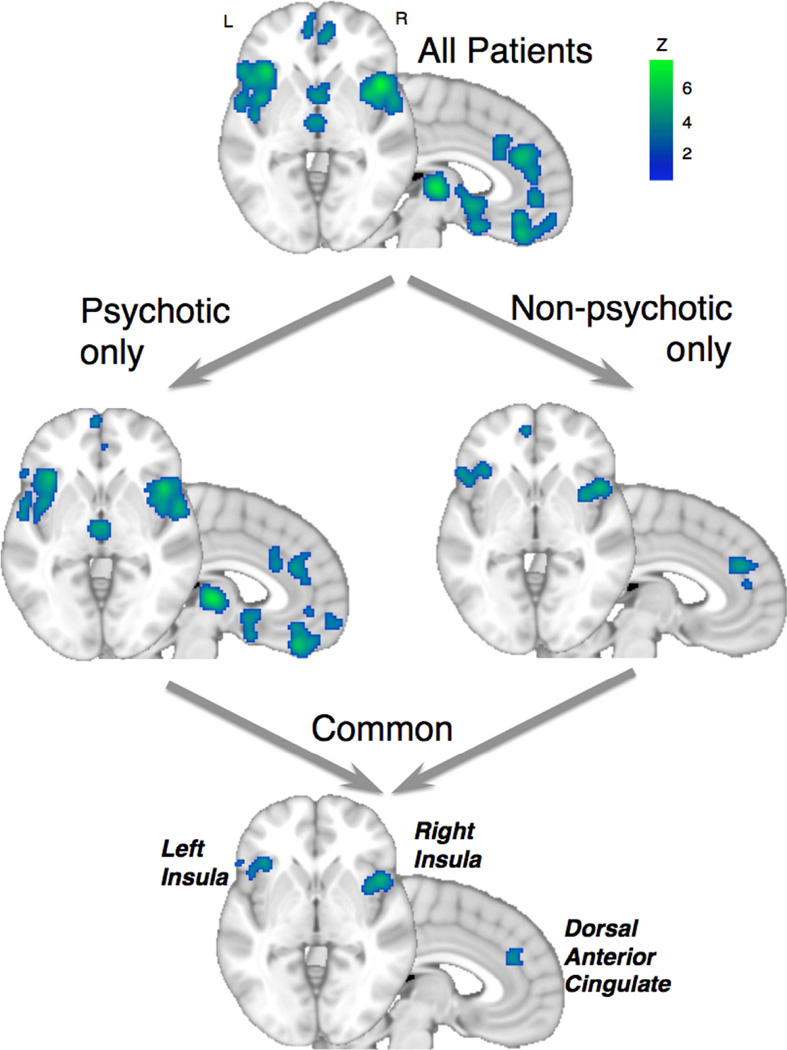
Across all studies, the clear majority (85%) of peak voxels represented decreased gray matter in patients compared with controls. Consistent gray matter decreases in patients were found in the bilateral anterior insula, dorsal anterior cingulate (dACC) and dorsomedial prefrontal cortex (dmPFC), ventromedial PFC (vmPFC), thalamus, amygdala, hippocampus, superior temporal gyrus and parietal operculum. By contrast, gray matter increases in patients were found exclusively in the striatum. To hone the set of regions to those common to both psychotic and non-psychotic disorders, we performed a conjunction which revealed a more circumscribed, shared pattern of gray matter loss in the bilateral anterior insula and dACC (Figure 1). Furthermore, presence of gray matter loss in one region predicted gray matter loss in the other regions (Robinson et al., 2010) suggesting that this gray matter loss might occur in a coordinated fashion across a structural network inclusive of these regions. By contrast, the gray matter increases in the striatum of patients were evident only in the psychotic disorders group.
Figure 1.
Shared patterns of decreased gray matter from the voxel-based morphometry (VBM) meta-analysis. Patient versus healthy participant comparisons (a) for studies pooled across all diagnoses, (b) separately by psychotic or non-psychotic diagnosis studies, and (c) from a conjunction across the psychotic and non-psychotic diagnostic groups. Common gray matter loss is evident across diagnoses in the anterior insula and dorsal anterior cingulate (dACC). Adapted from Goodkind et al. (2015).
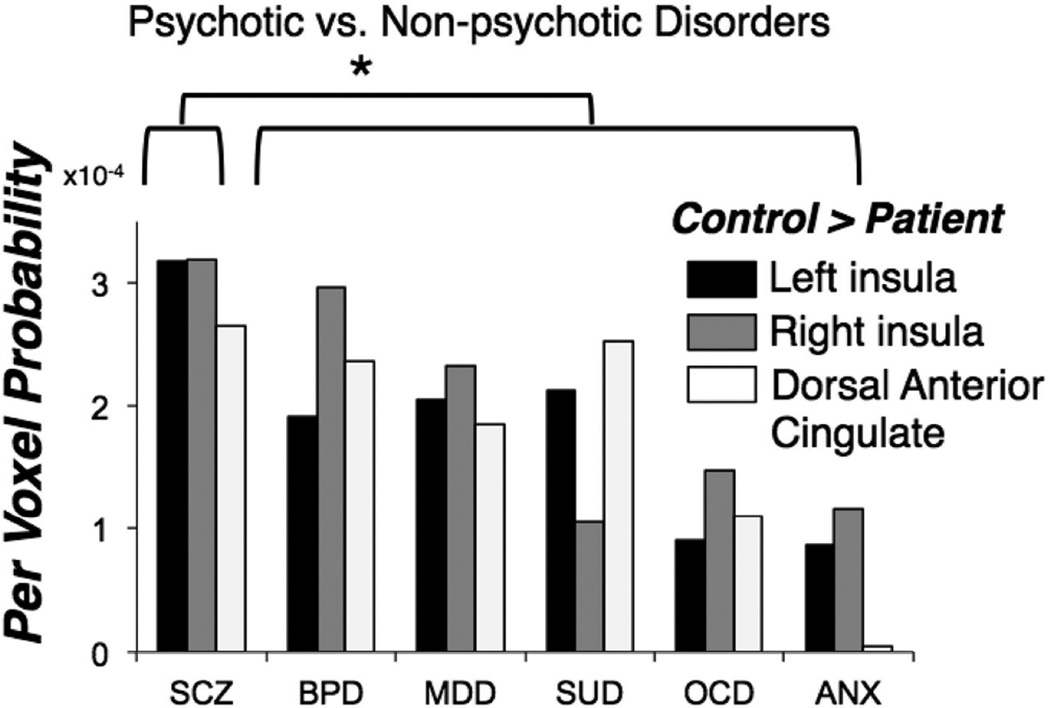
As the psychotic versus non-psychotic distinction does not account for more refined phenotypic differences, a follow-up analysis of the extracted per voxel probability of observing gray matter decreases in each of the three common regions (right and left insula, dACC) revealed similar magnitude effects across all non-psychotic diagnoses, as shown in Figure 2. Schizophrenia spectrum disorders showed significantly greater reductions of gray matter volume in these regions relative to the non-psychotic disorders as a whole. Notable given the dramatic differences in psychotropic use patterns between psychotic and non-psychotic disorders, these effects were not accounted for by current medication usage. In summary, these results suggest that anterior insula and dACC gray matter loss represent a transdiagnostic neural abnormality, most pronounced in disorders prone to psychosis.
Figure 2.
Extracted per voxel probabilities of decreased gray matter in the VBM meta-analysis, separated by diagnosis and common gray matter loss region (left and right anterior insula, dACC). Values represent the probability of identifying a gray matter abnormality for an average voxel within the region of interest, derived from the modeled activation maps. SCZ=schizophrenia, BPD=bipolar disorder, MDD=major depressive disorder/dysthymia, SUD=substance use disorder, OCD=obsessive-compulsive disorder, ANX=anxiety disorders. Adapted from Goodkind et al. (2015).
To assess whether cognitive control capacity may be determined, in part, by the structural integrity of this anterior insula/dorsal anterior cingulate–based network, we examined relationships between gray matter volume in these nodes in relation to neuropsychological performance. We utilized a dataset of 163 healthy adults from the BRAINnet database who had completed a computerized neurocognitive assessment battery that covered a broad range of basic and higher-level cognitive functions. As such, this served as a conservative test of whether reduced gray matter volume would predict behavioral variability/weaknesses even in the context of healthy cognitive functioning and mental wellbeing.
To reduce the array of tasks representing overlapping cognitive domains, we conducted principal components analysis, which yielded three principal components. Consistent with identification of an overriding cognitive control factor in latent variable analyses of cognitive performance data (Miyake et al., 2000), the principal component accounting for the most variance among test performance reflected general cognitive control (task switching, interference, inhibition, working memory, conflict). The second component reflected sustained attention (continuous performance task) while the third component reflected cognitive processing speed (choice reaction time, finger tapping).
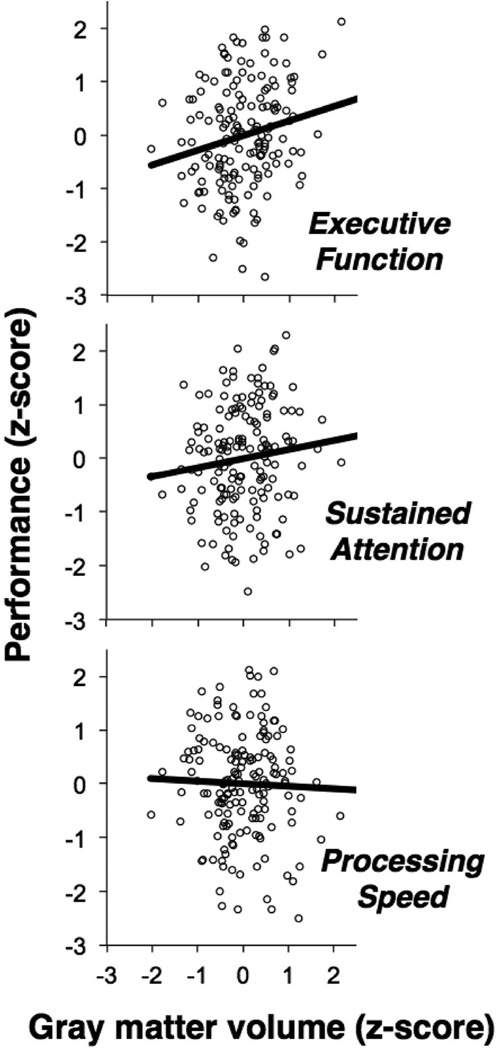
We then regressed individual behavioral performance on each of these components with subject-specific gray matter volumes, measured using whole brain volume-corrected VBM. Lower gray matter across the three common gray matter loss regions predicted worse performance in terms of general cognitive control with a similar-direction trend for sustained attention, but no effect on processing speed (Figure 3). Gray matter volume in primary visual cortex, a control region, was unrelated to any of the performance indices. These results suggest that lower gray matter in the common gray matter loss regions (i.e. more “patient-like” regional volume) is associated with worse higher-level cognitive control, but not with more rudimentary and less specific aspects of task performance.
Figure 3.
Among 163 healthy participants from the BRAINnet Foundation Database database who had completed a computerized neurocognitive assessment battery covering a broad range of basic and higher-level cognitive functions, lower gray matter across the three common gray matter loss regions (left and right anterior insula, dACC) predicted worse performance in terms of general executive function, with a similar trend for sustained attention, but no effect on general cognitive and performance speed.
In summary, in a VBM meta-analysis of nearly 16,000 patient and matched control participants, we identified a transdiagnostic pattern of gray matter loss in bilateral anterior insula and dACC. Further, lower gray matter volume in this network was systematically related to behavioral decrements on neuropsychological tasks— foremost for higher order cognitive control. Taken together, these findings suggest a coordinated structural perturbation of this cingulo-opercular network across disorders, likely contributing to transdiagnostic cognitive control deficits.
A Common Underlying Neurocircuit Disruption: Task Activation
Unknown from our VBM meta-analysis is whether these nodes of common gray matter loss show parallel functional disruptions. A corresponding meta-analysis of cognitive control tasks is clearly warranted. In the interim, we examine the extant disorder-specific literature for evidence of abnormal activation in patients during cognitive control tasks. In short, we consider whether findings augur a common functional substrate of cognitive dyscontrol, which overlaps with the disrupted structural integrity of the anterior insula/dorsal anterior cingulate–based network.
Cingulo-opercular, Fronto-parietal & Multiple Demand Networks & Cognition
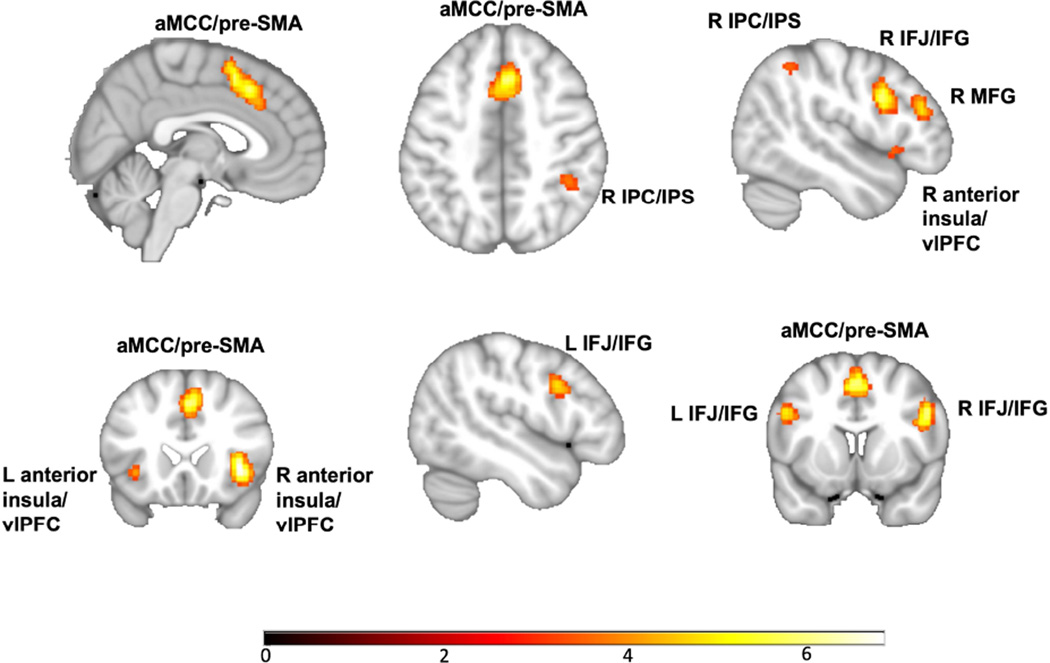
The insula and anterior cingulate (i.e., cingulo-opercular or “salience network”; Seeley et al., 2007) feature prominently in intact (Hayes et al., 2014) as well as disordered emotional responding (Etkin & Wager, 2007; Satterthwaite et al., 2015). Increasingly, however, anterior cingulate and insular cortices are recognized as more broadly deployed—assigning salience for the coordination of dynamic interaction of large-scale neural networks in response to contextual demands (Jiang et al., 2015; Medford & Critchley, 2010; Menon & Uddin, 2010; (Jiang et al., 2015; Medford & Critchley, 2010; Menon & Uddin, 2010; Power et al., 2013; Sridharan et al., 2008). Specific to the critical role of the cingulo-opercular network in cognitive control is its coordination with the frontal-parietal network to function as a superordinate or “multiple demand” cognitive control/processing network (Duncan, 2010; Duncan & Owen 2000; Müller et al., 2015). That is, in conjunction with the dACC (and the posteriorly adjacent medial frontal cortex) and bilateral anterior insula, a network of regions referred to as the fronto-parietal or central executive network (Dosenbach et al., 2008) are reliably recruited as part of the multiple demand network: the dorsolateral prefrontal cortex (dlPFC)/middle frontal gyrus, inferior frontal junction/gyrus, ventrolateral prefrontal cortex (vlPFC), inferior parietal cortex extending into intraparietal sulcus, and mid-cingulate cortex extending into pre-supplementary motor area (pre-SMA). An empirically derived multiple demand network is represented in figure 4. Müller & colleagues (2015) performed a conjunction across results from three large-scale meta-analyses of cognitive tasks in healthy participants, retrieved through ANIMA, a data sharing initiative (http://anima.fz-juelich.de)). Identifying regions reliably recruited across diverse cognitive domains thus yielded the multiple demand network.
Figure 4.
The multiple demand network as empirically derived by Müller & colleagues (2015) from conjunction of results across three large-scale meta-analyses of diverse cognitive tasks in healthy participants (retrieved through ANIMA (http://anima.fz-juelich.de)). aMCC/pre-SMA=anterior mid-cingulate cortex/ pre-supplementary motor area; IPC/IPS=intraparietal cortex/sulcus; MFG=middle frontal gyrus; IFJ/IFG=inferior frontal junction gyrus; vlPFC=ventrolateral prefrontal cortex.
Similar to the latent or common cognitive control factor observed in behavioral measures of cognitive processing, the activity of this network suggests a ‘common core’ recruited across diverse cognitive challenges and domains (Duncan, 2013). For example, the multiple demand network is activated in tasks ranging from performance monitoring, to focusing attention, to storing information in working memory, to inhibiting irrelevant information and selecting competing task-relevant responses (e.g., Fedorenko et al., 2013; Müller et al., 2015). Furthermore, the multiple demand network is sensitive to cognitive demand, showing activation proportional to increasing difficulty (Fedorenko et al., 2013). Importantly, the canonical pattern is observed not only at the single task level, but also the individual level, suggesting that the patterns observed at group (Duncan & Owen, 2000) and meta-analytic levels (Müller et al., 2015; Niendam et al., 2012) are not artifacts of aggregating findings.
Although findings have been mixed in terms of which multiple demand network components show dissociable sensitivity to phasic (i.e., moment-to-moment) versus sustained (i.e., set maintenance) demands (e.g., Braver, 2012; Cole & Schneider, 2007, Crittenden et al., 2016; Dosenbach et al., 2006; 2008; Sadaghiani & D’Esposito, 2012; Wilk et al., 2012), accumulating findings suggest that the broad multiple demand network can be differentiated into subnetworks—a cingulo-opercular (CO) network and a frontoparietal (FP) network (Dosenbach et al., 2008) whose close coupling provides the foundation for a broad array of cognitive processes. Analyses of resting-state (Dosenbach et al., 2007) as well as cognitive task-based fMRI (Crittenden et al., 2016) have shown that while coordination is high across the broad network, nodes within the CO and FP subnetworks show stronger connectivity within than between subnetworks. Additionally lesions to nodes of one of the subnetworks hampers functional connectivity within that network, while integrity of the other network remains relatively preserved (Nomura et al., 2012). Recent work on causal interactions between these subnetworks during cognitive tasks (Cai et al., 2015; Chen et al., 2015; Jiang et al., 2015) suggests the anterior insula amplifies salience detection in the anterior and mid-cingulate in a manner proportional to both cognitive demand and individual capacity, and, in turn prompts activation of the broader frontal-parietal network, particularly lateral frontal regions (dlPFC/IFJ) and parietal cortex. Furthermore, Cieslik and colleagues (2013) performed a coactivation-based parcellation of the left lateral frontal cortex across cognitive paradigms. Two functional subregions emerged with the anterior region preferentially connected to the CO subnetwork-based anterior cingulate and the posterior region with the FP subnetwork-based intraparietal sulci. In summary, the cingulo-opercular and frontal-parietal subnetworks functionally integrate to subserve a wide array of cognitive functions as a coordinated, broader multiple demand network.
Mental Illness & the Multiple Demand Network
Next we consider the possibility that across disorders there is evidence of functional impairment in the multiple demand network, including dACC and anterior insula. Relative to other Axis I disorders and consistent with the severity of neurocognitive and functional impairment, schizophrenia has received the most intensive examination of cognitive control/executive function neurocircuitry, including a handful of whole-brain meta-analyses. In the most recent, Minzenberg and colleagues (2009) analyzed functional magnetic resonance imaging and positron emission tomography findings from 41 studies of a broad array of tasks including delayed match-to-sample, delayed response, go/no-go, mental arithmetic, N-back, oddball, sequence recall, Stroop, Wisconsin Card Sort, and word generation tasks. Across tasks, patients showed pronounced hypoactivation relative to control participants in bilateral dlPFC, right vlPFC, visual, and right premotor cortices as well as inferior parietal lobule and medial frontal gyrus extending to dACC. Regarding subcortical regions, patient hypoactivation also occurred in the putamen and dorsomedial thalamus. In contrast, patient hyperactivation was observed in a more posterior region of the dACC extending to supplementary motor area. Increased mid-cingulate/pre-SMA among patients relative to control participants may reflect a compensatory process for maintaining intact performance amidst deficiencies in other multiple demand network nodes (i.e., adaptively balancing proactive versus reactive control; Jiang et al., 2015).
Very similar patterns of impairment seated within and extending beyond the multiple-demand network emerge in bipolar disorder, though with less pervasive disruption compared to schizophrenia. Wegbreit and colleagues (2014) implemented a whole-brain meta-analysis on 44 cognitive processing studies spanning numerous domains and tasks (e.g., stroop, n-back, go/no-go, paced motor task, sentence completion, delayed match-to-sample, stop signal). Patient hypoactivation emerged in bilateral inferior frontal gyrus/vlPFC, superior frontal gyrus, visual cortex, putamen, precuneus, lingual gyrus, and inferior parietal lobule. Patient hyperactivation was evident in pregenual ACC and mid-cingulate cortex. Underscoring the domain generality of these patterns, similar multiple demand network hypoactivation has been observed in meta-analyses of bipolar disorder circumscribed to episodic memory (Ragland et al., 2009) and response inhibition (Hajek et al., 2013).
Major depressive disorder also shows aberrant recruitment of multiple demand network nodes, though even less extensive than bipolar disorder and consistent with the moderate effect sized neuropsychological deficits. In a whole-brain meta-analysis of 19 cognitive control tasks similar in domain and task breadth to the schizophrenia and bipolar studies reviewed previously, patients showed hypoactivation in right inferior frontal gyrus and left thalamus. Patient hyperactivation emerged in medial and bilateral middle frontal gyrus, putamen, and left thalamus (Palmer et al., 2015). A separate meta-analysis of working memory tasks in major depression, demonstrated lateral and medial prefrontal hyperactivation was attributable to patient samples showing worse performance and thus possibly reflects compensatory efforts (Wang et al., 2015). In sum, across psychotic, bipolar, and unipolar depression disorders, the extent of network deficits are on par with observed neuropsychological impairments.
Both anxiety disorders and substance use disorders have less often been the subject of functional neuroimaging investigation of cognitive control and meta-analytical summaries are as yet unavailable. In the case of anxiety disorders, the majority of whole-brain functional imaging studies of cognitive control tasks have focused on DSM-IV defined OCD and PTSD, paralleling the neuropsychological publication biases. A search in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) for whole-brain neuroimaging investigations of cognitive control/executive function tasks in anxiety disorders was performed. Specific search terms are provided in the supplemental materials. A total of 1,469 articles were reviewed published through July 2016, revealing 29 studies with 35 tasks comparing neurocircuit activation during cognitive demand in patients compared to control participants. Twenty-one of these studies were of OCD, 7 of PTSD, and only one of generalized social anxiety disorder. Taken together, these anxiety disorders tended to show reduced lateral and medial prefrontal activation in tasks reflecting the canonical dimensions of shifting, inhibition, and updating (e.g., Chen, Li, Xu, & Liu, 2009; Falconer et al., 2008; Gu et al., 2008; Kang et al., 2013; Roth et al., 2007; Remijnse et al., 2013). In contrast, dACC/anterior insula involvement was alternately marked by hyper- (Fitzgerald et al., 2005; Maltby et al., 2005; Marsh et al., 2014; Roth et al., 2007) and hypoactivation (Chen et al., 2009; Gu et al., 2008; Kang et al., 2013; Remijnse et al., 2013) in different samples. The variability observed between these neuroimaging studies strongly underscores the need for additional investigations of cognitive control circuits in anxiety and related disorders, especially as PTSD, OCD, and traditionally defined anxiety disorders may be more heterogeneous than originally thought. In latent variable analysis of underlying symptom dimensions, no consensus has emerged regarding whether OCD and PTSD are better construed as predominantly fear or anxious-misery disorders (Cox, Clara, & Enns, 2002; Forbes et al., 2010). Additionally, PTSD and OCD were reclassified from anxiety disorders into separate spectra in DSM-5, Trauma and Stress-related Disorders and Obsessive-Compulsive and related disorders, respectively. This latter DSM revision suggests a particular paucity of data on contemporary nosologically defined anxiety disorders.
Similar to anxiety disorders, the literature on substance use disorders and functional cognitive control circuits suffers from a limited corpus and pronounced disorder heterogeneity. For example, central nervous system effects and neurotoxicity vary dramatically across substance(s) of abuse. The same PubMed search run for anxiety disorders was repeated for substance use disorders (see supplement for details). A total of 1,182 articles published through July 2016 were reviewed, revealing 37 studies comparing neurocircuit activation during cognitive functioning in patients compared to control participants. These studies included stimulant (n=13), alcohol (n=10), cannabis (n=6), polysubstance (n=4), opiate (n=3), and cocaine (n=1) abuse/dependence also varying in terms of active or abstinent users. Despite the variability in identified substances of use and abuse, these patients relative to other disorders tended to show more uniform attenuations of the multiple demand network and related regions (e.g., Akine et al., 2007; Bach et al., 2012; Bolla et al., 2003; Bolla et al., 2005).
Structure, Function, & the Multiple Demand Network
In summary, in a prior meta-analysis we observed reduced gray matter in the anterior dACC and bilateral anterior insula across disorders. The cingular-opercular (CO) network, a key subnetwork of the broader multiple demand network whose efficient deployment is essential for intact cognition, comprises these regions. A review of functional neuroimaging studies of cognitive control tasks across disorders suggested a functional parallel. That is, regions of both the CO and FP subnetworks of the multiple demand network were implicated in transdiagnostic cognitive control deficits. Consistent with the essential role of anterior insula as neural network switchboard coordinating large-scale network interactions, weakening of the structural integrity of this CO network would likely affect a multitude of processes, possibly contributing to the wide array of cognitive as well as affective dysfunction and symptom presentations in mental illness. Additionally, large-scale structural and functional connectomics have demonstrated that many of the multiple demand network nodes implicated in psychiatric cognitive dyscontrol serve as densely interconnected hubs. These hubs, namely superior frontal cortex, superior parietal cortex, and the insula (as well as bilateral precuneus, hippocampus, putamen and thalamus) form a central core or “rich club” (e.g., van den Heuvel & Sporns, 2011) undergirding information transfer and integration. In fact, direct comparisons of connectomics in schizophrenia patients and control participants has shown reduced density of rich club connections predominantly comprising the white matter pathways that link multiple demand network nodes, specifically the midline frontal, parietal, and insular hub regions (van den Heuvel et al., 2013). In essence aberrations in gray matter, functional activation and white matter connections likely interact to prompt the real-world impairment secondary to transdiagnostic neurocognitive impairment and affective distress.
Summary, Limitations, & Future Directions
Accumulating findings have revealed that psychiatric disorders share a common factor or vulnerability to dysfunction. Similarly, historic conceptualizations of distinct cognitive domains have been superseded by models that include a higher order common cognitive control/executive function factor. Separate studies suggest this common cognitive control factor may be related to deficits in a frontal-cingulate-parietal-insular network recruited for a wide diversity of cognitive demands. Additionally, the nodes in this multiple demand network (dACC and insula), which function as a switchboard for communication between large-scale networks, share a common vulnerability to gray matter reduction, more pronounced at extreme levels of general psychopathology.
While burgeoning evidence of common factors calls into question historic parcellations of cognition and disease, it is important to note that shared variance also reflects the inherent impurity in real world psychiatric distress and cognitive processing. For example, a bias for domain generality over specificity in brain function and behavior may in part reflect that study tasks are grouped into domains based on conceptualizations of the predominant underlying executive function. Naturally, however, performance of most tasks involves a synergy of cognitive processes that spans domains such as working memory, set shifting, and performance monitoring. In a related vein, robust transdiagnostic effects in part reflect that polythetic diagnostic schemes, which yield tremendous phenotypic heterogeneity, in combination with high comorbidity hamper detection of profiles specific to putatively “pure” disorder manifestations. Finally, it must be noted that both the corpus of task-based functional imaging and performance data on cognitive control is particularly limited for some individual disorders and cognitive domains, which biases findings towards common over distinct deficits. For example, among healthy control samples updating- and shifting-specific factors emerge with the common cognitive control factor (Miyake & Friedman, 2012). The emerging pattern of transdiagnostic neurocognitive and multiple demand network impairment does not preclude the presence of distinct deficits within individual disorders and individual brain regions. The appearance of common impairment could result from numerous distinct deficits. Elucidating this point necessitates more extensive investigation across disorders and across task domains to enable more powerful analyses of concurrent specific and common deficits in brain and behavior. In fact, future studies examining a single disorder focused on a single potential marker of executive function impairment would likely provide little to advance our understanding of neurocognitive impairments in mental illness.
Taken together, large-scale investigations that enable dimensional factor analysis of common and specific symptom and neurocognitive domains in conjunction with multivariate analysis of brain activation/deactivation patterns are warranted. At the forefront of this work and an exemplar for future investigations, Shanmugan and colleagues (2016) performed such an approach on data from a community sample of 1,129 individuals who completed a working memory task during fMRI acquisition. Severity on a general psychopathology factor reliably predicted attenuated recruitment of the multiple demand network. Symptom factors reflecting lower order dimensions were also considered. That is, psychosis spectrum symptoms were associated with hypoactivation of the dlPFC, externalizing symptoms with hypoactivation of the frontoparietal cortex and cerebellum, and anxious-misery symptoms with widespread hyperactivation of the executive network. Essentially, common and specific factors demonstrated dissociable patterns, which would have been obscured in a conventional nosological framework. While additional transdiagnostic investigations that enable more comprehensive latent variable modeling of common as well as potential distinct impairments are needed, findings to date of common factors across symptom, behavior and brain modalities have been robust and most importantly, have shown prospective prediction of distress and functional impairment that often exceed traditional “distinct” factors.
Conclusions
Consistent with principles of the Research Domain Criteria (RDoC) project (Kozak & Cuthbert, 2016), the current review implicates disruption of a cognitive control network across disorders. Importantly, this network parallels the multiple demand network intrinsic to adaptive, flexible cognition. Given our prior findings of transdiagnostic gray matter loss in overlapping regions of this network, a parallel is suggested across structural and functional measures of brain dysfunction. Also highlighted is the particular vulnerability of the anterior cingulate and insular cortices to perturbations especially in the context of highly burdensome general psychopathology. Most importantly, these findings signify promise for psychosocial, pharmacological, and neuromodulatory interventions that target the foundation of intact, dynamic cognition seated in the frontal-cingulate-parietal-insular network. Such targeted approaches could be powerful for ameliorating not only symptoms, but also the broad functional impairments and diminished quality of life prevalent across psychiatric disorders.
Supplementary Material
Acknowledgments
LM was supported by National Institute of Mental Health K23 MH104849. AE was supported by the Sierra-Pacific Mental Illness Research, Education and Clinical Center (MIRECC) at the Palo Alto VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrover-Roig D, Sesé A, Barceló F, Palmer A. A latent variable approach to executive control in healthy ageing. Brain and Cognition. 2012;78:284–299. doi: 10.1016/j.bandc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Akine Y, Kato M, Muramatsu T, Umeda S, Mimura M, Asai Y, Tanada S, Obata T, Ikehira H, Kashima H, Suhara T. Altered brain activation by a false recognition task in young abstinent patients with alcohol dependence. Alcohol: Clinical Experimental Research. 2007;31:1589–1597. doi: 10.1111/j.1530-0277.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Bach P, Vollstädt-Klein S, Frischknecht U, Hoerst M, Kiefer F, Mann K, Ende G, Hermann D. Diminished brain functional magnetic resonance imaging activation in patients on opiate maintenance despite normal spatial working memory task performance. Clinical Neuropharmacology. 2012;35:153–160. doi: 10.1097/WNF.0b013e31825c38f5. [DOI] [PubMed] [Google Scholar]
- Boelema SR, Harakeh Z, van Zandvoort MJ, Reijneveld SA, Verhulst FC, Ormel J, Vollebergh WA. Executive functioning before and after onset of alcohol use disorder in adolescence. A TRAILS study. Journal of Psychiatr Research. 2016;78:78–85. doi: 10.1016/j.jpsychires.2016.03.014. [DOI] [PubMed] [Google Scholar]
- London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophrenia Bulletin. 2014;40:744–755. doi: 10.1093/schbul/sbt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Science. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Cáceda R, Nemeroff CB, Harvey PD. Toward an understanding of decision making in severe mental illness. Journal of Neuropsychiatry Clinical Neuroscience. 2014;26:196–213. doi: 10.1176/appi.neuropsych.12110268. [DOI] [PubMed] [Google Scholar]
- Cai W, Chen T, Ryali S, Kochalka J, Li CS, Menon V. Causal interactions within a frontal-cingulate-parietal network during cognitive control: convergent evidence from a multisite-multitask investigation. Cerebral Cortex. 2016;26:2140–2153. doi: 10.1093/cercor/bhv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher N, Teesson M, Sunderland M, Newton NC, Krueger RF, Conrod PJ, Barrett EL, Champion KE, Nair NK, Slade T. The structure of adolescent psychopathology: a symptom-level analysis. Psychological Medicine. 2016;46:981–984. doi: 10.1017/S0033291715002470. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clinical Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li L, Xu B, Liu J. Insular cortex involvement in declarative memory deficits in patients with post-traumatic stress disorder. BMC Psychiatry. 2009;18:39. doi: 10.1186/1471-244X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Michels L, Supekar K, Kochalka J, Ryali S, Menon V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory-visual attention. European Journal of Neuroscience. 2015;41:264–274. doi: 10.1111/ejn.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorder. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cerebral Cortex. 2013;23:2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BJ, Clara IP, Enns MW. Posttraumatic stress disorder and the structure of common mental disorders. Depression Anxiety. 2002;15:168–171. doi: 10.1002/da.10052. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Crittenden BM, Mitchell DJ, Duncan J. Task encoding across the multiple demand cortex is consistent with a frontoparietal and cingulo-opercular dual networks distinction. Journal of Neuroscience. 2016;36:6147–6155. doi: 10.1523/JNEUROSCI.4590-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug and Alcohol Dependence. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JC, Marra CA, Najafzadeh M, Lui-Ambrose T. The independent contribution of executive functions to health related quality of life in older women. BMC Geriatrics. 2010;10:16–23. doi: 10.1186/1471-2318-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Science. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, Klebanov P, Pagani LS, Feinstein L, Engel M, Brooks-Gunn J, Sexton H, Duckworth K, Japel C. School readiness and later achievement. Developmental Psychology. 2007;43:1428–1446. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand MD system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Science. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Dunlop K, Hanlon CA, Downar J. Noninvasive brain stimulation treatments for addiction and major depression. Annals of the New York Academy of Science. 2016 doi: 10.1111/nyas.12985. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Gyurak A, O’Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues in Clinical Neuroscience. 2013;15:419–429. doi: 10.31887/DCNS.2013.15.4/aetkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM. The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry Neuroscience. 2008;33:413–422. [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proceeedings of the National Academy of Sciences of the United States of America. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Foxall GR. Cognitive requirements of competing neuro-behavioral decision systems: some implications of temporal horizon for managerial behavior in organizations. Frontiers in Human Neuroscience. 2014;8:184. doi: 10.3389/fnhum.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D, Parslow R, Creamer M, O’Donnell M, Bryant R, McFarlane A, Silove D, Shalev A. A longitudinal analysis of posttraumatic stress disorder symptoms and their relationship with fear and anxious-misery disorders: implications for DSM-V. Journal of Affective Disorders. 2010;127:147–152. doi: 10.1016/j.jad.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, Cohen R, Kahn JP, Limosin F. Executive functions in adult offspring of alcohol-dependent probands: toward a cognitive endophenotype? Alcoholism: Clinical and Experimental Research. 2013;37(Suppl 1):E356–E363. doi: 10.1111/j.1530-0277.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. International Journal of Methods in Psychiatric Research. 2014;23:41–57. doi: 10.1002/mpr.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A. Frontoparietal activation during response inhibition predicts remission to antidepressants in patients with major depression. Biological Psychiatry. 2015;79:274–281. doi: 10.1016/j.biopsych.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hajek T, Alda M, Hajek E, Ivanoff J. Functional neuroanatomy of responseinhibition in bipolar disorders--combined voxel based and cognitive performance meta-analysis. Journal of Psychiatric Research. 2013;47:1955–1966. doi: 10.1016/j.jpsychires.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. 2012;11:73–79. doi: 10.1016/j.wpsyc.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Duncan NW, Xu J, Northoff G. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neuroscience Biobehavioral Reviews. 2014;45:350–368. doi: 10.1016/j.neubiorev.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Morris DW, Osuji J, Moates AF, Carmody TJ, Thaker GK, Cullum M, Tamminga CA. Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Research. 2012;196:38–44. doi: 10.1016/j.psychres.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Beck J, Heller K, Egner T. An insula-frontostriatal network mediates flexible cognitive control by adaptively predicting changing control demands. Nature Communications. 2015;6:8165. doi: 10.1038/ncomms9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Jang JH, Han JY, Kim JH, Jung WH, Choi JS, Choi CH, Kwon JS. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology Biological Psychiatry. 2013;40:340–346. doi: 10.1016/j.pnpbp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. American Journal of Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Eaton NR. The hierarchical structure of common mental disorders: Connecting multiple levels of comorbidity, bifactor models, and predictive validity. Journal of Abnormal Psychology. 2015;124:1064–1078. doi: 10.1037/abn0000113. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim JW, Koo TH, Yun HR, Won SH. Shared and distinct neurocognitive endophenotypes of schizophrenia and psychotic bipolar disorder. Clinical Psychopharmacology and Neuroscience. 2015;13:94–102. doi: 10.9758/cpn.2015.13.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Chang SW, Fochtmann LJ, Mojtabai R, Carlson GA, Sedler MJ, Bromet EJ. Schizophrenia in the internalizing-externalizing framework: a third dimension? Schizophophrenia Bulletin. 2011;37:1168–1178. doi: 10.1093/schbul/sbq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria Initiative: Background, issues, and pragmatics. Psychophysiology. 2015 doi: 10.1111/psyp.12518. In press. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23:551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology. 2012;121:971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto JE, Juujarvi P, Kooistra L, Pulkkinen L. Dimensions of executive functioning: evidence from children. British Journal of Developmental Psychology. 2003;21:59–80. [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Markon KE. Modeling psychopathology structure: A symptom level analysis of Axis I and II disorders. Psychological Medicine. 2010;40:273–288. doi: 10.1017/S0033291709990183. [DOI] [PubMed] [Google Scholar]
- Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB. Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biological Psychiatry. 2014;75:615–622. doi: 10.1016/j.biopsych.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Addicott MA, Sweitzer MM. Smoking abstinence and neurocognition: implications for cessation and relapse. Current Topics in Behavioral Neuroscience. 2015;23:193–227. doi: 10.1007/978-3-319-13665-3_8. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure Function. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, McTeague LM, Gyurak A, Patenaude B, Williams LM, Grieve SM, Korgaonkar MS, Etkin A. Cognition-childhood maltreatment interactions in the prediction of antidepressant ouctomes in major depressive disorder patients: resuts from the ISPOT-D trial. Depression Anxiety. 2015;32:594–604. doi: 10.1002/da.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Structure & Function. 2015;220(4):2401–2414. doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive Affective Behavioral Neuroscience. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: explaining multifinality and divergent trajectories. Perspectives in Psychological Science. 2011;6:589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Palmer SM, Crewther SG, Carey LM START Project Team. A meta-analysis of changes in brain activity in clinical depression. Frontiers in Human Neuroscience. 2015;8:1045. doi: 10.3389/fnhum.2014.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Vollebergh WA, Wiers RW, Field M. Psychological changes and cognitive impairments in adolescent heavy drinkers. Alcohol and Alcoholism. 2014;49:182–186. doi: 10.1093/alcalc/agt162. [DOI] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. American Journal of Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195:286–293. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, van den Heuvel OA, Nielen MM, Vriend C, Hendriks GJ, Hoogendijk WJ, Uylings HB, Veltman DJ. Cognitive inflexibility in obsessive-compulsive disorder and major depression is associated with distinct neural correlates. PLoS One. 2013;8:e59600. doi: 10.1371/journal.pone.0059600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AT, Bzdok D, Genon S, Langner R, Müller VI, Eickhoff CR, Hoffstaedter F, Cieslik EC, Fox PT, Laird AR, Amunts K, Caspers S, Eickhoff SB. ANIMA: A data-sharing initiative for neuroimaging meta-analyses. Neuroimage. 2016;124:1245–1253. doi: 10.1016/j.neuroimage.2015.07.060. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Lahey BB, Waldman ID. Comorbidity among dimensions of childhood psychopathology: converging evidence from behavior genetics. Child Development Perspectives. 2015;9:26–31. doi: 10.1111/cdep.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs NR, Spruijt-Metz D, Sakuma KL, Chou CP, Pentz MA. Executive cognitive function and food intake in children. Journal of Nutrition Education Behavior. 2010;42:398–403. doi: 10.1016/j.jneb.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge JY, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, Aouizerate B, Burbaud P. Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35:686–691. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, D’Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebral Cortex. 2015;25:2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, Ruparel K, Elliott MA, Sheline YI, Gur RC, Gur RE, Davatzikos C, Leibenluft E, Thase ME, Wolf DH. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, Schweinsburg BC. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin. 2015;1411:105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TF, Nugent AC, Scherk H, Gruber O, Chen X, Sachdev PS, Dickstein DP, Malhi GS, Ha TH, Ha K, Phillips ML, McIntosh AM. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar disorders. 2012;14:135–145. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, Vandekar SN, Roalf DR, Elliott MA, Jackson C, Gennatas ED, Leibenluft E, Pine DS, Shinohara RT, Hakonarson H, Gur RC, Gur RE, Satterthwaite TD. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. American Journal of Psychiatry. 2016 doi: 10.1176/appi.ajp.2015.15060725. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry. 2016 doi: 10.1016/S2215-0366(16)00012-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophrenia Research. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Whisman MA, Turner AE, Guild RM, Munakata Y. Opposite effects of anxiety and depressive symptoms on executive function: the case of selecting among competing options. Cognition Emotion. 2014;285:893–902. doi: 10.1080/02699931.2013.859568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Frontiers in Psychology. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addiction Biology. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Tabarés-Seisdedos R, Balanzá-Martínez V, Sánchez-Moreno J, Martinez-Aran A, Salazar-Fraile J, Selva-Vera G, Rubio C, Mata I, Gómez-Beneyto M, Vieta E. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. Journal of Affective Disorders. 2008;109:286–299. doi: 10.1016/j.jad.2007.12.234. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. Journal of Neuroscience. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goñi J, Hulshoff Pol HE, Kahn RS. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- Wang XL, Du MY, Chen TL, Chen ZQ, Huang XQ, Luo Y, Zhao YJ, Kumar P, Gong QY. Neural correlates during working memory processing in major depressive disorder. Progress in Neuro-Psychopharmacology Biological Psychiatry. 2015;56:101–108. doi: 10.1016/j.pnpbp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Wegbreit E, Cushman GK, Puzia ME, Weissman AB, Kim KL, Laird AR, Dickstein DP. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71:926–935. doi: 10.1001/jamapsychiatry.2014.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E. Finding the elusive psychiatric “lesion” with 21st-century neuroanatomy: a note of caution. American Journal of Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15060753. in press. [DOI] [PubMed] [Google Scholar]
- Wilk HA, Ezekiel F, Morton JB. Brain regions associated with moment-to-moment adjustments in control and stable task-set maintenance. Neuroimage. 2012;59:1960–1967. doi: 10.1016/j.neuroimage.2011.09.011. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases v.9. World Health Organization; 1977. [Google Scholar]
- World Health Organization. International Classification of Diseases, v. 10. World Health Organization; 2007. http://apps.who.int/classifications/apps/icd/icd10online. [Google Scholar]
- World Health Organization. Global Health Observatory GHO data. 2013 http://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends/en/
- Wright AG, Krueger RF, Hobbs MJ, Markon KE, Eaton NR, Slade T. The structure of psychopathology: toward an expanded quantitative empirical model. Journal of Abnormal Psychology. 2013;122:281–294. doi: 10.1037/a0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.