Abstract
In patients with major depressive disorder (MDD) or bipolar disorder (BD), abnormalities in excitatory and/or inhibitory neurotransmission and neuronal plasticity may lead to aberrant functional connectivity patterns within large brain networks. Network dysfunction in association with altered brain levels of glutamate (Glu) and gamma-aminobutyric acid (GABA) have been identified in both animal and human studies of depression. In addition, evidence of an antidepressant response to subanesthetic dose ketamine has led to a collection of studies that have examined neurochemical (e.g. glutamatergic and GABA-ergic) and functional imaging correlates associated with such an effect. Results from these studies suggest that an antidepressant response in association with ketamine occurs, in part, by reversing these neurochemical/physiological disturbances. Future studies in depression will require a combination of neuroimaging approaches from which more biologically homogeneous subgroups can be identified, particularly with respect to treatment response biomarkers of glutamatergic modulation.
Keywords: major depressive disorder, bipolar disorder, mood disorder, glutamate, NMDA receptor antagonist, ketamine
Introduction
In rodent studies, pharmacological- and stress-induction paradigms that lead to depressive-like behaviors have been associated with alterations in cortical glutamate (Glu) (1–3); findings that have been reversed by monoaminergic antidepressants and electroconvulsive therapy (4, 5). As a result, a glutamatergic hypothesis of depression was posited that extends beyond monoaminergic dysfunction in patients with major depressive disorder (MDD) or bipolar disorder (BD) (6). Interestingly, clinical studies of depression using magnetic resonance spectroscopy (MRS) as well as positron emission tomography (PET) have identified alterations in Glu and gamma-aminobutyric acid (GABA) concentrations and activity, suggesting that dysfunction in excitatory and/or inhibitory neurotransmitter signaling mechanisms may play a critical role in depression. In this review, we examine studies that use electroencephalography (EEG), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI) techniques to identify aberrant functional neural circuitry patterns in patients with MDD and BD. We then hypothesize links between neurotransmitter abnormalities and functional neurocircuitry deficits in depression, from which we encourage future work using MRS or PET techniques in conjunction with functional imaging techniques to elucidate and characterize these relationships.
Furthermore, a pivotal role of glutamatergic neurotransmission in the pathophysiology of and treatment response in MDD and BD has been supported by studies that demonstrate antidepressant efficacy of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine (7) in preclinical and clinical studies (8, 9). Here we highlight ketamine as the best available molecular tool with which to probe the impact of glutamatergic modulation on excitatory/inhibitory neural circuitry dynamics in healthy and depressed subjects with MDD or BD due to evidence supporting ketamine’s efficacy and its lower burden of side effects compared to other glutamatergic modulating agents (9–14). Within this framework, we review clinical studies using MRS, PET, fMRI, and MEG to explore how glutamatergic modulation may alleviate aberrant functional neurocircuitry in depression and mediate antidepressant response to ketamine. Lastly, we emphasize the importance of utilizing a combinatorial approach to better identify and predict treatment responders to ketamine.
Dysregulation of Glutamatergic and GABAergic Neurotransmission in Depression
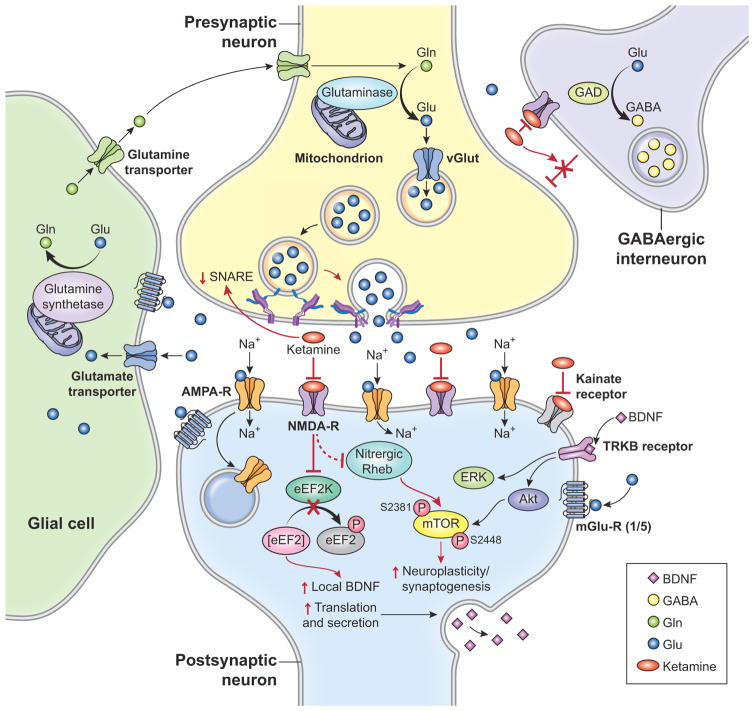
Proton MRS (1H-MRS) is an in vivo imaging technique for total tissue detection of neurochemicals, including N-acetylaspartate (NAA), GABA, Glu, glutamine (Gln), and a combination of Glu-Gln with a minor contribution from GABA (known as Glx) that is often reported due to poor signal resolution between these metabolites in weaker magnetic fields. 1H-MRS studies examining levels of GABA, Glu, Gln, and Glx in the brain require an appreciation of Glu metabolism, particularly the Glu/Gln cycle (15, 16) (see Figure 1). Briefly, Glu is produced in neurons from glucose-derived tricarboxylic acid cycle intermediates and branched-chain amino acids. Cytosolic Glu is packaged into vesicles via vesicular glutamate transporters (vGluTs) for exocytotic release. After neuronal depolarization and release into the synaptic cleft, Glu binds to one of three types of ionotropic Glu receptors: NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), or kainate, all of which are embedded in the postsynaptic membrane and clustered within postsynaptic densities. Glu also binds to metabotropic receptors (mGluRs), typically found extrasynaptically and presynaptically. To prevent synaptic spillover and excitotoxicity, Glu is removed from the synapse by Glu transporters in astrocytes and metabolically converted into Gln via glutamine synthetase. Gln is released by astrocytes into presynaptic neurons where it is converted back to Glu via cytosolic glutaminase (15, 16). Inhibitory GABAergic neurons also contain the enzyme glutamic acid decarboxylase (GAD), which converts Glu to GABA.
Figure 1. The Cellular and Molecular Effects of Ketamine on Glutamatergic and GABAergic Metabolism and Neurotransmission.
(A) Normal glutamatergic and GABAergic metabolism and neurotransmission: Glutamate (Glu) is packaged into vesicles via vesicular glutamate transporters (vGluTs) for exocytotic release. After neuronal depolarization and release into the synaptic cleft, Glu binds to NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate, or metabotropic receptors (mGluRs). Glu is removed from the synapse by Glu transporters in astrocytes and metabolically converted into glutamine (Gln) via glutamine synthetase. Gln is released by astrocytes into presynaptic neurons where it is converted back to Glu via cytosolic glutaminase. Inhibitory GABAergic neurons contain the enzyme glutamic acid decarboxylase (GAD), which converts Glu to GABA.
(B) Ketamine-induced changes, depicted in red: Ketamine antagonizes N-methyl-D-aspartate (NMDA) receptors on GABAergic interneurons and on post-synaptic neurons; the former disinhibits cortical glutamatergic neurons and the latter increases synthesis of brain-derived neurotrophic factor (BDNF). Via the kainate receptor, ketamine increases activity of mammalian target of rapamycin (mTOR) leading to neuroplasticity and synaptogenesis. Ketamine also increases BDNF via nitric oxide production, leading to stabilization of Nitrergic Rheb and enhancement of mTOR signaling.
In comparison to healthy subjects, aberrant amino acid neurotransmitter levels measured by 1H-MRS have been found in individuals with MDD (6, 17–19) and BD (20, 21). Specifically, in MDD patients, Glu and Glx reductions were found in the dorsolateral prefrontal cortex (dlPFC) (22) and other prefrontal cortical (PFC) areas such as the dorso-medial and dorso-anterolateral PFC (23) and anterior cingulate cortex (ACC) (24), with increased levels in the occipital cortex (OCC) (25) to a degree that may be related to duration of illness (26). In a meta-analysis of 17 1H MRS studies of MDD patients, reductions of Glx in the PFC were associated with number of failed antidepressant treatments, a measure of chronicity and proxy for severity of depressive illness course (19). Surprisingly, this study found no isolated reductions in Glu, implicating astrocyte-mediated metabolic alterations in Glu metabolism underlying the pathophysiology of MDD (19). A possible explanation may be related to abnormal mitochondrial energy production in glutamatergic neurons. In an in-vivo 13C MRS and 1H-MRS study that examined the potential relationship between the Glu/Gln cycle and mitochondrial energy production (27), patients with MDD showed a 26% reduction in mitochondrial energy production compared to healthy subjects, though no differences were found in Glu/Gln cycle rate. The authors suggested that reductions in energy production within glutamatergic neurons resulted from reduced synaptic strength via reductions in AMPA or NMDA receptors in the postsynaptic neuron. Additionally, although performed post-hoc without correction for multiple comparisons, the authors found a negative association between Glu concentrations and the number of depressive episodes (27), suggesting that reductions in synaptic strength may reduce Glu neurotransmission over successive episodes of depression.
Neurochemical studies in BD patients have shown mixed results. Although some studies found no differences in Glu between BD patients and healthy subjects (28–31), two meta-analyses of 1H MRS studies in BD noted increased Glx in the PFC regardless of mood state, as well as in the ACC in depressed states (20, 32). A recent study showed that elevated Glu in the ACC associated with BD patients in euthymic states was related to number of depressive/manic episodes (21); notably, this finding may allow for differentiation of depression between BD and MDD. Impaired oxidative metabolism in glutamatergic neurons has been hypothesized to explain elevated Glx and lactate levels associated with mixed or depressed states in BD (33), and further supports a contributory role of inflammation in the pathophysiology of depression (34). Wide and overlapping ranges of mood and neurocognitive states in BD (35) increase variation within and between study samples, contributing to challenges in interpreting neurochemical and functional imaging studies of BD. Therefore, further studies of BD may require a larger number of patients to better examine state- vs. trait-related brain correlates in addition to the development of novel and sophisticated models of BD subphenotypes (36). Lastly, in addition to known sources of heterogeneity between 1H-MRS studies of depression (37), such as subject selection, field strength, MRS sequences, and anatomical placement of the voxel of interest (18), studies of BD patients are further confounded by concurrent use of medications (e.g. valproate and lithium), which has been shown to alter Glu and GABA levels in the brain (20, 38). Unsurprisingly, 1H-MRS studies in MDD have consistently shown reduced levels of Glu, Gln, Glx, and GABA (39), whereas in BD studies, elevated levels of Glu have been observed inconsistently without differences in Gln, Glx, or GABA (20).
Detection and quantification of neurotransmitter receptors using PET imaging, in conjunction with administration of a radioactive ligand that is displaced by the endogenous neurotransmitter of interest at its receptor, provides complementary information to MRS studies regarding neurotransmitter signaling mechanisms. For example, reduced mGluR5 (see Figure 1) density in MDD patients was observed in two clinical PET studies that used an mGluR5-specific radioligand (40, 41). Taken together with animal studies showing antidepressant-like effects associated with the mGluR5-specific antagonists MPEP and MTEP (42–44), these findings suggest that abnormal mGluR5 signaling may be involved in the pathophysiology of depression. One of the studies was of patients aged 55 to 80 (40) and may be confounded by comorbid medical conditions, their treatment, and age-related brain changes. Nevertheless, a clear need exists to develop specific Glu receptor ligands for mGluRs and ionotropic Glu receptor subunits (45) to better understand abnormal glutamatergic neurotransmission and plasticity in depression.
Studies of inhibitory neurotransmitter systems in depression have also obtained mixed results. In 1H-MRS studies of MDD patients, lower GABA levels were reported in the PFC (23), ACC (37), and OCC (46) compared to healthy subjects, and these changes may be more pronounced in association with melancholia (25). One negative study found no group differences in GABA concentrations in remitted MDD patients (47), but the authors suggested that GABA concentrations (and activity) may be sensitive to the presence of a depressive episode. Moreover, a 1H-MRS study of MDD patients found no group differences in Glx concentrations within the ventromedial PFC (vmPFC) compared to healthy subjects, but observed an increased ratio of Glx to GABA associated with lower age of depression onset, suggesting that an increased excitatory/inhibitory neurotransmitter ratio may be associated with depression vulnerability (47). In studies of euthymic BD patients, decreased (48) and increased (49) GABA levels have been observed compared to healthy subjects, as have no differences in GABA levels (50).
Based on the hypothesized excitatory/inhibitory imbalance in depression (51), 1H-MRS studies of Glu and Glu/GABA ratios suggest that a deficiency of excitatory neurotransmission or an imbalance of excitatory/inhibitory neurotransmission characterizes a subset of depressed patients. Despite the clinical efficacy of GABAergic medications (e.g. valproic acid, lamotrigine, and carbamazepine) in BD (52, 53), inconsistent reports of GABA levels associated with BD does not support an isolated deficiency in GABA in depression. Abnormalities in glutamatergic neuronal metabolism may be experienced in a proximal stage of the underlying pathophysiology of MDD and BD. For example, in a study of schizophrenia patients randomized to receive pomaglumetad (a metabotropic glutamate 2/3 receptor agonist) versus placebo for 6 weeks, greater improvement in symptoms were experienced earlier in the course of illness or with history of a medication targeted at the D2 receptor (54). Future prospective investigations in larger clinical samples may allow identification of subgroups of depressed patients who better respond to GABA- or Glu-modulating therapies along the illness course. Moreover, innovations to current MRS techniques (27), such as application of novel pulse sequences at higher magnetic field strengths (e.g., 7 Tesla (T)) that can better resolve cerebral Glu, Gln, and GABA concentrations (55) could facilitate identification of biologically homogeneous and enriched subgroups (39) to which directed clinical interventions can be addressed (56).
Functional Circuitry Abnormalities in Depression
Neural circuitry describes the complex array of interconnected neurons in the brain from which simultaneous and coordinated information processing is refined and reorganized via experience-related synaptic changes (57). Neuroimaging methods that indirectly (e.g., fMRI blood-oxygen level dependent (BOLD) signal) or directly (e.g., EEG and MEG) examine neural activity aim to identify circuitry-level abnormalities and/or response to behavioral or neurochemical interventions (17). Considerations are necessary to address before links can be drawn between amino acid neurochemistry and functional imaging. First, MEG possesses greater spatial resolution than EEG (58, 59); thus, we focus primarily on MEG studies. Second, although functional neuroimaging can be used in conjunction with cognitive and/or emotionally-salient tasks, we focus on studies performed at resting-state (task-free) from which functional connectivity patterns are derived as EEG and MEG frequency findings will be reviewed in further detail. Third, functional connectivity patterns within larger interconnected neural circuits have emerged from novel statistical techniques (60, 61). Lastly, we examine studies of abnormal functional connectivity networks as they may relate to neurochemical studies of Glu and GABA in depression.
Abnormalities in resting state functional connectivity patterns in patients with MDD have been found within and between large brain networks. The default mode network (DMN), which includes the medial prefrontal cortex (mPFC) and posterior cingulate cortex, has been shown to deactivate during cognitive tasks, and is associated with introspection or self-referential thought when not actively recruited in task performance (62). The DMN and other networks involved in cognitive control of attention and emotion have been shown differ in MDD patients compared to healthy controls (63). In MDD, reduced connectivity has been shown in fronto-parietal brain networks and hyperconnectivity (increased positive or reduced negative connectivity) within the DMN and between the subgenual ACC (sgACC) and mPFC (63); regions that have been linked to abnormalities in GABA in animal models (64, 65), as well as GABA reductions (66) and Glx/GABA imbalances (47) in MDD patients.
In a systematic review of eight resting-state fMRI studies of BD patients in all mood states, abnormalities in functional connectivity were found in the mPFC and ACC with limbic-striatal regions (67). Compared to MDD patients, BD patients show significantly stronger functional connectivity within the dorsolateral PFC and ventrolateral PFC, as well as inferior frontal/dorsolateral PFC to ACC (68), suggesting that changes in functional connectivity between the ACC and PFC, as well as differences in Glu neurotransmission within the ACC, may differentiate the two disorders. This is supported by studies that compared BD and MDD patients and found differences in PFC activation during emotionally-laden tasks (69).
Similarities between functional connectivity patterns derived from resting state fMRI studies and MEG studies across multiple frequency bands were demonstrated in healthy subjects using a model-free independent component analysis (ICA) (70–72). Using a similar model on beta band filtered MEG data (73), our group identified decreased connectivity between the sgACC and a network within the precentral motor cortex and precuneus, and increased connectivity in limbic areas (i.e. amygdala and temporal cortex) in MDD patients compared to healthy subjects. These results support the role of the sgACC and other cortical and subcortical regions (17, 63, 74) in impaired cognitive control, psychomotor retardation, and other symptom clusters in depression (75). In a study using both 1H-MRS and fMRI in MDD patients, Glu levels in the mPFC associated with connectivity to subcortical regions (76), and Glx reductions in the sgACC predicted decreased functional connectivity between the sgACC and the anterior insula (77). Decreased Glu levels corresponded with decreased BOLD response to an emotional stimulus in MDD patients with prominent anhedonia (78), suggesting that reduced glutamatergic neurotransmission and/or signaling may contribute to alterations in functional connectivity (79) at specific electrophysiological oscillation frequencies relating to cognitive and limbic-related symptomatology in depression.
Ketamine in Depression
Ketamine’s antidepressant effects were demonstrated over a decade ago in a double-blind, placebo-controlled clinical study of eight depressed patients randomized to receive either a subanesthetic dose (0.5 mg/kg IV over 40 minutes) of ketamine or saline. Four of the eight patients (n=7 completers) had an antidepressant response to ketamine (defined as a reduction of 50% or greater on the Hamilton Depression Rating Scale (HAM-D)) (10). Subsequently, our group and others replicated IV ketamine’s antidepressant effects in both MDD and BD patients across single and repeated administrations under various study designs (8, 9). The time course of the antidepressant response is characterized by an initial reduction in depressive symptoms within 2 hours, a maximal reduction in depressive symptoms within 24 hours, and a sustained response for up to 1 week after administration (9, 11).
Ketamine’s effect on glutamatergic and GABAergic neurons has emerged from pre-clinical studies (80–83) (see Figure 1). Ketamine antagonizes NMDA receptors on GABAergic interneurons and on post-synaptic neurons; the former disinhibits cortical glutamatergic neurons (83) and the latter increases synthesis of intracellular growth factors, such as brain-derived neurotrophic factor (BDNF) (81, 82). Additionally, via the kainate receptor, ketamine increases activity of mammalian target of rapamycin (mTOR) and other molecules responsible for neuroplasticity and synaptogenesis (81, 82). A recent study examining both signaling pathways found that ketamine increased BDNF by generating nitric oxide, leading to the stabilization of Nitrergic Rheb, a small G-protein that enhances mTOR signaling (80). These findings have shifted our conceptualization of the pathophysiology and treatment of depression (17, 84), and encouraging the development of Glu-based treatments for depressive disorders (12, 56, 85, 86).
Antidepressant Response to Ketamine and Glutamate/GABA Neurotransmission
To date, five 1H-MRS studies have examined Glu, Gln, Glx, and/or GABA levels, and one PET study used an mGluR5 ligand before and after IV ketamine infusion; all were conducted in healthy subjects (87–90) (see Table 1). In a double-blind, placebo-controlled, crossover study of 10 males (n=8 completers), elevated Gln levels were observed in the ACC two hours post-infusion, correlating with psychotomimetic symptoms (87). In an open-label study of 13 males, significantly elevated Glu levels were observed in the ACC 35 minutes post-infusion that did not correlate with psychotomimetic symptoms (89). A third double-blind, placebo-controlled, parallel group design study of 17 males (ketamine: n=8; placebo: n=9) found that ketamine administration was not associated with changes in Glx, Glu, or GABA levels in the mPFC/ACC 40 minutes post-infusion (88). Inconsistent results in small samples make it challenging to draw definitive conclusions about ketamine-induced amino acid neurotransmitter changes in healthy volunteers. In addition, one study (87) did not measure Glu directly, and scan quality was too poor in another study (89) to measure Gln levels. Nevertheless, consistent with the “glutamate surge” hypothesis, Glu levels increased during ketamine infusion, leading to psychotomimetic effects, with a subsequent decrease of Glu to baseline levels after infusion ceased. In support of this hypothesis, Delorenzo and colleagues (40) examined pre- and post-infusion PET scans with an mGluR5 ligand to measure the degree of ketamine binding to the mGluR5 receptor in 10 healthy control subjects who received IV ketamine. Reduced mGluR5 ligand (or increased ketamine) binding was found in the ACC, mPFC, orbital PFC, ventral striatum, parietal lobe, dorsal putamen, dorsal caudate, amygdala, and hippocampus (90). The ligand used in this study (ABP-688) is an allosteric modulator, and therefore may not be a valid method of measuring Glu release. Although basimglurant (RO4917523, RG7090), a negative allosteric modulator at the mGlu5R, has shown promise as an adjuvant medication to traditional antidepressant medications (91) in one study, a significant reduction in MADRS was found in the self-reported rather than clinician-administered MADRS and therefore, replication is necessary to support its antidepressant efficacy.
Table 1.
Neural Correlates of Antidepressant Response to Ketamine
| Citation | Sample Size and Diagnostic Groups | Neuroimaging Method | Trial Design and Ketamine Dose | Significance and Findings |
|---|---|---|---|---|
| Rowland et al., 2005 (87) | Healthy subjects N = 10 (10M) |
1H-MRS, 4T MRI, 8cc voxel placed in the ACC bilaterally; quantification of Gln, NAA, choline, creatine, Glu; images acquired before, during, and at end of loading dose prior to maintenance dose | Double-blind, placebo-controlled, single infusion; IV ketamine 0.27 mg/kg loading dose over 10 min, 0.00225 mg/kg per min thereafter for up to 2 hrs until end | Increased Glu in the ACC associated with ketamine infusion |
| Taylor et al., 2012 (88) | Healthy subjects N = 17 (11M,6F) |
1H-MRS, 3T MRI, 30×20×20mm voxel placed in the ACC and mPFC; quantification of Glx, Glu, Gln; images acquired before, during, and after infusion | Double-blind, placebo-controlled, single infusion; IV ketamine 0.5 mg/kg over 40 min | No increase in Glu or Gln in the ACC or mPFC associated with ketamine infusion |
| Stone et al., 2012 (89) | Healthy subjects N = 13 (13M) |
1H-MRS, 3T MRI, 30×30×30 mm voxel placed over the thalami bilaterally and surrounding subcortical structures, 20×20×20 mm voxel placed over the ACC; quantification of Glu, Gln, GABA; images acquired before and after infusion at 25 min and 35 min | Open-label, single infusion; IV ketamine 0.5 mg/kg over 40 min | Increased Glu in the ACC but no effect on ACC Glu + Gln, or subcortical GABA levels associated with ketamine infusion |
| Valentine et al., 2011 (94) | MDD subjects N = 10 (4M,6F) |
1H-MRS, 3T MRI, 3.0×1.5×3.0 cm voxel placed in the OCC; quantification of Glu, Gln, GABA; images acquired before, 3 hrs after, and 48 hrs after infusion | Single-blind, placebo-controlled, two infusions 1 week apart; IV ketamine 0.5 mg/kg over 40 min | Improvement in depressive symptoms at 1 hr and for at least 7 days after ketamine infusion was not associated with baseline measures of, or changes in, occipital Glu, Gln, or GABA |
| Salvadore et al., 2012 (92) | MDD subjects N = 14 (9M,5F) |
1H-MRS, 3T MRI, 5×3×2 cm voxel placed in the DM/DA-PFC, 3×3×2 cm voxel placed in the vmPFC; quantification of Glu, Gln, Glx, GABA; images acquired 1–3 days before infusion | Open-label, single infusion; IV ketamine 0.5 mg/kg over 40 min | Pretreatment GABA or Glu was not associated with a decrease in depressive symptoms in either of the two ROIs; pretreatment Glx/glutamate ratio in the DM/DA-PFC was negatively correlated with improvement in depressive symptoms |
| Milak et al., 2015 (93) | MDD subjects N = 11 (3M,8F) |
1H-MRS, 3T MRI, 3.0×2.5×2.5 cm voxel placed in the mPFC and pgACC; quantification of Glu, Gln, Glx, GABA; images acquired before, during, and after infusion | Double-blind, placebo-controlled, two infusions 1 week apart; IV ketamine 0.5 mg/kg over 40 min | Increased Glx and GABA in the mPFC associated with ketamine infusion |
| Scheidegger et al., 2012 (96) | Healthy subjects N = 19 (9M,10F) |
fMRI, 3T MRI, SPM8, seed ROIs in the CCN, the DMN, and affective networks, bilateral DLPFC, dACC, sgACC, and amygdala; images acquired before and 24 hr after infusion | Double-blind, placebo-controlled, crossover of two infusions 2 weeks apart; IV ketamine0.5 mg/kg over 40 min | Decreased functional connectivity of the DMN to a network of dorsal brain regions and to the sgACC and mPFC via the dACC associated with ketamine infusion |
| Grimm et al., 2015 (95) | Healthy subjects N = 24 (12M,12F) |
fMRI, 3T MRI, SPM8, seed ROIs in the DLPFC and hippocampus bilaterally; images acquired before and 20 min after infusion | Single-blind, placebo-controlled, crossover of 3 single infusions; IV ketamine 0.5 mg/kg over 40 min | Increased functional connectivity between the DLPFC bilaterally and the left hippocampus associated with ketamine infusion |
| Carlson et al., 2013 (98) | MDD (TRD) subjects N = 26 (20M,6F) |
PET, 18[F]-FDG, 3T MRI, SPM5, whole-brain and ROI analyses at amygdala, anterior hippocampus, medial thalamus, habenula, and sgACC; images acquired before and after | Open-label, single infusion; IV ketamine 0.5 mg/kg over 40 min | Reduction in depressive symptoms associated with increased metabolism in STG/MTG, and cerebellum as well as decreased metabolism in ventral and medial loci within the STG/MTG, parahippocampal gyrus, inferior parietal cortex, and temporo-occipital cortex |
| Lally et al., 2015 (97) | MDD (TRD) subjects N = 20 (14M,6F) |
PET, 18[F]-FDG, 3T MRI, SPM5, whole-brain and ROI analyses at ventral striatum and OFC; images acquired before and at 240 min after infusion | Open-label, single infusion; IV ketamine 0.5 mg/kg over 40 min | Reduction in anhedonia was associated with increased metabolism in the hippocampus and dACC as well as decreased metabolism in the inferior frontal gyrus and OFC |
| Li et al., 2016 (99) | MDD (TRD) subjects N = 48 (no gender breakdown given) |
PET, 18[F]-FDG, 3T MRI, SPM5, whole-brain and ROI analyses at PFC and amygdala; images acquired before and at 40 min after infusion | Double-blind, placebo-controlled, single infusion; Group A: IV ketamine 0.5 mg/kg over 40 min, Group B: IV ketamine 0.2 mg/kg over 40 min, Group C: IV normal saline | Reduction of depressive symptoms was associated with increased metabolism in the PFC in Groups A and B, but not C; whole-brain analysis confirmed a group effect on the PFC (Group A<C, Group B<C); metabolic differences in the PFC predicted response at 40 and 240 min |
| Lally et al., 2014 (100) | BD-I and BD-II subjects N = 21 (6M,15F) (all on lithium or valproic acid) |
PET, 18[F]-FDG, 3T MRI, SPM5, whole-brain and ROI analyses at ventral striatum and OFC; images acquired before and at 120 min after infusion | Double-blind, placebo-controlled, crossover of two infusions 2 weeks apart; IV ketamine 0.5 mg/kg over 40 min | Reduction of depressive symptoms but not anhedonic symptoms was associated with increased metabolism in the ventral striatum; reduction in anhedonia was associated with increased metabolism in the dACC |
| Nugent et al., 2014 (101) | BD-I and BD-II subjects N = 21 (6M,15F) (all on lithium or valproic acid) |
PET, 18[F]-FDG, 3T MRI, SPM5, whole-brain and ROI analyses at left and right amygdala, hippocampus, vlPFC, anteromedial PFC, DLPFC, ventral striatum, pgACC, sgACC, medial thalamus, dACC, lateral orbital cortex, superior temporal gyrus, frontal polar cortex, habenula, and anterior insula; images acquired before and at 120 min after infusion | Double-blind, placebo-controlled, crossover of two infusions 2 weeks apart; IV ketamine 0.5 mg/kg over 40 min | Reduction in depressive symptoms was associated with increased metabolism in the right ventral striatum as well as reduced metabolism in left hippocampus in whole brain analysis |
| Shaw et al., 2015 (107) | Healthy subjects N = 18 (18M) |
MEG acquired during visuomotor task before and after infusion, 3T MRI | Single-blind, placebo-controlled, crossover of two infusions; initial IV ketamine bolus of 0.25 mg/kg delivered over 1 min, followed by maintenance infusion at a rate of 0.25 mg/kg | Increased beta amplitudes and decreased peak gamma frequency in the visual cortex as well as amplified gamma-band amplitudes in motor and visual cortices were associated with ketamine infusion |
| Muthukumaraswamy et al, 2015 (112) | Healthy subjects N = 25 (25M) |
MEG acquired at rest and during visuomotor task before and during infusion, 3T MRI | Single-blind, placebo-controlled, crossover of two infusions; initial IV ketamine bolus of 0.25 mg/kg delivered over ~1 min, followed by maintenance infusion at a rate of 0.375 mg/kg/h | Decreases in OCC, parietal, and ACC alpha power, increases in medial frontal theta power, and increases in parietal and cingulate cortex high gamma power in association with ketamine infusion; dynamic causal modeling showed that oscillatory changes were accompanied by temporally sustained reductions in frontoparietal effective connectivity |
| De Simoni et al., 2013 (114) | Healthy subjects N = 10 (10M) |
phfMRI, 3T MRI, BOLD signal, initiated 5 min prior to infusion, SPM5 | Open-label, two infusions, IV ketamine (target plasma levels 50 ng/mL and 75 ng/mL) | Widespread changes present in ACC, mid-posterior cingulate and paracingulate cortices, hippocampal and parahippocampal regions, bilateral insula, DLPFC, vlPFC, thalamus, supplementary motor area, bilateral operculum, precuneus and medial occipital lobes in association with ketamine infusion. |
| Doyle et al., 2013 (115) | Healthy subjects N = 16 (16M) |
phfMRI, 3T MRI, BOLD signal, initiated at 15 min prior to infusion, SPM5 | Double-blind, placebo-controlled, partial crossover design of four infusions, two with pre-treatment risperdone or lamotrigine or placebo; IV ketamine (target plasma levels of 75 ng/mL) initially at 0.003 mg/kg for 1 min, then at ~0.31 mg/kg/h | Lamotrigine and risperidone resulted in widespread attenuation of the ketamine-induced increases in signal, including the frontal and thalamic regions; a contrasting effect across both pretreatments was observed only in the sgPFC, in which ketamine reduced the signal |
ACC = anterior cingulate cortex; BD = bipolar disorder; BOLD = blood oxygen level dependent; CCN = cognitive control network; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral PFC; DM/DA-PFC = dorsomedial/dorsal anterolateral prefrontal cortex; DMN = default mode network; 18[F]-FDG = 18fluoro-deoxyglucose; fMRI = functional magnetic resonance; GABA = gamma-aminobutyric acid; Gln = glutamine; Glu = glutamate; Glx = combination of Glu and Gln; 1H-MRS = proton magnetic resonance; IV = intravenous; MDD = major depressive disorder; MEG = magnetoencephalography; mPFC = medial prefrontal cortex; MRI = magnetic resonance imaging; NAA = N-acetylaspartate; OCC = occipital cortex; OFC = orbitofrontal cortex; PET = positron emission tomography; PFC = prefrontal cortex; pgACC = perigenual ACC; phfMRI = pharmacologic fMRI; ROI = region of interest; sgACC = subgenual anterior cingulate cortex; sgPFC = subgenual PFC; SPM = statistical parametric mapping; STG/MTG = superior and middle temporal gyri; T = tesla; TRD = treatment resistant depression; vlPFC = ventrolateral prefrontal cortex; vmPFC = ventromedial prefrontal cortex.
Three 1H-MRS studies examined ketamine-related changes in Glu, Glx, and/or GABA in MDD patients (92–94) (see Table 1); two uncovered no neurochemical signature associated with antidepressant response to ketamine (93, 94). The third study observed improved depressive symptoms in 14 unmedicated, treatment-resistant MDD patients in association with increased pretreatment Glx/Glu ratio in the dorsomedial PFC/dorsal anterolateral PFC. Pretreatment measures of GABA or Glu did not correlate with reduction in depressive symptoms in either of these two regions of interest (p>0.1) (92). In another study of 11 unmedicated MDD patients, no association was observed between antidepressant response to ketamine and Glx or GABA levels in the mPFC before, during, or after ketamine infusion (93). Interestingly, in this study, ketamine was associated with increased Glx/water and GABA/water ratios, indicating target engagement and suggesting that ketamine transiently increases excitatory and inhibitory neurotransmission. Finally, in a single-blind study of 10 patients with MDD, ketamine was associated with reduced depressive symptoms at one hour to seven days post-infusion; however, antidepressant efficacy was not associated with baseline levels or change in any amino acid neurotransmitter within the OCC (94). Therefore, it is unclear whether amino acid neurometabolite levels or their ratios can help predict antidepressant response to ketamine due to inconsistent results in small samples across different imaging platforms.
Ketamine and Functional Neural Circuitry in Depression
Increased pre-treatment neural activity in the rostral ACC (rACC) associates with antidepressant response across different pharmacologic, electrophysiologic, and behavioral interventions (74), suggesting a common neurobiological signature may be associated with antidepressant response to treatment. In healthy subjects, functional connectivity between the rACC and mPFC increased acutely (95) and decreased 24 hours after ketamine infusion (96). Furthermore, using PET imaging methods, ketamine associated with increased glucose metabolism in the dorsal ACC (dACC) (97), altered glucose metabolism in PFC regions (97–99) in MDD, and increased glucose metabolism in the dACC and putamen in BD patients (100). Our group has shown that sgACC hypermetabolism predicted response to ketamine in BD patients (101). Taken together, the evidence suggests that specific activation patterns in the sgACC and dACC lead to disrupted functional engagement of PFC regions that is partly modifiable in patients with depression through glutamatergic interventions. This adds to the substantial and mounting evidence that the ACC is not only a key hub connecting limbic dysfunction with clinical symptomatology in MDD, but also that its functional response may correlate with ketamine’s antidepressant efficacy.
Excitatory/inhibitory neurotransmitter imbalances may be inferred through specific electrophysiological measures. For example, interactions between superficial excitatory pyramidal cells and inhibitory GABAergic interneurons are attributed to oscillations in the gamma-frequency band as measured by MEG (83, 102, 103). Increases in gamma-band power (104, 105), reduced coupling of spike-rate and local field potential power within the gamma band (105), and disruptions in neuronal plasticity within prefrontal-hippocampal circuits (106) have been associated with NMDA receptor blockade with ketamine in the rodent neocortex. Similarly, in humans, subanesthetic dose ketamine infusion associates with increased gamma-band amplitudes in motor and visual cortices (102), and decreased peak gamma frequency in the visual cortex (107). Given the correlation between gamma band oscillations and BOLD connectivity in both rodents (108) and humans (109), ketamine may be associated with abnormalities in functional connectivity and disruptions in neuronal plasticity, particularly in circuits interconnecting the hippocampus and PFC (108, 110). Glu levels in the posterior cingulate cortex strongly correlate with connectivity in the DMN (111), suggesting that Glu alterations via ketamine administration may give rise to alterations in brain function at rest (ie. non-task) as well as during a task. Finally, a MEG study in healthy volunteers (n=25 males) found that subanesthetic dose ketamine increased anterior theta and gamma power but decreased posterior theta, delta, and alpha power; these changes were sustained for up to 50 minutes post-ketamine infusion (that is, after the resolution of perceptual distortions) (112). The authors reported frontoparietal connectivity changes, with ketamine reducing NMDA- and AMPA-mediated frontoparietal connectivity. If replicated, the antidepressant effects of ketamine may depend upon acute and prolonged changes in MEG spectral power as well as in electrophysiological synchrony in frontoparietal circuitry. The extent to which gamma-band changes measured by MEG reflect altered glutamatergic neurotransmission and/or signaling in association with ketamine administration is unclear. Furthermore, given that current studies are limited to healthy subjects (see Table 1), it is not known whether ketamine-related electrophysiological changes are similar in depressed patients.
Ketamine as a Functional Neurocircuitry Modulator: Future Directions
In depressed patients, evidence from neurochemical studies of MDD and BD suggests that alterations in Glu-related excitatory neurotransmission exist in a subset of patients. Although evidence of isolated abnormalities in GABA-related inhibitory neurotransmission in depression has been less clear, a reduction in efficient energy metabolism in glutamatergic neurons may lead to an imbalance of Glu over GABA neurotransmission that manifests as network connectivity perturbations in BD and MDD. Moreover, given the small number of existing clinical studies with low-powered samples of MDD patients, it is unclear how ketamine’s antidepressant efficacy is associated with specific Glu and/or GABA levels. Further studies are needed at higher magnetic field strength with attention to specific regions of interest such as the sgACC, dACC, fronto-parietal cortices, mPFC, and limbic areas (see Figure 2). In addition, resting-state MEG studies—particularly those focused on beta and gamma band activity—may reveal connectivity changes that correlate with and/or predict ketamine’s antidepressant response.
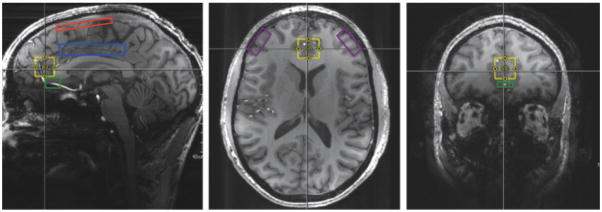
Figure 2. Proposed Regions of Interest to Identify Alterations in Glu/GABA and Abnormalities in Functional Connectivity.
Sagittal, axial, and coronal viewpoints showing the medial prefrontal cortex (mPFC) in yellow. Sagittal viewpoint showing the fronto-parietal cortical region in red and the dorsal anterior cingulate cortex (dACC) in blue. Sagittal and coronal viewpoints showing the subgenual anterior cingulate cortex (sgACC) in green. Axial viewpoint showing the dorsolateral prefrontal cortex (DLPFC) in purple. Hippocampus and amygdala not shown in figure.
*Modified with permission: http://onlinelibrary.wiley.com/doi/10.1002/jmri.24970/full#jmri24970-fig-0001
Evidence of large variations in Glu levels during and after ketamine infusion in healthy and depressed subjects warrants examination of changes in Glu/GABA neurochemistry in conjunction with functional neuroimaging before, during, and after ketamine infusion (87–89, 92–94). Pharmacodynamic fMRI (phfMRI) is a functional imaging technique that measures brain response during infusion of pharmacologic agents, thus providing early and longitudinal measures of drug action in the brain (113). phfMRI studies conducted in healthy volunteers have established test-retest reliability (114) and specificity (115) in association with ketamine infusions (see Table 1), further supporting that such an approach in future ketamine studies may help predict those who may experience an antidepressant response.
Lastly, identifying reliable diagnostic and treatment response biomarkers in MDD has been a challenge given the clinical heterogeneity of this disorder and the wide variability of treatment response (17, 84, 116). Neuroendocrine, neurochemical, and neurophysiological assays in the search for MDD biomarkers have been replicated in some, but not all, translational studies (117–120). Moreover, the general consensus within the field has been that neuroimaging techniques, in conjunction with other illness biomarkers, may be required to diagnose and treat psychiatric disorders (119–121). A combinatorial approach of diverse techniques and modalities (122), will be required to identify underlying neural system abnormalities that are unique to subgroups of MDD patients and/or correlate with antidepressant response.
Acknowledgments
The authors thank the 7SE research unit and staff for their support, and Ms. Ioline Henter for invaluable editorial assistance
Footnotes
Financial Disclosures: Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857). Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 2.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Musazzi L, Racagni G, Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011;59:138–149. doi: 10.1016/j.neuint.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 5.Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993;265:1380–1386. [PubMed] [Google Scholar]
- 6.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 8.Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 2014;231:3663–3676. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- 9.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 10.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 11.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 12.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messer M, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- 15.Niciu MJ, Ionescu DF, Richards EM, Zarate CA., Jr Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014;121:907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 17.Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015;1344:50–65. doi: 10.1111/nyas.12759. [DOI] [PubMed] [Google Scholar]
- 18.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol. 2015;25:1109–1117. doi: 10.1016/j.euroneuro.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Chitty KM, Lagopoulos J, Lee RS, Hickie IB, Hermens DF. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. Eur Neuropsychopharmacol. 2013;23:1348–1363. doi: 10.1016/j.euroneuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Ehrlich A, Schubert F, Pehrs C, Gallinat J. Alterations of cerebral glutamate in the euthymic state of patients with bipolar disorder. Psychiatry Res. 2015;233:73–80. doi: 10.1016/j.pscychresns.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 24.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 25.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 26.de Diego-Adelino J, Portella MJ, Gomez-Anson B, Lopez-Moruelo O, Serra-Blasco M, Vives Y, et al. Hippocampal abnormalities of glutamate/glutamine, N-acetylaspartate and choline in patients with depression are related to past illness burden. J Psychiatry Neurosci. 2013;38:107–116. doi: 10.1503/jpn.110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, et al. Glutamate metabolism in major depressive disorder. Am J Psychiatry. 2014;171:1320–1327. doi: 10.1176/appi.ajp.2014.14010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senaratne R, Milne AM, MacQueen GM, Hall GB. Increased choline-containing compounds in the orbitofrontal cortex and hippocampus in euthymic patients with bipolar disorder: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2009;172:205–209. doi: 10.1016/j.pscychresns.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Port JD, Unal SS, Mrazek DA, Marcus SM. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Res. 2008;162:113–121. doi: 10.1016/j.pscychresns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, et al. Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:427–434. doi: 10.1016/j.pnpbp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Frye MA, Watzl J, Banakar S, O’Neill J, Mintz J, Davanzo P, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 32.Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 33.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 34.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016 Jan 12; doi: 10.1038/mp.2015.206. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhi GS, Byrow Y, Fritz K, Das P, Baune BT, Porter RJ, et al. Mood disorders: neurocognitive models. Bipolar Disord. 2015;17(Suppl 2):3–20. doi: 10.1111/bdi.12353. [DOI] [PubMed] [Google Scholar]
- 36.Fears SC, Service SK, Kremeyer B, Araya C, Araya X, Bejarano J, et al. Multisystem component phenotypes of bipolar disorder for genetic investigations of extended pedigrees. JAMA Psychiatry. 2014;71:375–387. doi: 10.1001/jamapsychiatry.2013.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvadore G, Zarate CA., Jr Magnetic resonance spectroscopy studies of the glutamatergic system in mood disorders: a pathway to diagnosis, novel therapeutics, and personalized medicine? Biol Psychiatry. 2010;68:780–782. doi: 10.1016/j.biopsych.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLorenzo C, Sovago J, Gardus J, Xu J, Yang J, Behrje R, et al. Characterization of brain mGluR5 binding in a pilot study of late-life major depressive disorder using positron emission tomography and [(1)(1)C]ABP688. Transl Psychiatry. 2015;5:e693. doi: 10.1038/tp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Need AB, Baez M, Witkin JM. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther. 2006;319:254–259. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- 43.Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav. 2005;81:901–906. doi: 10.1016/j.pbb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golla SS, Klein PJ, Bakker J, Schuit RC, Christiaans JA, van Geest L, et al. Preclinical evaluation of [(18)F]PK-209, a new PET ligand for imaging the ion-channel site of NMDA receptors. Nucl Med Biol. 2015;42:205–212. doi: 10.1016/j.nucmedbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 47.Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, PMM, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 49.Brady RO, Jr, McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord. 2013;15:434–439. doi: 10.1111/bdi.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soeiro-de-Souza MG, Henning A, Machado-Vieira R, Moreno RA, Pastorello BF, da Costa Leite C, et al. Anterior cingulate Glutamate-Glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol. 2015;25:2221–2229. doi: 10.1016/j.euroneuro.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 52.Vigo DV, Baldessarini RJ. Anticonvulsants in the treatment of major depressive disorder: an overview. Harv Rev Psychiatry. 2009;17:231–241. doi: 10.1080/10673220903129814. [DOI] [PubMed] [Google Scholar]
- 53.Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- 54.Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78:754–762. doi: 10.1016/j.biopsych.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Lally N, An L, Banerjee D, Niciu MJ, Luckenbaugh DA, Richards EM, et al. Reliability of 7T (1) H-MRS measured human prefrontal cortex glutamate, glutamine, and glutathione signals using an adapted echo time optimized PRESS sequence: A between- and within-sessions investigation. J Magn Reson Imaging. 2016;43:88–98. doi: 10.1002/jmri.24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 57.Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 58.Cohen D, Cuffin BN. Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol. 1983;56:38–51. doi: 10.1016/0013-4694(83)90005-6. [DOI] [PubMed] [Google Scholar]
- 59.Reite M, Teale P, Rojas DC. Magnetoencephalography: applications in psychiatry. Biol Psychiatry. 1999;45:1553–1563. doi: 10.1016/s0006-3223(99)00062-1. [DOI] [PubMed] [Google Scholar]
- 60.Atasoy S, Donnelly I, Pearson J. Human brain networks function in connectome-specific harmonic waves. Nat Commun. 2016;7:10340. doi: 10.1038/ncomms10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 62.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 65.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 66.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vargas C, Lopez-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 68.He H, Yu Q, Du Y, Vergara V, Victor TA, Drevets WC, et al. Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. J Affect Disord. 2016;190:483–493. doi: 10.1016/j.jad.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rive MM, Mocking RJ, Koeter MW, van Wingen G, de Wit SJ, van den Heuvel OA, et al. State-Dependent Differences in Emotion Regulation Between Unmedicated Bipolar Disorder and Major Depressive Disorder. JAMA Psychiatry. 2015;72:687–696. doi: 10.1001/jamapsychiatry.2015.0161. [DOI] [PubMed] [Google Scholar]
- 70.Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci U S A. 2011;108:16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brookes MJ, Liddle EB, Hale JR, Woolrich MW, Luckhoo H, Liddle PF, et al. Task induced modulation of neural oscillations in electrophysiological brain networks. Neuroimage. 2012;63:1918–1930. doi: 10.1016/j.neuroimage.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 73.Nugent AC, Robinson SE, Coppola R, Furey ML, Zarate CA., Jr Group differences in MEG-ICA derived resting state networks: Application to major depressive disorder. Neuroimage. 2015;118:1–12. doi: 10.1016/j.neuroimage.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, et al. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS One. 2013;8:e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 79.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harraz MM, Tyagi R, Cortes P, Snyder SH. Antidepressant action of ketamine via mTOR is mediated by inhibition of nitrergic Rheb degradation. Mol Psychiatry. 2016;3:313–319. doi: 10.1038/mp.2015.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, et al. Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety. 2014;31:297–307. doi: 10.1002/da.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19:978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park M, Niciu MJ, Zarate CA., Jr Novel Glutamatergic Treatments for Severe Mood Disorders. Curr Behav Neurosci Rep. 2015;2:198–208. doi: 10.1007/s40473-015-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 88.Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012;26:733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C, et al. In vivo ketamine-induced changes in [(1)(1)C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biol Psychiatry. 2015;77:266–275. doi: 10.1016/j.biopsych.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuxe K, Borroto-Escuela DO. Basimglurant for treatment of major depressive disorder: a novel negative allosteric modulator of metabotropic glutamate receptor 5. Expert Opin Investig Drugs. 2015;24:1247–1260. doi: 10.1517/13543784.2015.1074175. [DOI] [PubMed] [Google Scholar]
- 92.Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2012;15:1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, et al. Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology (Berl) 2015;232:4231–4241. doi: 10.1007/s00213-015-4022-y. [DOI] [PubMed] [Google Scholar]
- 96.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA., Jr Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. 2015;29:596–607. doi: 10.1177/0269881114568041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li CT, Chen MH, Lin WC, Hong CJ, Yang BH, Liu RS, et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: A randomized controlled study. Hum Brain Mapp. 2016;37:1080–1090. doi: 10.1002/hbm.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2014;16:119–128. doi: 10.1111/bdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 105.Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23:1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Shaw AD, Saxena N, LEJ, Hall JE, Singh KD, Muthukumaraswamy SD. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol. 2015;25:1136–1146. doi: 10.1016/j.euroneuro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 108.Gass N, Schwarz AJ, Sartorius A, Schenker E, Risterucci C, Spedding M, et al. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology. 2014;39:895–906. doi: 10.1038/npp.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Front Hum Neurosci. 2012;6:339. doi: 10.3389/fnhum.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamiyama H, Matsumoto M, Otani S, Kimura SI, Shimamura KI, Ishikawa S, et al. Mechanisms underlying ketamine-induced synaptic depression in rat hippocampus-medial prefrontal cortex pathway. Neuroscience. 2011;177:159–169. doi: 10.1016/j.neuroscience.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 111.Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015;35:11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23:862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- 114.De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C, et al. Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage. 2013;64:75–90. doi: 10.1016/j.neuroimage.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 115.Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O’Daly OG, Williams SCR, et al. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J Pharmacol Exp Ther. 2013;345:151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- 116.Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T, et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry. 2015;172:124–138. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, et al. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- 120.Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mayberg HS. Neuroimaging and psychiatry: the long road from bench to bedside. Hastings Cent Rep. 2014;(Spec No):S31–36. doi: 10.1002/hast.296. [DOI] [PubMed] [Google Scholar]
- 122.Nugent AC, Martinez A, D’Alfonso A, Zarate CA, Theodore WH. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J Cereb Blood Flow Metab. 2015;35:583–591. doi: 10.1038/jcbfm.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]